Introduction
Calcium electroporation (CaEP) has demonstrated promising results for local treatment of cutaneous metastases from breast cancer, melanoma, and head-and-neck cancer in initial clinical trials [Citation1–3]. CaEP is an innovative cancer treatment that utilises local application of high-voltage pulses to permeabilise cell membranes, initiating a rapid influx of intratumourally injected calcium ions, to selectively kill cancer cells [Citation4,Citation5].
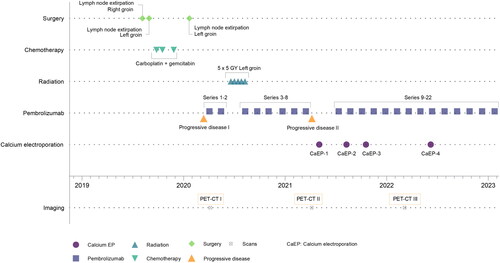
This case study reports an improved response to immunotherapy following CaEP treatment in a patient with metastasis from disseminated urothelial cancer. Long-term disease control of regional recurring metastases was achieved through a combination of systemic immunotherapy and local treatment with CaEP (). Notably, urothelial cancer with skin manifestation is typically associated with a poor prognosis [Citation6].
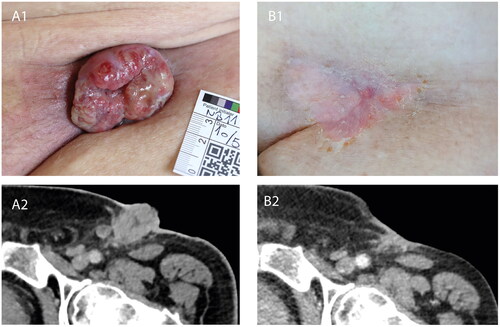
Figure 1. Tumour response to calcium electroporation and pembrolizumab. (A1) Clinical photograph of the left inguinal region prior to initial instatement of CaEP, showing clinical progression of recurring inguinal lymph node metastasis during first bout of pembrolizumab. (A2) Contrast-enhanced CT-scan axial view at the level of the left inguinal region, performed at approximately the same time as the clinical photograph in A1, confirming progression of lymph node metastasis. (B1) Clinical photograph of the left inguinal region following four treatments of local calcium electroporation and second bout of pembrolizumab, showing complete clinical response. (B2) Contrast-enhanced CT-scan axial view at the level of left inguinal region, performed at approximately the same time as the clinical photograph in B1, showing regression of both the exophytic and deeper seated parts of the lymph node metastases.
The combination of electroporation and calcium may be used for local tumour management, yet both preclinical and clinical studies have indicated an immunological effect of CaEP [Citation7]. The toxic calcium levels induce tumour necrosis, releasing cellular components such as immunostimulatory danger-associated molecular patterns (DAMPs), for example, HMGB1 (high-mobility group protein 1) and ATP (adenosine triphosphate) [Citation4,Citation8–10]. DAMPs can activate antigen-presenting cells (APCs), potentially leading to the recruitment of effector T-cells to the tumour microenvironment [Citation7,Citation11].
An immune-stimulatory effect of CaEP was described in a preclinical study using an immunocompetent mouse model where mice developed systemic anti-tumour immunity following treatment [Citation7]. Similarly, an abscopal response was reported in the first patient treated with CaEP for cutaneous melanoma metastases [Citation12]. These findings suggest that CaEP may hold potential in stimulating immune responses in cancer patients.
Anti PD-L1 (anti-programmed death-ligand 1) and other checkpoint inhibitors have expanded the scope of oncological treatments, enhancing survival rates for numerous cancer patients [Citation13]. However, many tumours remain unresponsive, particularly those described as excluded from immune-infiltration [Citation14,Citation15] or immunogenically ‘cold’ [Citation16]. In fact, response rates for patients with metastatic urothelial cancer have been reported as 15–21%, with long-term responses observed in only a few cases [Citation17]. Methods capable of converting ‘cold’ tumours into ‘hot’, inflamed ones, thereby improving beneficial immune cell infiltration, could potentially enhance the effectiveness of immunotherapeutic treatments [Citation18].
