Abstract
Background
Microscopically positive margins to lymph node metastases (R1LNM) are associated with poorer oncological outcomes in patients with Stage 3 colon cancer. These poorer outcomes were seen despite a greater proportion of these patients receiving adjuvant chemotherapy when compared to those with microscopically negative (R0) margins. We sought to determine if differences in the type or duration of adjuvant chemotherapy could account for the differences in outcomes seen between patients with R0 and R1LNM margins.
Methods
A multicentre retrospective study including patients undergoing surgery for Stage 3 colon cancer between 2016–2019 at specialist centres. Patients were stratified according to margins status (R0 vs R1LNM). Type/duration of chemotherapy and oncological outcomes were compared between groups.
Results
718 patients were included, of whom 100 had R1LNM margins (13.1%). Patients with R1LNM margins had significantly poorer 3-year distant metastases-free (R0 78.2% (95% CI 74.5–81.3) versus R1LNM 58.8% (95% CI 47.2–68.6), p < 0.001) and disease specific survival (R0 88.3% (95% CI 85.2–90.9) versus R1LNM 78.5% (95% CI 68.0–85.8), p < 0.001) when compared to those with R0 margins. No differences were noted in the proportion of patients who completed long-course chemotherapy or were treated with oxaliplatin-based combinations between the R1LNM and R0 groups. Differences in outcomes between R0 and R1LNM groups persisted even when only those patients who completed long-course chemotherapy were compared.
Discussion
Differences in adjuvant chemotherapy do not appear to account for the poorer oncological outcomes seen in patients with R1LNM margins after surgery for Stage 3 colon cancer. This suggests that adjuvant chemotherapy may be less effective in this patient group. Further studies to elucidate a potential biological basis for this difference are warranted.
Introduction
Microscopically positive (R1) resection margins have a recognised association with poorer oncological outcomes in patients with rectal cancer [Citation1,Citation2]. Similar associations between R1 margins and disease relapse are seen in patients with colon cancer [Citation3–6]. The impact of R1 margins appears to be dependent on their nature, with different patterns of relapse seen in patients with close margins to the primary tumour when compared to those to lymph node metastases or tumour deposits (referred to in either case as R1LNM margins). In a previous study from our group including more than 1000 patients with Stage III colon cancer, R1LNM margins are associated with increased risks of distant but not local relapse when compared to patients with R0 margins [Citation6]. Interestingly, the poorer outcomes associated with R1LNM margins occurred despite a greater proportion of patients receiving adjuvant chemotherapy than patients with R0 margins [Citation6].
There are two possible explanations for the finding above. The first is that patients with R1LNM margins may have been ‘under-treated’ when compared to those with R0 margins. Whilst adjuvant chemotherapy remains the standard treatment for patients with Stage III colon cancer following potentially curative surgery, determining the most appropriate type and duration of therapy can be challenging. Short-course chemotherapy with the CAPOX regimen (capecitabine/oxaliplatin) has been shown to be non-inferior to long-course chemotherapy in patients with ‘low-risk’ disease, whilst leading to fewer adverse events [Citation7]. In the study named above [Citation6], no details on the type or duration of adjuvant chemotherapy were available, so it is possible that the poorer outcomes in the R1LNM group are due to a greater proportion of patients being treated with short-course chemotherapy.
The second explanation may be that R1LNM margins are in fact a surrogate for more aggressive disease biology or a greater disease burden at the time of diagnosis. There are several lines of evidence that support this hypothesis. In our previous retrospective study of a national cohort including more than 4000 patients with Stage III colorectal cancer, R1LNM margins are associated with a number of poor histopathological prognostic factors, such as extramural venous invasion and lymphatic invasion, as well as more advanced nodal stage when compared to R0 margins [Citation5]. A subsequent analysis of oncological outcomes in over 1000 of these patients suggested that margin status also influences patterns of disease relapse, which differed not only between patients with R0 and R1 resections but also between subdivisions of R1 resections themselves [Citation6]. Furthermore, no difference in surgical quality was found when we compared patients with R0 and R1 margins in the same cohort, implying that technical failure of surgical treatment cannot explain the difference in outcomes between these two groups [Citation8]. If the use of adjuvant therapies does not differ between patients with R0 and R1LNM margins, this would imply that the R1LNM group are more resistant to chemotherapy adding further weight to the argument that R1 margins are surrogates for more aggressive disease.
