Abstract
Background
Population-based survival results after radical cystectomy (RC) are limited. Our objective was to report short and long-term survival results after RC for bladder cancer from Finland in a population-based setting.
Materials and methods
The Finnish National Cystectomy Database containing retrospectively collected essential RC data covering the years 2005–2017 was combined with the survival data from the Finnish Cancer Registry. Kaplan-Meier plots were used to estimate survival and the survival graphs were illustrated according to the final pathological staging. Centers were divided according to operational volume, and the results were then compared using Pearsons’s Chi-squared test.
Results
A total of 2047 patients were included in the study. 30-, and 90-day mortality was 1.3%, and 3.8%, respectively. The OS of the entire RC population at 5- and 10 years was 66% and 55%, and CSS was 74% and 72%, respectively. Center volume did not significantly associate with surgical mortality or long-term survival. The 5- and 10-year OS according to pT-category was 87% and 74% for pT0, 85% and 69% for pTa-pTis-pT1, 70% and 58% for pT2, 50% and 42% for pT3 and 41% and 30% for pT4. The corresponding 5- and 10-year CSS rates were 96% and 93% for pT0, 91% and 90% for pTa-pTis-pT1, 78% and 75% for pT2, 56% and 55% for pT3 and 47% and 44% for pT4. The 5- and 10-year OS rates in patients with no lymph node metastases (pN-) were 74% and 62%, and CSS 82% and 80%, respectively. If lymph nodes were positive (pN+), the corresponding OS rates were 44% and 34% and CSS 49% and 48%, respectively.
Conclusion
RC survival results have improved in contemporary series and are associated with the pTNM-status. The nationwide results from Finland demonstrate outcome comparable to high volume single-center series.
Background
Muscle-invasive bladder cancer (MIBC), which constitutes approximately 25% of newly diagnosed BCs, is an aggressive disease and radical cystectomy (RC) with pelvic lymph node dissection (PLND) is the gold standard of therapy [Citation1]. According to EAU non-muscle-invasive bladder cancer (NMIBC) guidelines, RC is also recommended immediately for certain patients with pTa- and pT1-staged BC with a high risk for disease progression [Citation2]. In Finland, approximately 1200 new BC cases are diagnosed, and about 180 RCs are performed annually [Citation3]. There has been growing evidence and demand for centralizing the treatment of MIBC to high-volume surgeons and hospitals [Citation4]. In Finland, the Finnish government gave a Government Decree on the centralization of demanding surgery in August 2017 [Citation5]. As a result, the centralization of complex cancer surgery in Finland began in 2018 and 2017 was the last year when low and medium-volume centers performed RCs in Finland.
The tumor-node-metastasis (pTNM) -status is the most important prognostic factor for overall survival (OS) [Citation6,Citation7]. Neoadjuvant chemotherapy (NAC) has been shown to downstage BC and improve prognosis [Citation8,Citation9]. Only a few national population survival analyses are available with some exclusions, for example, from Nordic countries, Australia, Italy, Austria, and the Netherlands results have been published [Citation10–17]. This has also been the situation in Finland, as only one center has previously reported survival rates [Citation18]. Specialized single-center long-term RC survival data have also been reported before [Citation6,Citation19–21]. These series contain up to 50-100 RCs performed annually, making them comparable to national series.
This study aimed to report short and long-term survival results after RC for bladder cancer from Finland in a population-based setting. A secondary objective was to evaluate perioperative mortality and critically assess if the centralization policy of demanding cancer surgery to high-volume centers is justified. This recommendation is also supported by the EAU MIBC guidelines but has not been investigated on a national level.
Materials and methods
The institutional Medical Ethics Committee of the Hospital District of Southwestern Finland (EMTK: 4/1802/2015) approved this retrospective study.
The Finnish National Cystectomy Database (FNCD) is an online platform, which was established by academic urologists to gather information from all 16 hospitals in Finland where RCs were carried out between 2005 and 2017 to complete the period before the centralization of RCs. The FNCD was designed for research purposes only and it was modeled to be secure and accessible through the Internet. The inclusion criteria for the study was that the RC was performed due to BC. The BC did not need to be of urothelial origin. Cystectomies performed for benign reasons were excluded from the study. The patients were identified using the ICD-10 codes (C67.*= malignant neoplasm of the bladder) and surgical procedure codes in each hospital, as per the Nordic Classification of Surgical Procedures.
