Abstract
Background
Trials reporting adverse health outcomes (AHOs) in terms of patient-reported outcome measures (PROMs) after contemporary curative treatment of prostate cancer (PC) are hampered by study heterogeneity and lack of new treatment techniques. Particularly, the evidence regarding toxicities after radiotherapy (RT) with the volumetric arc therapy (VMAT) technique is limited, and comparisons between men treated with surgery, primary radiotherapy (PRT) and salvage radiotherapy (SRT) are lacking. The aim of the study was to evaluate change in PROMs 3 months after treatment with robotic-assisted laparoscopic prostatectomy (RALP), PRT and SRT administered with VMAT.
Material and methods
A prospective cohort study of men with PC who received curative treatment at the University Hospital of North Norway between 2012 and 2017 for RALP and between 2016 and 2021 for radiotherapy was conducted. A cohort of 787 men were included; 406 men treated with RALP, 265 received PRT and 116 received SRT.
Patients completed the validated PROM instrument EPIC-26 before (pre-treatment) and 3 months after treatment. EPIC-26 domain summary scores (DSSs) were analysed, and changes from pre-treatment to 3 months reported. Changes were deemed clinically relevant if exceeding validated minimally clinically important differences (MCIDs).
Results
Men treated with RALP reported clinically relevant declining urinary incontinence DSS (−41.7 (SD 30.7)) and sexual DSS (−46.1 (SD 30.2)). Men who received PRT reported worsened urinary irritative DSS (−5.2 (SD 19.6)), bowel DSS (−8.2 (SD 15.1)) and hormonal DSS (−9.6 (SD 18.2)). Men treated with SRT experienced worsened urinary incontinence DSS (−7.3 (SD 18.2)), urinary irritative DSS (−7.5 (SD 14.0)), bowel DSS (−12.5 (SD 16.1)), sexual DSS (−14.9 (SD 18.9)) and hormonal DSS (−23.8 (SD 20.9)).
Conclusion
AHOs 3 months after contemporary curative treatment for PC varied according to treatment modality and worsened in all treatment groups, although most in SRT.
Introduction
For men with localised or locally advanced prostate cancer (PC), curative treatment options include surgery or primary radiotherapy (PRT) in combination with endocrine therapy [Citation1,Citation2]. There are no randomised trials comparing prostatectomy with radiotherapy for men with high-risk disease, and survival differences has not been shown [Citation3]. Consequently, until data from the ongoing trial by the Scandinavian PC Group (SPCG-15) [Citation4] become available, patient treatment decisions are made based on knowledge about the treatment’s impact on adverse health outcomes (AHOs).
RALP is a minimally invasive technique that has shown less peri-operative complications than open surgery and is safe in the long run (similar functional and oncological outcome) [Citation5,Citation6]. At the University Hospital of North Norway (UNN), robotic-assisted laparoscopic prostatectomy (RALP) was established in 2012. However, 17–31% experience a biochemical failure after treatment and are offered salvage radiotherapy (SRT) as a second curative treatment [Citation7,Citation8]. In November 2016, our radiotherapy department implemented volumetric arc therapy (VMAT) and simultaneous integrated boost (SIB) in the curative treatment of PC, with delivery of a precisely sculptured three-dimensional dose distribution to facilitate improved treatment precision while further limiting the doses to healthy tissue [Citation9]. Toxicity within 3–6 months after radiotherapy is defined as acute or subacute toxicity. The pathophysiology of radiation-induced toxicity involves an acute inflammatory response and tissue oedema [Citation10].
Patient-reported outcomes (PROs) are collected through various patient-reported outcome measures (PROMs) and are powerful tools to inform clinicians and policy-makers about AHOs. EPIC-26 is an internationally validated tool for evaluating PROs in men with PC. The instrument focuses on the same five domains as the original EPIC questionnaire; urinary incontinence, urinary irritative/obstructive, bowel, sexual, and hormonal [Citation11]. Most published studies addressing potential side effects after radical treatment for PC during the last decades have used EPIC-26 [Citation12].
Available studies reporting PROMs after curative treatment of PC are limited by heterogeneous study populations and/or lack of details regarding radiotherapy doses and techniques [Citation13–16]. Particularly, the evidence regarding toxicities after RT with the VMAT technique is limited [Citation17], and comparisons between men treated with surgery, PRT and SRT are lacking. Both surgical and radiation techniques have improved the recent years, hence updated information on both short-term and long-term AHOs after treatment is warranted.