The purpose of this study was to investigate whether the type and duration of adjuvant chemotherapy differed according to margin status in patients with Stage III colon cancer and to what extent such differences may account for the association of R1LNM with poorer oncological outcomes.
Methods
This was a retrospective cohort study based on data from the Danish Colorectal Cancer Group (DCCG) database and is reported according to STROBE guidelines [Citation9]. The DCCG database is a national cancer registry that includes at least 95% of all patients with newly diagnosed colorectal cancers in Denmark and has been recently validated, with an overall data accuracy of > 95% [Citation10]. Patients diagnosed with Union for International Cancer Control (UICC) Stage III colorectal between 01/01/2016 and 31/12/2019 at 4 specialist colorectal centres were identified. Patients undergoing potentially curative surgery were included in the study cohort. Patients who received neoadjuvant chemotherapy and those undergoing palliative operations, stents or local excisions were excluded, alongside those who died within 90 days of the operation.
Clinicopathological variables were extracted directly from the DCCG database. Data regarding the type and duration of adjuvant/post-operative chemotherapy as well as oncological outcomes (local recurrence, distant metastases and the cause of death) were retrieved from electronic patient journals. Patients were stratified into 2 groups according to their resection margins. Margin status was defined by pathologists at each centre according to national guidelines, with the inking of the non-peritonealised and radial margins prior to sectioning of the specimen. R1LNM resections were defined as the presence of viable cancer cells from a metastatic lymph node or tumour deposit ≤ 1 mm of the resection margin. Tumour deposits were defined as discrete tumour foci in the mesocolon that are distinct from the primary tumour. Patients with R1 margins to the primary tumour were excluded due to the low number of patients in this group (see ). Patients in whom margin status was not assessed or the type of R1 margin was not specified were also excluded. R0 resections were defined as the absence of viable cancer cells ≥ 1 mm from the resection margin. Additional subgroup analyses were performed according to the risk of relapse, defined according to the system outlined in the current European Society of Medical Oncology (ESMO) guidelines (low risk: T stage 1-3 and N Stage 1; high risk: T stage 4 or N stage 2) [Citation11].
The primary outcome of this study was the duration of adjuvant chemotherapy according to resection margin status. Secondary outcomes included the type of chemotherapy administered (single-agent versus oxaliplatin-based combination); and oncological outcomes (local recurrence-free survival (LRFS), distant metastases-free survival (DMFS) and disease-specific survival (DSS)). Oncological outcomes were defined as the time from surgery to the event or point of the last follow-up. Local recurrence was defined as recurrence at the site of the anastomosis. Distant recurrences were defined as recurrences at any other anatomical site. Follow-up was conducted according to national guidelines, with computed tomography scanning at 12 and 36 months after surgery.
Univariable analyses of the differences in clinicopathological variables according to margin status were performed using the Chi-square test for categorical data and the Kruskal Wallis test for continuous data. All analyses were two-sided and considered statistically significant with a p-value < 0.05. LRFS, DMFS and DSS were calculated using the Kaplan Meier method and compared using the log-rank test. Univariable Cox regression analyses were performed to identify prognostic factors of DMFS and DSS. Significant factors (p < 0.05) were combined using backwards selection methods in multivariable analyses. The results of the Cox regression analyses are presented as hazard ratios (HR) with 95% confidence intervals (CI). All analyses were performed using SPSS version 27.0 (IBM, Armonk, New York, USA).
Results
A total of 832 patients were identified, of whom 718 met the final inclusion criteria (). 100 patients had R1LNM margins (13.9%). Clinicopathological demographics stratified according to margin status are shown in . A greater proportion of patients with R1LNM margins had advanced T and N stage, resulting in a greater proportion of these patients being assessed as high-risk for relapse when compared with patients with R0 margins (77.0% versus 46.1%, p < 0.001). The proportion of patients with extramural venous invasion and lymphatic invasion was also higher in the R1LNM group. As seen in our previous studies, R1LNM were also more common in right-sided cancers [Citation5,Citation8]. A greater proportion of patients with R1LNM margins had deficient mismatch repair (dMMR) status, although this was not statistically significant (23.0% versus 15.5%, p = 0.170). The median follow-up for the cohort was 34 months (interquartile range 23–48 months).
Table 1. Clinicopathological characteristics of the patient cohort.