The collected data included patient characteristics (age, gender, BMI, ASA class, smoking status, CCI), tumor features (TURB-T and RC specimen histology, stage, grade, concomitant prostate cancer), and treatment details (NAC, surgical modality, diversion method). Comprehensive details about surgery and hospitalization, along with the use of potential adjuvant therapies, were documented. Postoperative complications and mortality were assessed using the Clavien Dindo-grading system [Citation22].
The data was collected retrospectively by urologists dedicated to BC care and each individual medical record was comprehensively examined to ensure accuracy. If possible, a urologist not responsible for performing RCs was selected to collect the data to avoid distortion of the results. In larger centers, the data inserter had a supervisor. In select low-volume centers, the patient files were sent to the main investigator in Turku who was responsible for inputting the data into the database.
The centers were divided into low (< 5 RCs performed annually), medium (5-20 RCs performed annually), and high volume centers (>20 RCs performed annually). By doing this, four university hospitals (Turku, Tampere, Helsinki, and Oulu) were classified as high-volume centers.
We combined the data from the FNCD with survival data from the Finnish Cancer Registry (FCR). The FCR contains information on all diagnosed cancer cases in Finland since 1953, including the unique personal ID number and diagnostic details. The registry covers 96% of solid and 86% of non-solid tumors [Citation23]. The Finnish Cancer Organizations maintain the registry, and data is collected by compulsory notifications of cancer diagnoses made by all Finnish healthcare units. The FCR data includes the site and date of cancer, the basis of diagnosis, TNM stage, histology, and information on treatment. The registry is annually matched with the Cause of Death Registry and the Finnish Central Population Registry. In the latter, the personal identifier digit is also checked annually, and the complete name, vital status, possible date of death, emigration status, and the official place of residence prior to the date of diagnosis are obtained. Patients were identified using the ICD-10-codes and the patients’ social security numbers with strict data security policies [Citation23].
Kaplan-Meier plots were used to estimate the OS and CSS for all patients in the database. Survival was calculated from the time of RC and the end of follow-up was 2018. Death from BC was defined from the FCR data when the main cause of death was inserted as C67.*.The survival graphs were illustrated according to the final pathological TNM-classification. The log-rank test was used to compare subgroups of patients in terms of survival, and hazard ratios (HR) were calculated. The Wilcoxon rank sum test and Pearsons’s Chi-squared test were used to compare centers.
Results
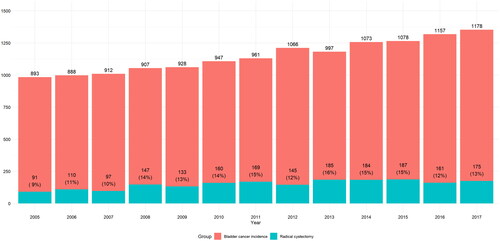
Basic characteristics of the study population are presented in . The study population included a total of 2047 patients. Of these, 1648 (81%) were men, and the median age of the patients was 69 (range 62–75) years. Overall, 384 (19%) patients had no smoking history, and the median ASA-score was 3. At the time of RC, 437 (22%) of patients had NMIBC (pTa-Tis-T1), and 356 (18%) had no evidence of tumor (pT0) in the RC specimen. A muscle-invasive (pT2) tumor was present in 372 (19%) patients, and 824 (41%) patients had an invasion beyond the detrusor muscle (pT3-4). Positive LNs were found in 395 (19%) patients. Patients had more advanced tumors and LND was more commonly performed in high and medium-volume centers. In low-volume centers LND was not performed in 20 (11%) of the cases and not reported in 58 (31%). NAC has used in 370 (18%) cases in the entire study population. . represents the incidence of BC and RCs performed in Finland during 2005–2017.
Table 1. Basic characteristics of the study population.