Case report
A 74-year-old male was initially diagnosed with non-invasive urothelial high-grade papilloma (stage pTa). Two years later, the patient presented with new papillomas in the bladder and prostate, and underwent curative treatment with intravesicular BCG (Bacillus Calmette-Guerin).
Sometime later, the patient discovered a lump in his right inguinal region. A computed tomography scan (CT-scan) revealed lymph node metastases in both inguinal regions and along the right iliac vessels. Surgical removal of a lymph node in the right inguinal region confirmed metastasis from urothelial carcinoma. The patient received three series of neoadjuvant carboplatin and gemcitabine, from which he experienced side effects of severe chest pain and abdominal pain following treatment. After chemotherapy, a cystoscopy was performed to locate a primary tumour, but no abnormal findings were found. Residual tumour in a lymph node in the left inguinal region was surgically removed four months later ().
Five months afterward, lymph node metastasis recurred in the left inguinal region, and the patient received his first treatment with pembrolizumab immunotherapy. Positron emission tomography-computed tomography scan (PET-CT scan) evaluated the tumour in the left inguinal region to approximately 54 mm in depth (see Supplementary Figure S1A). Due to the extent of the disease, the inguinal recurrence was not surgically resected, but was irradiated (5 Gy × 5) (Progressive disease I, ). The patient continued a regimen of pembrolizumab immunotherapy every six weeks, with initial moderate effect ().
Four months later, there was a recurrence of lymph node metastasis in the left inguinal region, which progressed rapidly and erupted through the skin as an exophytic tumour, causing infection and ongoing secretion (Progressive disease II, and ). The patient was disturbed by pain, smell, bleeding from the tumour, and secretion from a central fistula. Pembrolizumab was discontinued as the patient’s disease had progressed during treatment.
A repeated PET-CT scan evaluated the tumour in the left inguinal region to approximately 49 mm in depth (Supplementary Figure S1B), with close relation to the left femoral nerves and vessels. As surgery was not possible due to the dissemination of the disease, and since the area had already been irradiated, the patient was offered local protocol treatment with CaEP three months later (). The patient was informed that the protocol treatment was palliative, and the aim of treatment was to shrink the exophytic component to relieve symptoms, as well as obtaining sequential biopsies and blood samples.
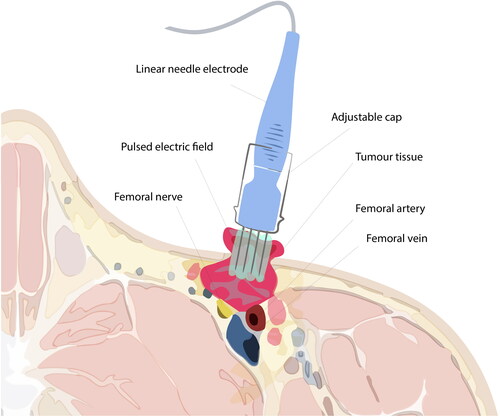
CaEP used 15 ml 220 mM CaCl2, injected directly into the tumour with a margin, and application of 52 rounds of eight 1000 V/cm, 100 microsecond pulses with a linear electrode and pulse generator (Cliniporator, IGEA, Italy) [Citation19]. As the electrode used for CaEP in the protocol was 3 cm, deeper tissues could not be reached, and caution regarding pressure of the needles was imperative, so as not to cause damage to the inguinal structures ().
Figure 3. Illustration of electroporation treatment of superficial inguinal tumour. Electroporation is conducted subsequently after calcium injection of the tumour and margin area. The electrode, at full length (3 cm), cannot reach the most profound parts of the tumour. Caution is required during treatment due to the anatomical structures, e.g. the femoral vein, nerve, and artery, adjacent to the tumour tissue. Note: Exaggerated dimensions, features and anatomical structures for illustration purposes in this image, which is not an exact representation of the patient.
