Introduction
Advances in chemotherapy have significantly improved the outcomes of cancer patients. However, some drugs are associated with a high incidence of cardiotoxicity, resulting in an asymptomatic decline in left ventricular ejection fraction (LVEF) or the development of clinical heart failure [Citation1]. Anthracyclines are associated with an increased risk of left ventricular dysfunction and heart failure with significant mortality and morbidity, considered one of the most serious cancer therapy-related side effects [Citation2,Citation3]. Other HER-2-targeted therapies such as trastuzumab have been noted to cause cardiac dysfunction [Citation4]. The estimated incidence of anthracycline and/or trastuzumab cardiotoxicity ranges from 1 to 18.6%, depending on the definition of toxicity used [Citation5].
Several mechanisms have been proposed to explain chemotherapy-induced cardiotoxicity, such as oxidative stress, apoptosis, and mitochondrial dysfunction [Citation6]. In addition, anthracyclines form a complex with topoisomerase II and DNA strands. Topoisomerase IIβ is expressed in cardiac tissue, and the specific complexes involving this isoenzyme may lead to cardiomyocyte necrosis and cell death [Citation7].
Consequently, various prevention or mitigation strategies were designed using B blockers and/or angiotensin-converting enzyme inhibitors (ACEi)/angiotensin receptor blockers (ARB) in randomized controlled trials, with modest or no effect of the intervention on the predefined primary outcome of LVEF drop prevention [Citation8–11]. Given the pleiotropic anti-inflammatory properties of statins, their use has also been proposed to reduce cardiotoxicity [Citation12]. Previously, few small randomized clinical trials have evaluated the efficacy of statins in preventing anthracycline-induced cardiotoxicity [Citation13–15]. Recently, a new clinical trial has provided new evidence [Citation16].
Therefore, the main objective of the present meta-analysis was to analyze the protective effect of statins on anthracycline-induced cardiotoxicity.
Materials and methods
A systematic review was conducted, based on the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [Citation17]. This systematic review was registered in PROSPERO.
Two independent reviewers searched the electronic PubMed/MEDLINE, Embase, Science Direct, Scopus, and Cochrane Controlled Trials databases for the terms ‘statin’, ‘chemotherapy’, ‘anthracycline’, ‘cardiotoxicity’, and ‘cancer treatment-related cardiac dysfunction’. Our meta-analysis included only randomized trials of statin therapy versus placebo, with a follow-up duration ≥ 3 months, which reported cardiotoxicity by chemotherapy in cancer patients. There were no idiomatic, geographical, or publication restrictions. Excluded studies included case-series, cross-sectional, case-control, and observational prospective studies. The search ended in March 2023.
The primary endpoint was defined as chemotherapy-induced cardiotoxicity based on the decrease in LVEF estimated by echocardiography or cardiovascular magnetic resonance. Outcomes were analyzed dichotomously and were defined according to the reported events within the selected studies (different definitions of LVEF decrease).
The Cochrane risk-of-bias tool for randomized trials (RoB 2) was used to assess the potential risk-of-bias in each included trial [Citation18].
Measures of effect size were expressed as odds ratio (OR) and their corresponding 95% confidence interval (CI). The I2 statistic was calculated to quantify between trial heterogeneity and inconsistency. The fixed-effects model was chosen because heterogeneity was low. Statistical analyses were performed using the R software for statistical computing version 3.5.1 with additional specific packages [Citation19]. A two-tailed p value < .05 was considered statistically significant.
Analysis of publication bias was not made because our research included few studies. In this context, the power of the tests is too low to distinguish chance from real asymmetry.
A sensitivity analysis for the primary endpoint was performed.
Results
The search included 260 potentially relevant articles after title screening, and 243 studies were excluded after abstract screening. After a full textual analysis, 14 studies were removed because they did not include the population, treatment, or outcomes relevant to our study. The final analysis included four randomized clinical trials with a total of 622 patients [Citation13–16]. Regarding the quality of the studies, two studies showed a low risk of bias [Citation13,Citation14], while another study showed some concerns [Citation15]. The remaining study [Citation16] could not be evaluated because the complete data has not been published to date.
All studies used anthracyclines as chemotherapy. On the other hand, rosuvastatin and atorvastatin were the statins used. The follow-up ranged from 6 to 24 months. The characteristics of the studies included in this meta-analysis are shown in .
Table 1. Characteristics of the studies included in the meta-analysis.
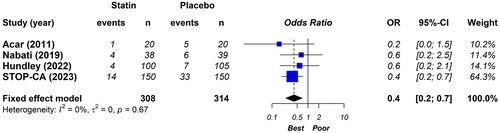
The main result of our study shows that statin therapy was associated with lower anthracycline-induced cardiotoxicity (OR: 0.4; 95%CI: 0.2–0.7, I2 0%) (). In other words, compared to placebo, patients who received statins less frequently showed a decreased LVEF after treatment with anthracyclines.