Use of post-operative chemotherapy
Post-operative chemotherapy use according to margin status is shown in . A total of 613 patients (74.3%) were referred for chemotherapy following surgery, with 472 (56.7%) receiving it. Further details regarding the reasons for the omission of chemotherapy in those patients who were referred for it are provided in Supplementary Table 1. No statistically significant differences in the proportion of patients referred for or receiving post-operative chemotherapy according to margin status were noted. No difference in the time from surgery to the start of chemotherapy was noted between groups, with similar proportions of patients receiving single or oxaliplatin-based combination chemotherapy. The proportion of patients who completed long-course chemotherapy (6 months) was similar in both R0 and R1LNM groups.
Table 2. Pattern of adjuvant/post-operative chemotherapy.
Chemotherapy use in patients with high- and low-risk cancers is shown in Supplementary Table 2. A greater proportion of patients with high-risk cancers and R1LNM margins received chemotherapy when compared to those with R0 margins (79.2% versus 66.7%, p = 0.034). However, no differences in the type or duration of chemotherapy were noted. Of note, only 12 patients (52.2%) with low-risk cancers and R1LNM margins received chemotherapy, and only 3 of those completed a long course (13.0%).
Oncological outcomes
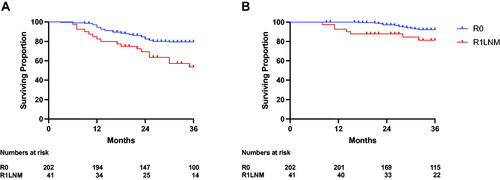
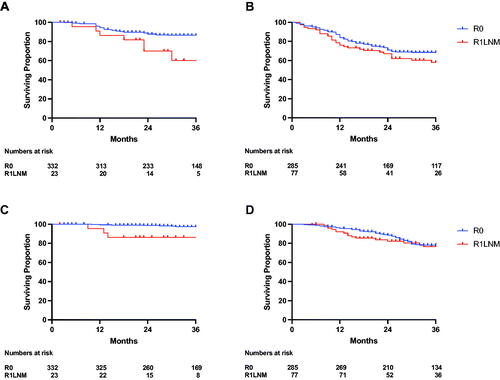
Oncological outcomes stratified according to margin status are shown in . No difference in 3-year LRFS was noted between the two groups (R0 95.4% (95% CI 93.2–96.9) versus R1LNM 97.8% (95% CI 91.5–99.4), p = 0.336). However, R1LNM margins were associated with significantly poorer 3-year DMFS when compared with R0 margins (R0 margins 78.2% (95% CI 74.5–81.3) versus R1LNM margins 58.8% (95% CI 47.2–68.6), p < 0.001). Accordingly, R1LNM margins were also associated with significantly poorer 3-year DSS (R0 margins 88.3% (95% CI 85.2–90.9) versus R1LNM margins 78.5% (95% CI 68.0–85.8), p < 0.001). The differences in both DMFS and DSS were greatest when only patients with low-risk cancer were compared (), whereas no statistically significant difference was found in these outcomes when only patients with high-risk cancers were compared (). Accordingly, while there were clear differences in DMFS and DSS between the low- and high-risk groups in patients with R0 margins, no difference was seen between these risk groups in patients with R1LNM margins (Supplementary Figure 1). Finally, the differences in DMFS and DSS between the R0 and R1LNM groups were unaltered when only patients who completed long-course chemotherapy were included ().
Figure 2. 3-year oncological outcomes in patients with Stage III colon cancer stratified according to margin status (R0 = red, R1LNM = blue). (A) Local recurrence-free survival - R0 95.4% (93.2–96.9) versus R1LNM 97.8% (91.5–99.4), p = 0.336 (B) Distant metastases-free survival - R0 78.2% (74.5–81.3) versus R1LNM 58.8% (47.2-68.6), p < 0.001. (C) Disease-specific survival - R0 88.3% (85.2–90.9) versus R1LNM 78.5% (68.0–85.8), p < 0.001.
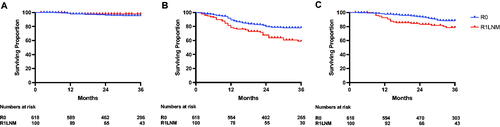
Figure 3. The impact of margin status on oncological outcomes according to perceived risk of relapse in patients with Stage III colon cancer.