In this prospective population-based cohort study, the aim was to evaluate the impact of RALP, PRT and SRT (both with VMAT) on AHOs measured by EPIC-26 before and 3 months after treatment, with a special emphasis on change in AHOs.
Patients and methods
Patients
Together with implementing new curative treatment techniques of prostate cancer at our hospital, prospective registries for registration of PROs were established. A quality registry for men treated with RALP at UNN was established in September 2012 after the implementation of RALP as a standard procedure. A quality registry for men treated with curative radiotherapy for PC was established at UNN in November 2016 after the implementation of new radiotherapy techniques including VMAT and SIB. In both registries, PROMs were evaluated by the questionnaire EPIC-26. The first EPIC-26 was filled out at the first patient visit at UNN, immediately before surgery or radiotherapy, but after initiation of endocrine treatment (for primary irradiated patients, endocrine treatment was initiated at local hospital). Patients completed EPIC-26 before treatment and 3 months after treatment and returned the questionnaire by mail. All men who completed EPIC-26 before and/or 3 months after treatment were included in this study.
The study was approved by the data protection officer (0637 and 0345) and the Regional Committee for Medical and Research Ethics North (2018/1849 and 2018/369) and informed written consent was obtained from all participants.
Medical and treatment characteristics
The study obtained information about disease, treatment characteristics and comorbidity from medical records. Prostate specific antigen (PSA) was reported at start of primary treatment and at start of RT. International Society of Urological Pathology (ISUP) 2014 grade [Citation18] and American Joint Committee of Cancer TNM-classification, as well as risk group stratification [Citation19] was registered (). Most high-risk patients were screened using bone scintigraphy or MRI scan of the spine.
Table 1. Patient characteristics according to type of curative treatment.
Curative surgical treatment in terms of RALP included removal of the prostate gland, seminal vesicles and in high-risk patients, pelvic lymph nodes [Citation20]. Curative radiotherapy was administered with the VMAT and SIB techniques and applied in combination with endocrine treatment (Supplementary Table 1) [Citation4,Citation21–24].
Outcomes
The Norwegian version of EPIC-26 has shown acceptable reliability and validity in psychometric testing [Citation25]. EPIC-26 covers five domains (urinary incontinence, urinary irritative/obstructive, bowel, sexual and hormonal; Supplementary Table 3) [Citation26]. Further, EPIC-26 comprises three questions on overall problem; urinary, bowel and sexual. Except for overall urinary problem, the problem scores are included as an item in the domain summary scores (DSSs), but as they are of high clinical relevance, several studies report problem scores in addition to DSSs.
DSSs and problem scores were computed by the standardised EPIC-26 scoring instructions in which individual items are transformed linearly to a 0-100 scale and high scores represent better function or lower problems [Citation27]. Corresponding DSSs were not calculated if ≥ 20% of the items were missing, and g [Citation27] missing data were not imputed. Number of patient replies for each EPIC-26 DSS is presented in Supplementary Table 2. For patients who completed EPIC both pre-treatment and 3 months after treatment, change in DSS and problem score were calculated as the score 3 months post-treatment minus the pre-treatment score.
Minimally clinically important differences (MCIDs) are thresholds for clinically relevant differences. Previously published validated cut-off values of MCIDs for each EPIC-26 domain are: 6 points for urinary incontinence, 5 points for urinary irritation, 4 points for bowel function, 10 points for sexual function, and 4 points for hormonal function [Citation28].
Statistical analysis
Categorical variables are presented as counts (proportion) and continuous variables are presented as median (interquartile range [IQR]) or mean (standard deviation [SD]). Categorical variables were compared using the chi-square test and continuous variables were compared using one-way ANOVA or t-tests depending on the number of groups being compared. EPIC-26 problem-scores were compared between time points using paired t-tests. Two-sided p-values of < 0.05 were considered statistically significant. Data were analysed using IBM SPSS version 27.
Results
Patient characteristics
Between September 2012 and October 2017, 533 men with PC treated with curative surgery were enrolled in the surgical registry of whom 127 were excluded (), Between November 2016 and March 2021, 463 men with PC were treated with curative radiotherapy, of whom 422 (91%) were included in the radiotherapy registry, and among them 41 men were excluded (). Overall, 406 patients who underwent RALP and 381 patients who received radiotherapy, including 265 who received PRT and 116 who received SRT, were included in the study, for a total of 787 patients.