The entire population’s 30- and 90-day perioperative mortality was 1.3% and 3.8%, respectively. The 30-day mortality in high, medium, and low volume centers was 1.3%, 1.6%, and 0.5%, respectively. The 90-day mortality rates in high, medium, and low-volume centers were 3.4%, 4.4%, and 4.9%, respectively. There was no statistically significant difference concerning mortality between high, medium, and low-volume centers.
and summarize the survival results. The OS of the entire RC population at 5- and 10 years was 66% and 55%, and CSS was 74% and 72%, respectively. There was no statistically significant difference between the OS or CSS rates in low, medium, and high-volume centers. The 5- and 10-year OS according to pT-category was 87% and 74% for pT0, 85% and 69% for pTa-pTis-pT1, 70% and 58% for pT2, 50% and 42% for pT3 and 41% and 30% for pT4. The corresponding 5- and 10- year CSS rates were 96% and 93% for pT0, 91% and 90% for pTa-pTis-pT1, 78% and 75% for pT2, 56% and 55% for pT3 and 47% and 44% for pT4. The 5- and 10-year OS rates in patients with no lymph node metastases (pN-) were 74% and 62%, and CSS 82% and 80%, respectively. In node-positive cases (pN+) the corresponding OS rates were 44% and 34% and CSS 49% and 48%, respectively. The OS and CSS Kaplan-Meier curves according to the pT-category are presented in , respectively.
Figure 2. A. Overall survival according to pT-category B. Cancer specific survival according to pT-category.
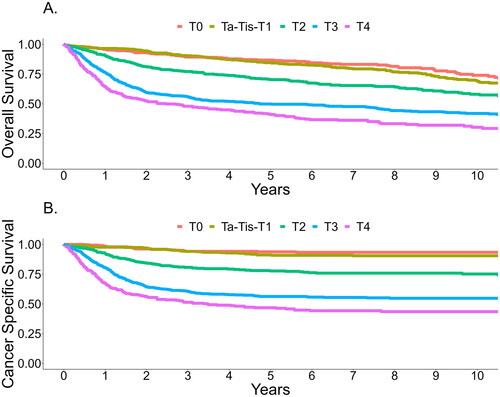
Table 2. Survival rates of the study population according to center volume.
Table 3. Survival rates of the study population according to pT- and pN-category.
Discussion
Our study reports the survival results for patients treated with RC in Finland in 2005–2017. The five-year OS and CSS for patients with pTa, pT1, or pTis were 85% and 91%, and the corresponding results for patients with pT2 were 70% and 78%. When extravesical tumor growth is present, survival decreases dramatically as patients with pT3 staged disease had 5-year OS and CSS rates of 50% and 56%, respectively. Patients with positive LNs had the worst OS and CSS, with 5-year results of 44% and 49%.
In the 2012 study by Hautmann et al. the five-year CSS for patients with pTa,pT1, pTis and pT2, pT3, pT4 was 93%, 74%, 66%, and 46%, respectively [Citation6]. This study is regarded as one of the landmark papers reporting ‘surgery only’ survival. Selection biases and treatment approach differences make direct comparisons difficult, but survival results are comparable. Similar results have been reported by research groups lead by Shariat, Madersbacher, and Stein [Citation19–21]. Our study’s national results were similar or better in some aspects. The most notable difference is in patients with positive LNs as in the aforementioned studies the 5-year CSS ranges from 22–34% and is an impressive 49% in our study. One can only speculate the reasons for the difference. Firstly, our study period is more modern as all patients were operated on in the twenty-first century in comparison to patients operated on in the 70s in the study by Stein et al [Citation21]. RC survival results in general seem to have improved in the more modern studies even though no major breakthroughs in MIBC treatment have appeared. Similar to our results in a study published in 2016 (patients operated 2007–2009) a 3-year CSS of 46% in patients with positive regional LNs was reported [Citation24]. There are likely several factors that may contribute to the improvements in survival after RC, including earlier detection of BC, the adaptation of national cancer management protocols, the use of consistent cancer care guidelines across the country, and the establishment of standardized patient pathways [Citation25]. An interesting study by Palumbo et al. in 2019 reported that the HR for cancer-specific mortality decreased to non-significant levels at three years of follow-up for patients with positive LNs after surgery [Citation26]. A study by Bruins et al. showed that a considerable number of patients with low-volume lymph node metastasis (2–3 positive nodes) remained free of recurrence during follow-up. They concluded that LN density was associated with higher recurrence risk than merely positive nodes [Citation27]. This is also seen in our results as the 5- and 10-year CSS for pN + patients are nearly alike. Thus, it seems that