The patient tolerated the treatment well. Apart from discomfort from local anaesthesia, no other adverse events were reported. The clinical evolution of the CaEP-treated area can be followed in . CaEP (CaEP-1, ) elicited a clear response, with the majority of the patient’s cutaneous tumour-component eradicated in two weeks (). The patient experienced immediate symptom relief from bleeding and secretion from the affected area. After CaEP treatment, physicians reinstated pembrolizumab therapy for systemic palliative disease control ().
Three months later, a recurrence occurred, and the patient was re-treated with CaEP with a good response (CaEP-2, ). After another two months, a small exophytic recurrence grew, and the patient was again re-treated for the third time, with a good response (CaEP-3, ).
A PET-CT scan was performed just after the patient’s third CaEP treatment, which showed remission in deep-seated tumour tissue of the inguinal area, not directly affected by the electroporation device, and the tumour now measured approximately 26 mm (compared to approximately 49 mm prior to CaEP treatment) (Supplementary Figure S1C). This area had previously progressed on immunotherapy but seemed to respond to the re-established pembrolizumab treatment.
The patient displayed stable disease with histological and clinical complete response in the skin after four CaEP treatments ( and ).
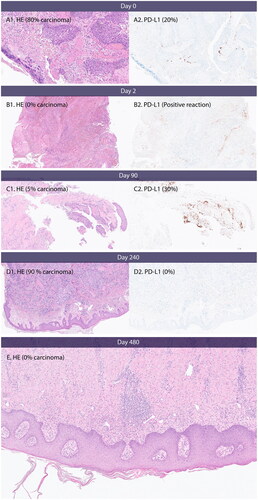
Figure 4. PD-L1 expression, necrosis and inflammation. (A) Day 0, baseline before first CaEP treatment. A1 shows carcinoma with necrosis and inflammation. (B) Day 2, after one CaEP treatment. B1 shows necrosis and inflammation. (C) Day 90, before second CaEP treatment. C1 shows pronounced inflammation and no necrosis. C2 shows PD-L1 expression of tumour tissue. (D) Day 240, before third CaEP treatment. D1 shows a biopsy from a recurrence with low inflammation of tumour tissue and no PD-L1 expression. (E) Biopsy from clinical complete response two months after fourth CaEP treatmen. E shows inflammation and fibrosis and no residual tumour.
Almost two years after the patient’s first CaEP treatment, and eight months since his last CaEP treatment, stable disease with complete response in the skin has been clinically confirmed. Since initial progression on last-line treatment for urothelial cancer, the patient remains active without distant metastasis and continues to live with mild symptoms on pembrolizumab therapy.
PD-L1 expression and tumour necrosis
The patient’s primary diagnostic biopsy showed urothelial metastasis. Upon staining for PD-L1, the majority of the tumour cells were negative with scattered positive lymphocytes and macrophages. The CPS (combined positive score) was assessed at seven percent. Biopsies taken at baseline and day 2, month 3 and 8 after CaEP-1, were analysed for PD-L1 expression from SP14-stained samples. Mitoses, necrosis, inflammatory reaction and amount of tumour-infiltrating lymphocytes (TILs) were assessed from haematoxylin-eosin stained samples. The results are displayed in and .
Table 1. Cellular effects in biopsies taken before and after treatment with calcium EP.
The results in show a high degree of inflammation after CaEP (day 2 and 90). The inflammation was decreased at day 240 where only a small recurrence remained in the skin, and the patient had been treated three times with CaEP. The PD-L1 signature of the remaining tumour tissue was 0 at day 240 compared to 20 at baseline. There was no residual tumour in biopsy from day 480. The samples are shown in .
Discussion
CaEP is quickly gaining recognition as a selective tool to eradicate cutaneous tumours. Reports of cases with long lasting disease control, even in patients with metastatic and recurring cancer, are emerging [Citation2,Citation6,Citation12,Citation19]. This is the first report of reversal of response on pembrolizumab after calcium electroporation.
If a patient requires local cancer management, and radiation or surgery is not possible, CaEP is a feasible option, even if the patient is undergoing simultaneous systemic oncological treatment [Citation20]. The treatment has the benefit of being repeatable, allowing for control of multiple, recurring or bulky cancer lesions in the skin [Citation21,Citation22]. The patient responded well to CaEP treatment, exhibiting complete remission of skin symptoms after four treatments, consistent with findings from recent studies [Citation23,Citation24].