Figure 1. Flow chart presenting the study population. RALP = Robotic-assisted laparoscopic prostatectomy; n = number; EPIC-26 = Expanded Prostate Cancer Index Composite 26-item. 1The database for surgical patients were administered by different personnel than the radiotherapy database and information about reasons for exclusion is missing. 2Data are presented as n (%). 349 of the RALP patients were later included in the radiotherapy registry as they received salvage radiotherapy.
Men treated with PRT were older (median age 73.5 years) than men treated with RALP (median age 65.1 years) and SRT (median age 66.3 years, ), with both differences being statistically significant, p < 0.001). Men treated with PRT or SRT had higher ISUP grade group, higher T stage and a larger proportion were high-risk patients compared to men treated with RALP. Hypertension, coronary disease and diabetes mellitus were more prevalent among men treated with PRT (p < 0.001). Overall, 27.5% of men treated with PRT and 56.6% of men treated with SRT received pelvic lymph node irradiation ().
Table 2. Treatment details.
Urinary function and problem
Pre-treatment urinary incontinence DSSs were similar for men treated with RALP and PRT ((90.2 (SD 17.8) and 88.8 (SD 17.0) respectively), while men treated with SRT had lower scores (69.4 (SD 27.2), p < 0.001, , ). Men treated with RALP and SRT reported clinically important changes in DSS (mean change RALP −41.7 (SD 30.7), mean change SRT −7.3 (SD 18.2), ).
Figure 2. EPIC-26 mean domain summary scores across treatment groups before and 3 months after treatment. EPIC-26 = Expanded Prostate Cancer Index Composite 26-item; SD = standard deviation.
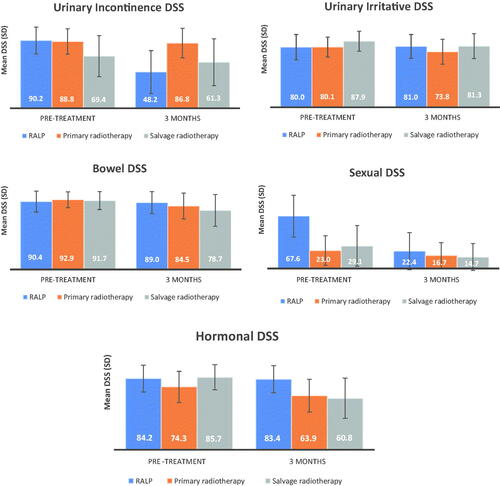
Figure 3. EPIC-26 mean domain summary score changes across treatment groups from pre-treatment to 3 months after treatment. EPIC-26 = Expanded Prostate Cancer Index Composite 26-item. Brackets indicate standard deviation. *indicate change exceeding the minimally clinically important differences. Changes in domain score was calculated for patients who completed EPIC-26 both pre-treatment and at three months after treatment
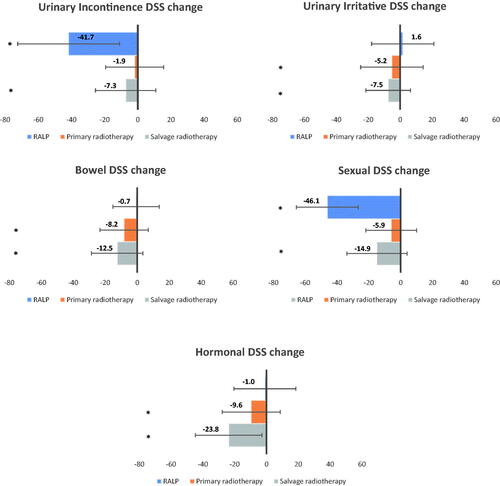
Table 3. EPIC-26 domain summary scores and problem scores.
Pre-treatment urinary irritative DSS was significantly higher for men treated with SRT compared with RALP and PRT (mean 87.9 (SD 13.3) vs 80.0 (SD 16.8) and 80.1 (SD 16.5), p < 0.001). After RALP there was a small improvement, with no significant change in DSS. For patients treated with radiotherapy, the urinary irritative DSS was decreased (mean changes PRT −5.2 (SD 19.6); SRT −7.5 (SD 14.0)).
Mean overall urinary problem score before treatment was similar across treatment groups (, Supplementary Table 3). Mean overall urinary problem scores changes were statistically significant (p < 0.001) for RALP (-21.8 (SD 34.7)), PRT (-8.3 (SD 22.3)) and SRT (-15.5 (SD 29.3)).