patients who survive the first two to three years after surgery are unlikely to die due to BC. It can be argued that these patients most likely have a low LN density and a clinical N0 –status after surgery, and oncological follow-up could be stopped around 3–5 years after RC. Secondly, the difference in survival rates observed in our study may also be attributed to patient selection. Although our study included cN + patients, cM + patients were mostly excluded, and these patients were more likely to receive NAC. NAC was introduced as standard treatment for patients with cT2 disease in 2008 in the Turku University Hospital and gained interest nationally from there-on. Our entire population had a NAC utilization rate of 18%, but this is prior to any national cancer care guidelines and national acceptance later on. In the reports by Stein et al. and Shariat et al. patients receiving NAC were also included [Citation20,Citation21]. The survival results are more comparable with our results, and the survival for patients with a positive LN status is also improved compared to the results by Hautmann. A comparison of our results with previous large RC series and other large RC studies is presented in the Supplementary material.
National RC survival studies have been published from Australia, England, the Netherlands, Italy, Sweden, Denmark, and Iceland with an approximately 50% 5-year OS for all RC patients [Citation10–16,Citation28]. Mainly survival results of the entire study population have been reported, and survival according to pT-category is lacking. Our study reports the survival rates according to pT-category. It shows that a tumor growing beyond the bladder wall at the time of surgery, or positive lymph nodes, is the leading cause for poorer survival rates. A Swedish study focusing on patients with clinically suspected LN metastases reported a 5-year CSS and OS of 37% and 29%, respectively [Citation29]. The national results from Iceland showed a 5-year OS of 54% for patients with a positive LN status [Citation10]. Our results are consistent with these studies.
The importance of hospital volume as a major determinant of peri-operative morbidity and mortality has been shown in a large systematic review [Citation4] and is also supported by the EAU guidelines [Citation1]. As 2017 was the last year medium and low-volume centers performed RCs in Finland, we now have an excellent view on RC survival prior to centralization. In the comparison between centers, there was no significant difference in the long-term survival results. Centralization may, however, be advocated with a broader spectrum of diversion methods which are introduced to more advanced staged patients. In the comparison between centers, patient characteristics are different which may unjustify the direct comparison of survival results concerning center volume. High-volume centers had more patients with extravesical tumor growth and positive LNs. In the systematic review by Bruins et al. the LND rate was significantly higher in high volume centers [Citation4] and in our study, information whether or not a LND was even performed was missing in 20–30% of patients in medium and low-volume centers. However, all available pathological reports were evaluated and while the quantity of removed LNs was missing in the aforementioned cases, LN positivity is in line with that reported in BC literature [Citation21]. Therefore, we consider that our data concerning LN metastases and LN positivity can be in general considered reliable.
The study population had 30- and 90-day perioperative mortality of 1.3% and 3.8%, respectively. Center volume did not affect mortality statistically significantly. However, there seems to be a tendency to favor larger operational volumes as the 90-day mortality was 3.4% in high-volume centers and 4.9% in low-volume centers. These results compare favorably with international standards and studies. In unselected series 30-day mortality up to 4.5% has been reported, although there is variation in the literature of 0–9% with the highest mortality rates in patients with many comorbidities [Citation4]. It is also notable that 4% of the patient population had an ASA-class of 4, defined as a patient with severe systemic disease that is a constant threat to life. This is reflected in the perioperative mortality rate, which is nearly the same at 90 days.