When tumours metastasise, they have often evolved in ways to evade the immune system. Over time, they may present fewer recognisable tumour antigens, and downregulate MHC (major histocompatibility complex) on their membranes. In addition to being less inflammatory, they may also secrete inhibitory cytokines [Citation25,Citation26]. If antigens are not presented adequately to the immune system, or if co-stimulation is inhibited by the presence of PD-L1, tumours may benefit from a more tolerant tumour microenvironment [Citation25].
One of the greatest challenges of immunotherapy are ‘cold’ tumours with scarce lymphocyte infiltration [Citation16], which can be a result of lack of antigen and T-cell activation. However, induction of immunogenic cell death may stimulate homing of T-cells to the tumour site. When tumours are infiltrated by T-cells, the immunotherapy such as pembrolizumab, can offset inhibitory ligands, e.g. PD-L1 and PD-L2, and cytotoxic T-cells are free to destroy surrounding cancer cells [Citation27]. Indeed this case showed both inflammation and decreased expression of PD-L1 over time following CaEP. Tumour reduction in untreated areas has been described following radiotherapy [Citation28,Citation29], and observed in trials investigating radiation in combination with check-point inhibitors in phase II trials [Citation30,Citation31].
We know that CaEP induces tumour necrosis [Citation4]. We hypothesise, that this necrosis is a way to create an immediate burst of tumour antigens and DAMPs, which may stimulate APCs present in the tumour microenvironment [Citation11,Citation32].
In this case, the patient presented with a tumour, where the deepest parts could not be reached with an electroporation device, as the tumour vicinity reached important anatomical structures. However, these areas, which had previously progressed on immunotherapy with pembrolizumab, responded to reinstated pembrolizumab treatment after CaEP (). At the time of resumed immunotherapy, the patient received a second CaEP treatment. If indeed, the first treatment had elicited an immune response, the second treatment may have acted as a booster, similar to mechanisms known from vaccines [Citation33]. The phenomena of so-called pseudo progression is well-known, but in this case there was initial regression followed by true progression with a skin penetrating tumour.
The first clinical trials have investigated CaEP in a palliative setting, e.g. for cutaneous metastases or inoperable primary tumours [Citation1–3]. Future trials should investigate CaEP in randomised trials, to provide evidence for CaEP as a booster for anti-cancer immunogenicity.
Conclusion
This case report describes the first effective treatment of cutaneous metastasis from urothelial cancer with CaEP. Our finding was supported by histological analyses and warrants further investigations of this novel cancer treatment for urogenital cancers. It is our experience that CaEP has very few side effects, low toxicity, and spares healthy tissues and has satisfactory cosmetic outcomes.
This is, to our knowledge, the first report of an indication, that CaEP has improved the outcome after immunotherapy with pembrolizumab. The patient also experienced abscopal response in tumour which had previously progressed on pembrolizumab treatment.
Ethical approval
The CaEP-B study protocol is approved by the European Union Drug Regulating Authorities (EudraCT no: 2019-004315-31), the Danish Data Protection Agency (REG-114-2019) and Ethics committee for Region Zealand (SJ-811) and registered on Clinicaltrials.gov (NCT04259658). The trial is performed according to Good Clinical Practice (GCP). Clinicaltrials.gov identifier: NCT04259658.
Author contributions
MV was the primary author of the manuscript and performed data curation and data visualisation. MV and JG treated the patient and curated data. LML curated data and performed photo documentation, AJC managed patient treatment and relevant sections in the manuscript. LÖ analysed images and SP and AVL performed histological analyses. All authors read and approved the final manuscript.
Consent for publication
In addition to securing written and oral consent for participation in the CaEP-B trial, the authors also obtained signed informed consent specifically for the publication of the case report. This consent encompasses the inclusion of photographs and PET-CT images.