Bowel function and problem
Mean bowel DSSs before treatment were similar across treatment groups (, ). Men treated with PRT and SRT reported clinically important declines in bowel function (mean DSS changes of −8.2 (SD 15.1) and −12.5 (SD 16.1), respectively, ).
Overall bowel problem score before treatment was similar across treatment groups (, Supplementary Table 3). Mean overall bowel problem score changes were statistically significant (p < 0.001) for all treatment groups, most pronounced for men treated with PRT and SRT (-10.8 (SD 26.4) and −17.1 (SD 25.4), respectively, p < 0.001).
Sexual function and problem
Mean pre-treatment sexual DSS for men undergoing RALP was 67.6 (SD 26.9), compared to 23.0 (SD 19.0) and 29.1 (SD 26.3), for PRT and SRT respectively (p < 0.001, , ). Mean sexual DSS changes for men treated with RALP and SRT were clinically important; −46.1 (SD 30.2) and −14.9 (SD 18.9), respectively ).
Overall pre-treatment sexual problem varied across treatment groups, with the lowest bother before RALP (, Supplementary Table 3). Mean score changes for overall sexual problem for patients treated with RALP and SRT were statistically significant (p < 0001) and were −39.3 (SD 42.7), and −8.7 (SD 33.1), respectively.
Hormonal function
The mean DSS before treatment for PRT was 74.3 (SD 18.4) compared to 84.2 (SD 15.9) and 85.7 (SD 14.8) for RALP and SRT respectively, p < 0.001, , ). The mean hormonal DSS changes for PRT and SRT were −9.6 (SD 18.2) and −23.8 (SD 20.9), respectively, ).
Discussion
In this prospective cohort study, we report PROMs in terms of EPIC-26 DSSs immediately before and 3 months after contemporary curative treatment for PC with RALP, PRT or SRT. Men treated with RALP experienced reduced urinary continence and sexual function. Men treated with PRT reported reduced urinary irritative, bowel and hormonal function. Men treated with SRT experienced worsening of all five functional domains. Studies reporting EPIC-26 DSSs following radiotherapy with VMAT are scarce. To the best of our knowledge, there are no other studies reporting PROs after SRT using the VMAT technique, and no studies which compare PROs after RALP, primary and salvage radiotherapy with VMAT.
Urinary incontinence DSS after RALP was reduced, in line with other studies [Citation15,Citation29] (Supplementary Table 4A). Urinary continence function after PRT was not reduced herein, while other studies are diverging [Citation15,Citation29–32] (Supplementary Table 4B). For men treated with SRT, we observed a low pre-treatment urinary incontinence DSS as could be expected due to previous surgery. In contrast to other studies, we noted reduced urinary incontinence DSS after SRT [Citation33–36] (Supplementary Table 4C).
Urinary irritative function was not changed after RALP, in line with the unfavourable risk group of Hoffman et al. [Citation15], but in contrast to Sanda et al. [Citation29] and the favourable risk group of Hoffmann [Citation15], reporting improved function. We observed a decline in urinary irritative function after PRT and SRT, in contrast to comparable studies reporting a stable [Citation29,Citation30,Citation32–34] or improving irritative function [Citation15,Citation35]. Overall urinary problem worsened in all treatment groups, most for men treated with RALP, consistent with Stensvold et al. [Citation37]. We observed worsened urinary problem after PRT in line with Caumont et al. [Citation31]. In contrast to previous studies [Citation34,Citation36] we also noted worsened urinary problem after SRT.
Bowel function for men treated with RALP was not altered, consistent with other studies [Citation6,Citation15,Citation29]. Our reduced DSS after PRT is in line with most comparable studies [Citation15,Citation29,Citation31,Citation32], however Ischii et al. [Citation30] observed a stable bowel function. In our study, bowel DSS was reduced after SRT, while other studies reported a smaller but clinically relevant decline [Citation34] or a stable bowel function [Citation33,Citation35,Citation36]. We observed a worsened bowel problem after both PRT and SRT, which was more pronounced than comparable studies [Citation31,Citation34,Citation36].