This study has several limitations common to retrospective studies, such as selection and misclassification bias, and patients being lost to follow-up. However, the FNCD data covers every RC performed due to BC during the study period. In addition, the risk of underreporting mortality or cause of death is tolerable as follow-up data from different centers was verified when combined with the FCR-data. Also, during the follow-up period, there may have been a shift from local institutions toward tertiary centers with the most demanding patients, which was impossible to adjust. This may hinder the potentially existing difference in survival and perioperative mortality between centers with different operational volumes.
In conclusion, the Finnish national RC results demonstrate that long-term survival is associated with pTNM- status at the time of surgery. We report promising survival outcomes after RC for BC from a nationwide population. This comprehensive national series also suggests that operational volume is not as important as the pTNM – status at the time of surgery and that RC survival results have improved in contemporary series.
Supplemental Material
Download MS Word (31.3 KB)Supplemental Material
Download MS Word (21.3 KB)Acknowledgements
The corresponding author would like to thank Jarkko Alajääski, PhD for reviewing statistical analyses used in the current manuscript.
Disclosure statement
The authors report there are no conflicts of interest to declare.
Data availability statement
The data that support the findings of this study are available from the corresponding author, Ilkka Nikulainen, upon reasonable request.
STROBE statement—checklist of items that should be included in reports of observational studies.
Additional information
Funding
References
- Witjes JA, Bruins M, Cathomas R, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer 2022. European association of urology guidelines. 2022 Edition. Vol. presented at the EAU Annual Congress Amsterdam 2022. Arnhem, The Netherlands: European Association of Urology Guidelines Office; 2022.
- Babjuk M, Burger M, Compérat E, et al. EAU guidelines on non-muscle-invasive bladder cancer (TaT1 and CIS) 2022. European association of urology guidelines. 2022 Edition. Vol. presented at the EAU Annual Congress Amsterdam 2022. Arnhem, The Netherlands: European Association of Urology Guidelines Office; 2022.
- Suomen Syöpärekisteri/The Finnish Cancer Registry. https://cancerregistry.fi/statistics/cancer-statistics/. 2020.
- Bruins HM, Veskimae E, Hernandez V, et al. The importance of hospital and surgeon volume as major determinants of morbidity and mortality after radical cystectomy for bladder cancer: a systematic review and recommendations by the european association of urology muscle-invasive and metastatic bladder cancer guideline panel. Eur Urol Oncol. 2020;3(2):131–144. doi: 10.1016/j.euo.2019.11.005.
- Finlex online database on legislative and other judicial information of Finland. https://www.finlex.fi/fi/laki/smur/2017/20170582. 2017.
- Hautmann RE, de Petriconi RC, Pfeiffer C, et al. Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: long-term results in 1100 patients. Eur Urol. 2012;61(5):1039–1047. doi: 10.1016/j.eururo.2012.02.028.
- Brierley J.D. ea. TNM classification of malignant tumors. 8th ed. Geneva: UICC International Union Against Cancer; Wiley 2017.
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859–866. doi: 10.1056/NEJMoa022148.
- Yin M, Joshi M, Meijer RP, et al. Neoadjuvant chemotherapy for muscle-invasive bladder cancer: a systematic review and two-step meta-analysis. Oncologist. 2016;21(6):708–715. doi: 10.1634/theoncologist.2015-0440.
- Bjornsson O, Gudmundsson EO, Marteinsson VT, et al. Radical cystectomy in the treatment of bladder cancer in Iceland: a population-based study. Scand J Urol. 2016;50(1):65–70. doi: 10.3109/21681805.2015.1085088.
- Coughlin GD, Youl PH, Philpot S, et al. Outcomes following radical cystectomy: a population-based study from Queensland, Australia. ANZ J Surg. 2019;89(6):752–757. doi: 10.1111/ans.15259.
- Fedeli U, De Paoli A, Corti MC, et al. Perioperative mortality and long-term survival after radical cystectomy: a population-based study in a Southern European country on 4,389 patients. Urol Int. 2020;104(7–8):559–566. doi: 10.1159/000506240.
- Goossens-Laan CA, Visser O, Hulshof MC, et al. Survival after treatment for carcinoma invading bladder muscle: a dutch population-based study on the impact of hospital volume. BJU Int. 2012;110(2):226–232. doi: 10.1111/j.1464-410X.2011.10694.x.