Supplemental Material
Download MS Word (12.2 KB)Supplemental Material
Download JPEG Image (10 MB)Supplemental Material
Download JPEG Image (3.9 MB)Acknowledgements
The authors would like to thank research nurses Tina Sørensen and Cathrine Wolf Lorenzen for their assistance in treatment and follow up. This work did not employ writing assistance.
Disclosure statement
JG is co-inventor of a patent regarding calcium electroporation: Therapeutic applications of calcium electroporation to effectively induce tumour necrosis. Granted. PCT/DK2012/050496. No other authors declare any conflict of interest.
Data availability statement
De-identified participant data are available from the corresponding author upon reasonable request. Reuse of data requires approval from the pertinent ethics committee.
Additional information
Funding
References
- Falk H, Matthiessen LW, Wooler G, et al. Calcium electroporation for treatment of cutaneous metastases; a randomized double-blinded phase II study, comparing the effect of calcium electroporation with electrochemotherapy. Acta Oncol. 2018;57(3):311–319. doi: 10.1080/0284186X.2017.1355109.
- Plaschke CC, Gehl J, Johannesen HH, et al. Calcium electroporation for recurrent head and neck cancer: a clinical phase I study. Laryngoscope Investig Otolaryngol. 2019;4(1):49–56. doi: 10.1002/lio2.233.
- Agoston D, Baltas E, Ocsai H, et al. Evaluation of calcium electroporation for the treatment of cutaneous metastases: a double blinded randomised controlled phase II trial. Cancers. 2020;12(1):179. doi: 10.3390/cancers12010179.
- Frandsen SK, Gissel H, Hojman P, et al. Direct therapeutic applications of calcium electroporation to effectively induce tumor necrosis. Cancer Res. 2012;72(6):1336–1341. doi: 10.1158/0008-5472.CAN-11-3782.
- Hansen EL, Sozer EB, Romeo S, et al. Dose-dependent ATP depletion and cancer cell death following calcium electroporation, relative effect of calcium concentration and electric field strength. PLoS One. 2015;10(4):e0122973. doi: 10.1371/journal.pone.0122973.
- Stranzenbach R, Doerler M, Scholl L, et al. Calcium electroporation in primary cutaneous marginal zone lymphoma. J Dtsch Dermatol Ges. 2021;19(10):1510–1512. doi: 10.1111/ddg.14583.
- Falk H, Forde PF, Bay ML, et al. Calcium electroporation induces tumor eradication, long-lasting immunity and cytokine responses in the CT26 Colon cancer mouse model. Oncoimmunology. 2017;6(5):e1301332. doi: 10.1080/2162402X.2017.1301332.
- Frandsen SK, Gehl J, Tramm T, et al. Calcium electroporation of equine sarcoids. Animals. 2020;10(3):517. doi: 10.3390/ani10030517.
- Szewczyk A, Gehl J, Daczewska M, et al. Calcium electroporation for treatment of sarcoma in preclinical studies. Oncotarget. 2018;9(14):11604–11618. doi: 10.18632/oncotarget.24352.
- Pakhomova ON, Gregory B, Semenov I, et al. Calcium-mediated pore expansion and cell death following nanoelectroporation. Biochim Biophys Acta. 2014;1838(10):2547–2554. doi: 10.1016/j.bbamem.2014.06.015.
- Gerlini G, Di Gennaro P, Borgognoni L. Enhancing anti-melanoma immunity by electrochemotherapy and in vivo dendritic-cell activation. Oncoimmunology. 2012;1(9):1655–1657. doi: 10.4161/onci.21991.
- Falk H, Lambaa S, Johannesen HH, et al. Electrochemotherapy and calcium electroporation inducing a systemic immune response with local and distant remission of tumors in a patient with malignant melanoma – a case report. Acta Oncol. 2017;56(8):1126–1131. doi: 10.1080/0284186X.2017.1290274.
- Wu M, Huang Q, Xie Y, et al. Improvement of the anticancer efficacy of PD-1/PD-L1 blockade via combination therapy and PD-L1 regulation. J Hematol Oncol. 2022;15(1):24. doi: 10.1186/s13045-022-01242-2.
- Hegde PS, Karanikas V, Evers S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin Cancer Res. 2016;22(8):1865–1874. doi: 10.1158/1078-0432.CCR-15-1507.