We observed a decline in sexual function after RALP, consistent with other studies [Citation15,Citation29]. Pre-treatment sexual DSS were low for patients treated with PRT most probably due to older age, more comorbidity and ongoing neoadjuvant ADT. Pre-treatment sexual function for men treated with SRT was low, probably related to the previous prostatectomy with impact on erectile function and corroborate results from other studies. We observed a clinically relevantly reduced sexual function after SRT, in contrast to other studies [Citation33–36]. The reduced sexual function after SRT could partly be explained by hormonal treatment, as LHRH-agonist was initiated at start of radiotherapy, whereas comparable studies applied neoadjuvant ADT. Sexual problem worsened in all treatment groups, in agreement with most other studies [Citation31,Citation34,Citation37].
Hormonal function was not changed after RALP, in line with Hoffman et al. [Citation15] and Sanda et al. [Citation29]. Pre-treatment hormonal DSS was lower for patients treated with PRT compared to SRT and RALP, probably due to ongoing treatment with neoadjuvant ADT. Still we observed further reduction of hormonal function after PRT, in line with other studies [Citation15,Citation29]. The large decline in hormonal function after SRT, is probably related to treatment with LHRH-agonist initiated at start of radiotherapy.
In contrast to comparable studies [Citation33–36], we observed clinically reductions of EPIC-26 DSSs after SRT in all five functional categories. Comparisons are challenging due to heterogeneity of patient inclusion (e.g., high-risk patients) as well as treatment regimens (e.g., radiation techniques and doses). Others have used lower prostate bed doses (66–68 Gy) compared to our study, while doses to elective pelvic nodes varied (50.4–56 Gy). Our study comprises a high number of SRT patients receiving pelvic nodal irradiation (57%) compared to van Gysen (15.6%) [Citation36]. The percentage of SRT patients receiving concomitant/adjuvant ADT was high in our study (92.2%). Some of the reported toxicity discrepancy could be due to a larger proportion of more heavily treated patients than in other studies. The reduction of bowel function is especially worrying, but also a reduced urinary and sexual function is of note, since previous surgery already has influenced these functions. The number of men eligible for treatment with SRT will probably increase, as more patients with high-risk disease undergo surgery [Citation38]. EAU has proposed a risk stratification for biochemical recurrence of PC after prostatectomy into low-risk and high risk based on PSA-doubling time and ISUP grade [Citation39,Citation40]. For some patients with low-risk biochemical recurrence, the risk of side effects probably outweighs the potential benefit of SRT.
When advising men with PC on curative treatment options, knowledge of PROs after treatment is important. Our study adds important information, especially regarding the decline in all five domains in the SRT group. Thorough information about AHOs to be expected may increase patient acceptance of symptoms. However, we observed large inter-individual differences of AHOs, which also must be communicated to the patient.
Strengths of our study include the prospective and longitudinal design and the relatively large number of patients treated in a consistent manner throughout the study period. Of note, the contemporary radiotherapy technique VMAT was applied in all irradiated patients. Furthermore, the pre-treatment PROMs available enables us to calculate changes for the individual patient and compare treatment groups. Limitations include variation in the completion rates of the EPIC questionnaire, lack of data before start of neoadjuvant endocrine treatment and the reporting of PROMs only 3 months after treatment. One should also be careful to directly compare our results to the other mentioned studies, since several studies lack calculation of the individual DSS changes. An example is the Hoffmann study [Citation15], which is older with different pre-treatment staging, differences in surgical and radiotherapy techniques, however their strength is reporting of data with a longer follow-up (5 years). Contemporary information on long-term AHOs and especially longitudinal assessment of patients treated with prostatectomy and subsequent SRT is warranted.
Conclusions
Contemporary curative PC therapy is associated with significant and specific AHOs according to treatment modality. The combined treatment of prostatectomy and SRT is especially associated with short-term reduced urinary, bowel, sexual and hormonal function. The risk of increased symptom burden must be communicated to the patient when making decisions on primary treatment modality, especially when discussing surgery in patients with high-risk disease.
Supplemental Material
Download MS Word (21.8 KB)Supplemental Material
Download MS Word (18.5 KB)Supplemental Material
Download MS Word (18 KB)Supplemental Material
Download MS Word (17.6 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data are not available due to restrictions from the data protection officer and the Regional Committee for Medical and Research Ethics.
References
- Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352(19):1977–1984. doi: 10.1056/NEJMoa043739.
- Widmark A, Klepp O, Solberg A, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373(9660):301–308. doi: 10.1016/S0140-6736(08)61815-2.