Distant-metastases-free survival in (A) low-risk patients (R0 86.4% (82.1–89.8) versus R1LNM 60.1% (31.3–80.0), p = 0.014) and (B) high-risk patients (R0 68.6% (62.5–73.8) versus R1LNM 58.0% (45.1–68.9), p = 0.358). Disease-specific survival in (C) low-risk patients (R0 97.4% (94.5–98.8) versus R1LNM 86.4% (63.4–95.4), p < 0.001) and (D) high-risk patients (R0 78.1% (72.2–82.9) versus R1LNM 76.6% (64.4–85.1), p = 0.452). Low risk was defined as T1-3 and N1 disease. High risk was defined as T4 or N2 disease.
Prognostic factors for oncological outcomes
Univariable (Supplementary Table 3) and multivariable analyses () were then performed to identify factors associated with DMFS and DSS. R1LNM margins were found to be independently associated with poorer DMFS (HR 1.66, 95% CI 1.07–2.60, p = 0.025) alongside risk group, venous invasion, and lymph node yield. However, only the risk group and venous invasion were found to be associated with poorer DSS, with R1LNM margins not found to have a statistically significant association (HR 1.80, 95% CI 0.97–3.34, p = 0.065).
Table 3. Multivariable analyses of factors associated with DMFS and DSS in patients receiving chemotherapy following potentially curative surgery for Stage III colon cancer.
Discussion
Current guidelines for adjuvant therapy in Stage III colon cancer recommend a combination of fluoropyrimidine and oxaliplatin for 3 months in low-risk patients and 6 months of oxaliplatin-based combination therapy in high-risk patients [Citation11]. At present, the only risk stratification in routine use for treatment allocation in these patients is based on T and N stages. However, the current study shows that R1LNM margins are independently associated with increased risks of systemic relapse and disease-related death in patients with Stage III colon cancer when compared with R0 resection. The choice of adjuvant chemotherapy did not appear to differ between these two groups, implying that margin status is not routinely considered in treatment allocation for these patients. This also suggests that the differences in outcome seen between patients with R0 and R1LNM margins cannot be explained by less intensive adjuvant treatment of the latter group, adding further weight to the argument that R1LNM margins may well be a surrogate for more advanced or aggressive disease.
Of particular interest, the impact of R1LNM margins in the current study was most marked in patients who would otherwise be regarded as low-risk for relapse, where R1LNM margins increased the risk of systemic metastases almost 3-fold when compared to R0 resection. Although adjuvant chemotherapy use in these patients did not differ significantly from ‘low-risk’ patients with R0 margins, less than 15% completed long-course chemotherapy. Determining the most appropriate type and duration of adjuvant chemotherapy for individual patients is extremely challenging. The limitations of the current approach to risk stratification are well recognised and there is much interest in identifying factors that can improve risk stratification in patients with colon cancer. Gene expression profiles and the Immunoscore have shown prognostic value in identifying patients at higher risk of relapse [Citation12–14]. Furthermore, there has been much interest in the presence of circulating tumour DNA (ctDNA) as a prognostic factor, with recent studies showing increased risks of relapse in patients with positive ctDNA following surgery [Citation15,Citation16]. R1LNM margins are also of prognostic value, at least in patients with Stage III colon cancer, and, as a standard part of the histopathological assessment of colon specimens in Denmark, is not associated with any additional costs, in contrast to the other methods named above. The results of the current study provide a strong rationale for the consideration of margin status alongside T and N stages in the identification of patients with Stage III colon cancer who are at high risk of relapse. Margin status may be of particular relevance in ’low-risk’ patients, where current guidelines would otherwise recommend short-course adjuvant chemotherapy.
A more pressing challenge is in identifying patients who will benefit most from currently available adjuvant therapies, in particular long-course adjuvant therapy. This dilemma is well illustrated by a recent study of dynamic changes in ctDNA before and after adjuvant chemotherapy in patients with Stage III colon cancer, where positive ctDNA after surgery was associated with increased risks of relapse [Citation15]. However, adjuvant chemotherapy failed to clear ctDNA in most of these patients, with recurrent disease developing in all patients who had persistently positive ctDNA. As such, one may argue that ctDNA-positive patients derived little benefit from adjuvant chemotherapy in that study. In a similar vein, while R1LNM margins were associated with increased risks of relapse in the current study, these poorer oncological outcomes persisted even when only those patients who completed long-course chemotherapy were included. This implies that currently available adjuvant therapies may be less effective in this high-risk group of patients and emphasises the need for the development of more effective systemic therapies for colon cancer.