- Teng MW, Ngiow SF, Ribas A, et al. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75(11):2139–2145. doi: 10.1158/0008-5472.CAN-15-0255.
- Bonaventura P, Shekarian T, Alcazer V, et al. Cold tumors: a therapeutic challenge for immunotherapy. Front Immunol. 2019;10:168. doi: 10.3389/fimmu.2019.00168.
- Resch I, Shariat SF, Gust KM. PD-1 and PD-L1 inhibitors after platinum-based chemotherapy or in first-line therapy in cisplatin-ineligible patients: dramatic improvement of prognosis and overall survival after decades of hopelessness in patients with metastatic urothelial cancer. Memo. 2018;11(1):43–46. doi: 10.1007/s12254-018-0396-y.
- Coulie PG, Van den Eynde BJ, van der Bruggen P, et al. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer. 2014;14(2):135–146. doi: 10.1038/nrc3670.
- Jensen KB, Lonkvist CK, Gehl J, et al. Calcium electroporation for management of cutaneous metastases in HER2-Positive breast cancer: a case report. Case Rep Dermatol. 2022;14(3):330–338. doi: 10.1159/000526157.
- Vissing M, Ploen J, Pervan M, et al. Study protocol designed to investigate tumour response to calcium electroporation in cancers affecting the skin: a non-randomised phase II clinical trial. BMJ Open. 2021;11(6):e046779. doi: 10.1136/bmjopen-2020-046779.
- Bastrup FA, Vissing M, Gehl J. Electrochemotherapy for metastatic cutaneous melanoma. Acta Oncol. 2022;61(5):531–532. doi: 10.1080/0284186X.2022.2057199.
- Falk H, Vissing M, Wooler G, et al. Calcium electroporation for keloids: a first-in-Man phase I study. Dermatology. 2021;237(6):961–969. doi: 10.1159/000514307.
- Vestergaard K, Vissing M, Gehl J, et al. Qualitative investigation of experience and quality of life in patients treated with calcium electroporation for cutaneous metastases. Cancers. 2023;15(3):599. doi: 10.3390/cancers15030599.
- Vissing M, Pervan M, Pløen J, et al. Calcium electroporation in cutaneous metastases – A non-randomised phase II multicentre clinical trial. European Journal of Surgical Oncology. 2023; doi: 10.1016/j.ejso.2023.04.024.
- Stewart TJ, Abrams SI. How tumours escape mass destruction. Oncogene. 2008;27(45):5894–5903. doi: 10.1038/onc.2008.268.
- Gooden MJ, de Bock GH, Leffers N, et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105(1):93–103. doi: 10.1038/bjc.2011.189.
- Wahid M, Akhter N, Jawed A, et al. Pembrolizumab’s non-cross resistance mechanism of action successfully overthrown ipilimumab. Crit Rev Oncol Hematol. 2017;111:1–6. doi: 10.1016/j.critrevonc.2017.01.001.
- Fuks Z, Strober S, Bobrove AM, et al. Long term effects of radiation of T and B lymphocytes in peripheral blood of patients with Hodgkin’s disease. J Clin Invest. 1976;58(4):803–814. doi: 10.1172/JCI108532.
- Stewart CC, Perez CA. Effect of irradiation on immune responses. Radiology. 1976;118(1):201–210. doi: 10.1148/118.1.201.
- Theelen W, Chen D, Verma V, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med. 2021;9(5):467–475. doi: 10.1016/S2213-2600(20)30391-X.
- Ho AY, Barker CA, Arnold BB, et al. A phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer. 2020;126(4):850–860. doi: 10.1002/cncr.32599.
- Dudda JC, Martin SF. Tissue targeting of T cells by DCs and microenvironments. Trends Immunol. 2004;25(8):417–421. doi: 10.1016/j.it.2004.05.008.
- Van Damme P, Van Herck K. A review of the long-term protection after hepatitis A and B vaccination. Travel Med Infect Dis. 2007;5(2):79–84. doi: 10.1016/j.tmaid.2006.04.004.