- Haggstrom C, Garmo H, de Luna X, et al. Survival after radiotherapy versus radical cystectomy for primary muscle-invasive bladder cancer: a swedish nationwide population-based cohort study. Cancer Med. 2019;8(5):2196–2204. doi: 10.1002/cam4.2126.
- Lund L, Jacobsen J, Clark P, et al. Impact of comorbidity on survival of invasive bladder cancer patients, 1996-2007: a Danish population-based cohort study. Urology. 2010;75(2):393–398. doi: 10.1016/j.urology.2009.07.1320.
- Patel MI, Bang A, Gillatt D, et al. Contemporary radical cystectomy outcomes in patients with invasive bladder cancer: a population-based study. BJU Int. 2015;116 Suppl 3(Suppl 3):18–25. doi: 10.1111/bju.13152
- Madersbacher S, Bauer W, Willinger M, et al. Radical cystectomy for bladder cancer in the 70+ population: a nation-wide registry analysis of 845 patients. Urol Int. 2010;85(3):287–290. doi: 10.1159/000316100.
- Bostrom PJ, Mirtti T, Kossi J, et al. Twenty-year experience of radical cystectomy for bladder cancer in a medium-volume centre. Scand J Urol Nephrol. 2009;43(5):357–364. doi: 10.3109/00365590902939387.
- Madersbacher S, Hochreiter W, Burkhard F, et al. Radical cystectomy for bladder cancer today–a homogeneous series without neoadjuvant therapy. J Clin Oncol. 2003;21(4):690–696. doi: 10.1200/JCO.2003.05.101.
- Shariat SF, Karakiewicz PI, Palapattu GS, et al. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: a contemporary series from the bladder cancer research consortium. J Urol. 2006;176(6 Pt 1):2414–2422; discussion 2422. doi: 10.1016/j.juro.2006.08.004.
- Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19(3):666–675. doi: 10.1200/JCO.2001.19.3.666.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae.
- Leinonen MK, Miettinen J, Heikkinen S, et al. Quality measures of the population-based Finnish cancer registry indicate sound data quality for solid malignant tumours. Eur J Cancer. 2017;77:31–39. doi: 10.1016/j.ejca.2017.02.017.
- Salama A, Abdelmaksoud AM, Shawki A, et al. Outcome of muscle-invasive urothelial bladder cancer after radical cystectomy. Clin Genitourin Cancer. 2016;14(1):e43-7–e47. doi: 10.1016/j.clgc.2015.07.007.
- Lundberg FE, Andersson TM, Lambe M, et al. Trends in cancer survival in the Nordic countries 1990–2016: the NORDCAN survival studies. Acta Oncol. 2020;59(11):1266–1274. doi: 10.1080/0284186X.2020.1822544.
- Palumbo C, Mistretta FA, Knipper S, et al. How cancer-specific mortality changes over time after radical cystectomy: conditional survival of patients with nonmetastatic urothelial carcinoma of the urinary bladder. Urol Oncol. 2019;37(12):893–899. doi: 10.1016/j.urolonc.2019.05.020.
- Bruins HM, Huang GJ, Cai J, et al. Clinical outcomes and recurrence predictors of lymph node positive urothelial cancer after cystectomy. J Urol. 2009;182(5):2182–2187. doi: 10.1016/j.juro.2009.07.017.
- John JB, Varughese MA, Cooper N, et al. Treatment allocation and survival in patients diagnosed with nonmetastatic muscle-invasive bladder cancer: an analysis of a national patient cohort in England. Eur Urol Focus. 2021;7(2):359–365. doi: 10.1016/j.euf.2020.01.013.
- Aljabery F, Liedberg F, Haggstrom C, et al. Management and outcome of muscle-invasive bladder cancer with clinical lymph node metastases. A nationwide population-based study in the bladder cancer data base Sweden (BladderBaSe). Scand J Urol. 2019;53(5):332–338. doi: 10.1080/21681805.2019.1681504.