- Hamdy FC, Donovan JL, Lane JA, et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415–1424. doi: 10.1056/NEJMoa1606220.
- Surgery Versus Radiotherapy for Locally Advanced Prostate Cancer (SPCG-15). ClinicalTrials.gov Identifier: NCT02102477. 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT02102477.
- Yaxley JW, Coughlin GD, Chambers SK, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet. 2016;388(10049):1057–1066. doi: 10.1016/S0140-6736(16)30592-X.
- Coughlin GD, Yaxley JW, Chambers SK, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: 24-month outcomes from a randomised controlled study. Lancet Oncol. 2018;19(8):1051–1060. doi: 10.1016/S1470-2045(18)30357-7.
- Connolly SS, Cathcart PJ, Gilmore P, et al. Robotic radical prostatectomy as the initial step in multimodal therapy for men with high-risk localised prostate cancer: initial experience of 160 men. BJU Int. 2012;109(5):752–759. doi: 10.1111/j.1464-410X.2011.10548.x.
- Vatne K, Stensvold A, Myklebust T, et al. Pre- and post-prostatectomy variables associated with pelvic post-operative radiotherapy in prostate cancer patients: a national registry-based study. Acta Oncol. 2017;56(10):1295–1301. doi: 10.1080/0284186X.2017.1314006.
- Teoh M, Clark CH, Wood K, et al. Volumetric modulated arc therapy: a review of current literature and clinical use in practice. Br J Radiol. 2011;84(1007):967–996. doi: 10.1259/bjr/22373346.
- Smit SG, Heyns CF. Management of radiation cystitis. Nat Rev Urol. 2010; 7(4):206–214. doi: 10.1038/nrurol.2010.23.
- Szymanski KM, Wei JT, Dunn RL, et al. Development and validation of an abbreviated version of the expanded prostate cancer index composite instrument for measuring health-related quality of life among prostate cancer survivors. Urology. 2010;76(5):1245–1250. doi: 10.1016/j.urology.2010.01.027.
- Ávila M, Patel L, López S, et al. Patient-reported outcomes after treatment for clinically localized prostate cancer: a systematic review and meta-analysis. Cancer Treat Rev. 2018;66:23–44. doi: 10.1016/j.ctrv.2018.03.005.
- Chien GW, Slezak JM, Harrison TN, et al. Health-related quality of life outcomes from a contemporary prostate cancer registry in a large diverse population. BJU Int. 2017;120(4):520–529. doi: 10.1111/bju.13843.
- Barocas DA, Alvarez J, Resnick MJ, et al. Association Between radiation therapy, surgery, or observation for localized prostate cancer and Patient-Reported outcomes After 3 years. Jama. 2017;317(11):1126–1140. doi: 10.1001/jama.2017.1704.
- Hoffman KE, Penson DF, Zhao Z, et al. Patient-Reported outcomes Through 5 years for active surveillance, surgery, brachytherapy, or external beam radiation With or Without androgen deprivation therapy for localized prostate cancer. Jama. 2020;323(2):149–163. doi: 10.1001/jama.2019.20675.
- Donovan JL, Hamdy FC, Lane JA, et al. Patient-Reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375(15):1425–1437. doi: 10.1056/NEJMoa1606221.
- Lawrie TA, Green JT, Beresford M, et al. Interventions to reduce acute and late adverse gastrointestinal effects of pelvic radiotherapy for primary pelvic cancers. Cochrane Database Syst Rev. 2018;1(1):Cd012529.
- Epstein JI, Egevad L, Amin MB, et al. The 2014 international society of urological pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40(2):244–252. doi: 10.1097/PAS.0000000000000530.
- European Association of Urology Guidelines Staging. Available from: https://uroweb.org/guideline/prostate-cancer/chapter/classification-and-staging-systems.
- De Carlo F, Celestino F, Verri C, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: surgical, oncological, and functional outcomes: a systematic review. Urol Int. 2014;93(4):373–383. doi: 10.1159/000366008.
- Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047–1060. doi: 10.1016/S1470-2045(16)30102-4.
- Siegmann A, Bottke D, Faehndrich J, et al. Dose escalation for patients with decreasing PSA during radiotherapy for elevated PSA after radical prostatectomy improves biochemical progression-free survival: results of a retrospective study. Strahlenther Onkol. 2011;187(8):467–472. doi: 10.1007/s00066-011-2229-3.
- Briganti nomogram 2018. https://www.evidencio.com/models/show/1555.