Determining which adjuvant therapy is most appropriate for patients with R1 margins is further complicated by the fact that this patient group was not represented in randomised trials of current adjuvant regimens [Citation17–19]. The absence of any other evidence necessitates the extrapolation of these results to patients with R1 margins. However, it should not be assumed that patients with R0 and R1 margins are truly comparable, as our previous study found that patients with R1 margins appear to have different patterns of relapse, being less likely to present with oligometastatic disease, and therefore less likely to be treated with curative intent [Citation6].
An interesting question is whether these differences are due to genuinely more aggressive cancer biology in the R1 margins group or due to a greater burden of currently undetectable micrometastases at the time of diagnosis. While the R1LNM group had more patients with advanced T and N stages in the current study, which may suggest more advanced disease at diagnosis, this group also had a higher incidence of cancers with deficient mismatch repair (dMMR), implying that differences in cancer biology may also exist. Furthermore, R1LNM were more common in patients with right-sided colon cancers. Given that a previous study did not demonstrate differences in surgical quality between R0 and R1LNM groups, it may be that the higher incidence of R1LNM margins in right-sided cancers is yet another indicator of the increasingly recognised differences in cancer biology between right- and left-sided colon cancers [Citation8,Citation20]. Additional studies with a larger cohort of patients are underway to determine the extent to which the R1LNM margin group is associated with dMMR status and other common gene mutations. Further translational studies to unravel potential biological differences between the R0 and R1LNM groups would also be of great interest and may even identify novel therapeutic targets.
It may also be the case, that more attention should be given to identifying which patients would benefit from neoadjuvant rather than adjuvant chemotherapy. In a randomised trial of peri-operative chemotherapy versus standard adjuvant chemotherapy in patients with T3 or T4 colon cancers, those patients who started chemotherapy prior to surgery had lower rates of R1 resections (4% versus 20%, p = 0.002) [Citation21]. Although R1 resections were not specifically defined in that study, and likely refer only to those close to the primary tumour, it would be reasonable to assume that increased use of pre- or peri-operative chemotherapy may reduce the incidence of R1LNM margins, with potential improvements in patient prognosis. One potential issue with this approach is the limited accuracy of clinical staging in patients with colon cancer, particularly for nodal metastases, which complicates patient selection for a neoadjuvant approach [Citation22]. Patient selection may be less of an issue for those with dMMR cancers, which have been shown to be exquisitely sensitive to neoadjuvant immune checkpoint blockade [Citation23]. Given the higher incidence of dMMR cancers in the R1LNM group, widespread adoption of neoadjuvant immunotherapy may reduce the incidence of R1LNM margins and improve the outcomes of this subgroup of high-risk patients.
The authors acknowledge the limitations of the current study. Despite the high fidelity of the DCCG database, this study is retrospective and is subject to the biases inherent in that design. The study period started in 2016, when comprehensive pathology data were first coupled to the DCCG database. As a consequence, the follow-up for the current study is relatively short. Although the majority of relapses in patients with colon cancer occur within the first 2 years after surgery [Citation24,Citation25], it would be of interest to see whether the poorer oncological outcomes seen in the R1LNM margins group persist at 5-year’s follow-up. An additional limitation is that the DCCG database does not differentiate between R1 margins to metastatic lymph nodes and those to tumour deposits. Tumour deposits are increasingly recognised as poor prognostic factors in their own right but the current study cannot comment on whether outcomes differ between this further subdivision of R1 margins [Citation26]. A further point to consider is that routine reporting of R1 subdivisions may not be commonplace in other nations, which may limit both comparisons of the results of the current study and the potential to validate these results in a larger international cohort.
In conclusion, margin status did not appear to affect the choice of adjuvant chemotherapy in the current study of patients undergoing potentially curative surgery for Stage III colon cancer. Although based on a retrospective cohort, these data suggest that R1LNM margins are an independent prognostic factor for systemic relapse and should be used to identify a high-risk patient group. Further work should focus on the inclusion of patient with R1 margins in future trials of adjuvant therapies and investigating potential biological differences between these patient groups.
Ethical approval
This study was approved by the Danish Patient Safety Authority (R-20061154) and the Danish Data Protection Agency (P-2020-902).