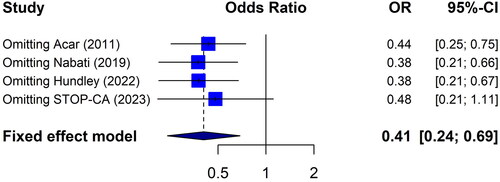
Figure 1. Effect of statin on the incidence of cardiotoxicity based on the decrease in LVEF. Fixed effects model, odds ratio, 95% confidence interval (CI), and I2 statistics.
The sensitivity analysis showed the same directionality and magnitude of results when studies were excluded one by one ().
Discussion
In this meta-analysis, which included patients with cancer under treatment with anthracyclines, we find an association between the statin therapy and the incidence of chemotherapy-induced cardiotoxicity.
Anthracyclines are proven effective drugs to treat especially breast cancer and lymphomas but could induce cardiotoxicity with consequent drop in the LVEF which, if left untreated, can evolve into advanced heart failure [Citation20].
Cardiovascular imaging has an important role in identifying patients with subclinical left ventricular dysfunction [Citation21]. Transthoracic echocardiography is the preferred imaging technique for baseline risk stratification as it provides quantitative assessment of LVEF during treatment and long-term follow-up. Current definitions of chemotherapy-induced cardiotoxicity are based on a reduction of LVEF [Citation20,Citation21]. Relative changes in global longitudinal strain have been proposed as an additional tool to identify patients at risk. Alternatively, in subjects with poor-quality echocardiography windows, cardiac magnetic resonance should be considered. Three studies evaluated in this meta-analysis used a definition of cardiotoxicity based on changes in LVEF estimated by echocardiography, while the remaining study used cardiovascular magnetic resonance. However, the definition was not exactly the same. The studies published by Acar et al. [Citation13] and Hundley et al. [Citation15] used an LVEF cutoff point of 50%. On the other hand, the study published by Nabati et al. [Citation14] and the STOP-CA trial [Citation16] considered different variations of the LVEF, although they coincided in the LVEF cutoff point of 55%. It is important to note that the main result of the study published by Hundley et al. [Citation15] showed negative results when they evaluated the difference in 24-month LVEF between placebo and treatment groups (continuous variable) as the primary endpoint. In this case, the administration of atorvastatin 40 mg per day did not affect mean LVEF values 6 and 24 months after initiating doxorubicin in patients with breast cancer or lymphoma. However, data on the number of patients with an absolute LVEF value <50% at the end of the trial were incorporated into this meta-analysis.
Statins are the most commonly used effective drugs to treat hyperlipidemia. These classes of drugs can significantly lower low-density lipoprotein cholesterol and have pleiotropic anti-inflammatory effects [Citation22]. The inflammatory effect is greater in lipophilic statins and in high-intensity regimens, such as atorvastatin 40–80 mg or rosuvastatin 20–40 mg [Citation23]. All the studies evaluated in this meta-analysis considered high-intensity statin schemes, with a greater anti-inflammatory effect. Therefore, extrapolating the results of this meta-analysis to other less potent statin regimens might be inappropriate.
This systematic review has some limitations. First, a few studies were included. Second, we were unable to assess publication bias given the low number of included studies. Third, clinical heterogeneity was observed (types of cancer, chemotherapy and statins schemes used, definitions of cardiac dysfunction, and follow-up times). In addition, there could be considerable variability in the measurement of LVEF depending on the method used [Citation24]. However, the sensitivity analysis suggests that the results are robust. Finally, this meta-analysis considered LVEF as a dichotomous variable. Carrying out a quantitative analysis with LVEF as a continuous variable could show other results. Despite these limitations, this study analyzed the best evidence available to date.
The results of our meta-analysis are consistent with previous observational studies [Citation25]. However, we believe that more research is needed to clarify this issue given the low number of randomized studies reported to date. The ongoing PREVENT (Preventing Anthracycline Cardiotoxicity With Statins; NCT01988571) trial and the SPARE-HF (Statins for the Primary Prevention of Heart Failure in Patients Receiving Anthracyclines Pilot Study; NCT03186404) trial can contribute by providing more evidence in the near future.
Conclusions
Our data suggest that, in a population with cancer under treatment with anthracyclines, the use of statins was associated with a significant reduction in chemotherapy-induced cardiotoxicity. These results must be confirmed in future clinical trials.
Ethical approval
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author, W.M, upon reasonable request.
Additional information
Funding
References
- Perez IE, Taveras Alam S, Hernandez GA, et al. Cancer therapy-related cardiac dysfunction: an overview for the clinician. Clin Med Insights Cardiol. 2019;13:1179546819866445. doi: 10.1177/1179546819866445.
- Hu W, Song M, Li L. Grading evaluation of cardiotoxicity in patients with breast cancer treated with adjuvant paclitaxel anthracycline/cyclophosphamide chemotherapy: a meta-analysis. Comput Math Methods Med. 2022;2022:7963146. doi: 10.1155/2022/7963146.
- Omland T, Heck SL, Gulati G. The role of cardioprotection in cancer therapy cardiotoxicity: JACC: cardioOncology state-of-the-art review. JACC CardioOncol. 2022; 4(1):19–37. doi: 10.1016/j.jaccao.2022.01.101.
- Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20(5):1215–1221. doi: 10.1200/JCO.2002.20.5.1215.
- Bloom MW, Hamo CE, Cardinale D, et al. Cancer therapy-related cardiac dysfunction and heart failure part 1: definitions, pathophysiology, risk factors, and imaging. Circ Heart Fail. 2016;9(1):e002661. doi: 10.1161/CIRCHEARTFAILURE.115.002661.
- Albini A, Pennesi G, Donatelli F, et al. Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst. 2010;102(1):14–25. doi: 10.1093/jnci/djp440.
- Lyu YL, Kerrigan JE, Lin CP, et al. Topoisomerase IIβ–mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res. 2007;67(18):8839–8846. doi: 10.1158/0008-5472.CAN-07-1649.
- Gulati G, Heck SL, Ree AH, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2x2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37(21):1671–1680. doi: 10.1093/eurheartj/ehw022.
- Vila MS, Ayub-Ferreira SM, de Barros Wanderley MR, Jr, et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY trial. J Am Coll Cardiol. 2018;71(20):2281–2290. doi: 10.1016/j.jacc.2018.02.049.
- Ayub-Ferreira SM, Avila M, Brandao S, et al. Carvedilol for prevention of chemotherapy induced cardiotoxicity: final results of the prospective, randomized, double-blind, placebo controlled CECCY trial. J Am Coll Cardiol. 2020;75(11):658. doi: 10.1016/S0735-1097(20)31285-7.
- Cardinale D, Ciceri F, Latini R, et al. Anthracycline- induced cardiotoxicity: a multicenter randomised trial comparing two strategies for guiding prevention with enalapril: the International Cardio Oncology Society-one trial. Eur J Cancer. 2018;94:126–137. doi: 10.1016/j.ejca.2018.02.005.
- Choudhary A, Rawat U, Kumar P, et al. Pleotropic effects of statins: the dilemma of wider utilization of statin. Egypt Heart J. 2023;75(1):1. doi: 10.1186/s43044-023-00327-8.
- Acar Z, Kale A, Turgut M, et al. Efficiency of atorvastatin in the protection of anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2011;58(9):988–989. doi: 10.1016/j.jacc.2011.05.025.
- Nabati M, Janbabai G, Esmailian J, et al. Effect of rosuvastatin in preventing chemotherapy-induced cardiotoxicity in women with breast cancer: a randomized, single-blind, placebo-controlled trial. J Cardiovasc Pharmacol Ther. 2019;24(3):233–241. doi: 10.1177/1074248418821721.
- Hundley WG, D’Agostino R, Jr, Crotts T, et al. Statins and left ventricular ejection fraction following doxorubicin treatment. NEJM Evid. 2022;1:10. doi: 10.1056/evidoa2200097.
- STOP-CA Trial: Statin Therapy Associated With Reduced Heart Dysfunction From Anthracyclines. 2023 scientific session of the American College of Cardiology. https://www.acc.org/latest-in-cardiology/articles/2023/03/01/22/45/sat-930am-stop-ca-acc-2023. (Accessed 03/13/2023).
- Arya S, Kaji AH, Boermeester MA. PRISMA reporting guidelines for meta-analyses and systematic reviews. JAMA Surg. 2021;156(8):789–790. doi: 10.1001/jamasurg.2021.0546.
- Sterne JAC, Savovi J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898.
- Viechtbauer W. Conducting meta-analyses in R with the metaphor package. J Stat Soft. 2010;36(3):1–48. doi: 10.18637/jss.v036.i03.
- Lyon AR, López-Fernández T, Couch LS, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43(41):4229–4361. doi: 10.1093/eurheartj/ehac244.
- Čelutkienė J, Pudil R, López-Fernández T, et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: a position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC). Eur J Heart Fail. 2020;22(9):1504–1524. doi: 10.1002/ejhf.1957.
- Woźniak E, Broncel M, Niedzielski M, et al. The effect of lipid-lowering therapies on the pro-inflammatory and anti-inflammatory properties of vascular endothelial cells. PLoS One. 2023;18(2):e0280741. doi: 10.1371/journal.pone.0280741.
- Koushki K, Shahbaz SK, Mashayekhi K, et al. Anti-inflammatory action of statins in cardiovascular disease: the role of inflammasome and toll-like receptor pathways. Clin Rev Allergy Immunol. 2021;60(2):175–199. doi: 10.1007/s12016-020-08791-9.
- Pellikka PA, She L, Holly TA, et al. Variability in ejection fraction measured by echocardiography, gated single-photon emission computed tomography, and cardiac magnetic resonance in patients with coronary artery disease and left ventricular dysfunction. JAMA Netw Open. 2018;1(4):e181456. doi: 10.1001/jamanetworkopen.2018.1456.
- Obasi M, Abovich A, Vo JB, et al. Statins to mitigate cardiotoxicity in cancer patients treated with anthracyclines and/or trastuzumab: a systematic review and meta-analysis. Cancer Causes Control. 2021;32(12):1407–1409. doi: 10.1007/s10552-021-01487-1.