- European Association of Urology Guidelines Treatment. https://uroweb.org/guidelines/prostate-cancer/chapter/treatment.
- Fosså SD, Storås AH, Steinsvik EA, et al. Psychometric testing of the norwegian version of the expanded prostate cancer index composite 26-item version (EPIC-26). Scand J Urol. 2016;50(4):280–285. doi: 10.3109/21681805.2016.1163617.
- The Expanded Prostate Cancer Index Composite - Short Form. https://medicine.umich.edu/dept/urology/research/epic.
- Scoring Instructions for the Expanded Prostate cancer Index Composite Shurt Form (EPIC-26). https://medicine.umich.edu/sites/default/files/content/downloads/Scoring%20Instructions%20for%20the%20EPIC%2026.pdf.
- Skolarus TA, Dunn RL, Sanda MG, et al. Minimally important difference for the expanded prostate cancer index composite short form. Urology. 2015;85(1):101–105. doi: 10.1016/j.urology.2014.08.044.
- Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250–1261. doi: 10.1056/NEJMoa074311.
- Ishii K, Yamanaga T, Ogino R, et al. Bowel and urinary quality of life after whole-pelvic versus prostate-only volumetric-modulated arc therapy for localized prostate cancer. Pract Radiat Oncol. 2018;8(2):e49–e55. doi: 10.1016/j.prro.2017.10.005.
- Caumont F, Conti G, Hurwitz LM, et al. A prospective analysis of health-related quality of life in intermediate and high-risk prostate cancer patients managed with intensity modulated radiation therapy, with vs. without hormonal therapy. Urol Oncol. 2020;38(10):794.e1-794–e9. doi: 10.1016/j.urolonc.2020.02.007.
- Bai M, Gergelis KR, Sir M, et al. Comparing bowel and urinary domains of patient-reported quality of life at the end of and 3 months post radiotherapy between intensity-modulated radiotherapy and proton beam therapy for clinically localized prostate cancer. Cancer Med. 2020;9(21):7925–7934. doi: 10.1002/cam4.3414.
- Akthar AS, Liao C, Eggener SE, et al. Patient-reported outcomes and late toxicity After postprostatectomy intensity-modulated radiation therapy. Eur Urol. 2019;76(5):686–692. doi: 10.1016/j.eururo.2019.05.011.
- Berlin A, Cho E, Kong V, et al. Phase 2 trial of guideline-based postoperative image guided intensity modulated radiation therapy for prostate cancer: toxicity, biochemical, and patient-reported health-related quality-of-life outcomes. Pract Radiat Oncol. 2015;5(5):e473–e482. doi: 10.1016/j.prro.2015.02.015.
- Corbin KS, Kunnavakkam R, Eggener SE, et al. Intensity modulated radiation therapy after radical prostatectomy: early results show no decline in urinary continence, gastrointestinal, or sexual quality of life. Pract Radiat Oncol. 2013;3(2):138–144. doi: 10.1016/j.prro.2012.05.005.
- van Gysen KL, Kneebone AB, Guo L, et al. Health-related quality of life using intensity-modulated radiation therapy for post-prostatectomy radiotherapy. J Med Imaging Radiat Oncol. 2013;57(1):89–96. doi: 10.1111/j.1754-9485.2012.02464.x.
- Stensvold A, Dahl AA, Brennhovd B, et al. Bother problems in prostate cancer patients after curative treatment. Urol Oncol. 2013;31(7):1067–1078. doi: 10.1016/j.urolonc.2011.12.020.
- Nezolosky MD, Dinh KT, Muralidhar V, et al. Significant increase in prostatectomy and decrease in radiation for clinical T3 prostate cancer from 1998 to 2012. Urol Oncol. 2016;34(2):57.e15-22–57.e22. doi: 10.1016/j.urolonc.2015.09.002.
- Van den Broeck T, van den Bergh RCN, Arfi N, et al. Prognostic value of biochemical recurrence Following treatment with curative intent for prostate cancer: a systematic review. Eur Urol. 2019;75(6):967–987. doi: 10.1016/j.eururo.2018.10.011.
- Tilki D, Preisser F, Graefen M, et al. External validation of the european association of urology biochemical recurrence risk groups to predict metastasis and mortality After radical prostatectomy in a european cohort. Eur Urol. 2019;75(6):896–900. doi: 10.1016/j.eururo.2019.03.016.