Supplemental Material
Download MS Word (14.7 KB)Supplemental Material
Download MS Word (11.7 KB)Supplemental Material
Download PDF (31.4 KB)Acknowledgements
None.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
In accordance with Danish law, the data on which the findings of this study are based cannot be made available for sharing.
Additional information
Funding
References
- Birbeck KF, Macklin CP, Tiffin NJ, et al. Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg. 2002;235(4):449–457.
- Nagtegaal ID, Marijnen CA, Kranenbarg EK, et al. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol. 2002;26(3):350–357.
- Khan MA, Hakeem AR, Scott N, et al. Significance of R1 resection margin in colon cancer resections in the modern era. Colorectal Dis. 2015;17(11):943–953.
- Scott N, Jamali A, Verbeke C, et al. Retroperitoneal margin involvement by adenocarcinoma of the caecum and ascending colon: what does it mean? Colorectal Dis. 2008;10(3):289–293.
- Smith HG, Chiranth D, Mortensen CE, et al. The significance of subdivisions of microscopically positive (R1) margins in colorectal cancer: a retrospective study of a national cancer registry. Colorectal Dis. 2022;24(2):197–209.
- Smith HG, Skovgaards DM, Chiranth D, et al. The impact of subdivisions of microscopically positive (R1) margins on patterns of relapse in stage III colorectal cancer - A retrospective cohort study. Colorectal Dis. 2022;24(7):828–837.
- Grothey A, Sobrero AF, Shields AF, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med. 2018;378(13):1177–1188.
- Smith HG, Chiranth DJ, Schlesinger NH. Do differences in surgical quality account for the higher rate of R1 margins to lymph node metastases in right- versus left-sided stage III colon cancer: a retrospective cohort study. Colorectal Dis. 2023;25(4):679–687 .
- von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349.
- Klein MF, Gogenur I, Ingeholm P, et al. Validation of the Danish colorectal cancer group (dccg.dk) database - on behalf of the Danish colorectal cancer group. Colorectal Dis. 2020;22(12):2057–2067.
- Argiles G, Tabernero J, Labianca R, et al. Localised colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(10):1291–1305.
- Pages F, Mlecnik B, Marliot F, et al. International validation of the consensus immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391(10135):2128–2139.
- Niedzwiecki D, Frankel WL, Venook AP, et al. Association between results of a gene expression signature assay and recurrence-free interval in patients with stage II colon cancer in cancer and leukemia group B 9581 (alliance). J Clin Oncol. 2016;34(25):3047–3053.
- Gray RG, Quirke P, Handley K, et al. Validation study of a quantitative multigene reverse transcriptase-polymerase chain reaction assay for assessment of recurrence risk in patients with stage II colon cancer. J Clin Oncol. 2011;29(35):4611–4619.
- Henriksen TV, Tarazona N, Frydendahl A, et al. Circulating tumor DNA in stage III colorectal cancer, beyond minimal residual disease detection, toward assessment of adjuvant therapy efficacy and clinical behavior of recurrences. Clin Cancer Res. 2022;28(3):507–517.
- Tie J, Cohen JD, Lahouel K, et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer. N Engl J Med. 2022;386(24):2261–2272.
- Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27(19):3109–3116.
- Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29(11):1465–1471.
- Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25(16):2198–2204.
- Mukund K, Syulyukina N, Ramamoorthy S, et al. Right and left-sided colon cancers–specificity of molecular mechanisms in tumorigenesis and progression. BMC Cancer. 2020;20(1):317.
- Foxtrot Collaborative G. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol. 2012;13(11):1152–1160.
- Nerad E, Lahaye MJ, Maas M, et al. Diagnostic accuracy of CT for local staging of colon cancer: a systematic review and meta-analysis. AJR Am J Roentgenol. 2016;207(5):984–995.
- Chalabi M, Fanchi LF, Dijkstra KK, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020;26(4):566–576.
- McCall JL, Black RB, Rich CA, et al. The value of serum carcinoembryonic antigen in predicting recurrent disease following curative resection of colorectal cancer. Dis Colon Rectum. 1994;37(9):875–881.
- Tsai HL, Chu KS, Huang YH, et al. Predictive factors of early relapse in UICC stage I-III colorectal cancer patients after curative resection. J Surg Oncol. 2009;100(8):736–743.
- Brouwer NPM, Nagtegaal ID. Tumor deposits improve staging in colon cancer: what are the next steps? Ann Oncol. 2021;32(10):1209–1211.