Abstract
Background
Convincing results from randomized controlled trials (RCTs) have led to increasing use of immune checkpoint inhibitors (ICI) as part of standard therapies in real-world (RW) scenarios. However, RW patients differ clinically from RCT populations and might have reduced long-term survival. Currently, only sparse data on 3-5-year survival rate for RW patients with advanced non-small cell lung cancer (NSCLC) treated with ICI exist.
Materials and methods
A multicenter study was performed including 729 patients with advanced NSCLC receiving monotherapy with ICI (retrospective data (n = 566) and prospective data (n = 163)). Detailed baseline clinical characteristics, programmed death-ligand 1 (PD-L1) tumor proportion score (TPS), and baseline haematological count were registered. Kaplan–Meier estimates and log-rank test were used for survival analyses, Cox regression for determination of prognostic factors.
Results
Median time of follow-up (FU) was 48.7 months (IQR 37.2–54.3). Median overall survival (OS) in first line treatment was 20.4 months (IQR 8.5–45.0) compared to 11.4 months (IQR 4.6–27.1) in ≥2nd line (HR 1.48, 95% CI 1.25–1.75). Estimated probability of OS was 30% at 3 years, 23% at 4 years, and 13% at 5 years in first line compared to 17, 13, and 11% in ≥2nd line, respectively. For those with performance status (PS) 2, the 2-year OS rate was 32% (95% CI 0.22–0.43) compared to 5% (95% CI 0.01–0.15) in patients with PD-L1 ≥ 50% versus <50%, respectively.
Conclusions
Compared to RCTs, long-term OS and PFS rates are lower in real-world patients treated with ICI in first line but much improved compared to historic rates on chemotherapy. A promising flattening of both the OS and progression free survival curves illustrates that also a subset of real-world patients obtain long-term remission. Patients with PS 2 and PD-L1 ≥ 50% may obtain clinically meaningful 2-year PFS and OS rates.
Background
In Denmark, as of 2022, lung cancer has become the most frequent and lethal cancer, with almost 4000 people dying annually [Citation1]. Non-small cell lung cancer (NSCLC) constitutes around 85% of all lung cancer cases, and historically, the 5-year survival rate of patients with advanced or metastatic NSCLC has been very dismal [Citation2]. Even with standard first line systemic chemotherapy the median overall survival (mOS) was only 7–10 months [Citation3,Citation4] and the 5-year survival rate close to zero [Citation2,Citation5,Citation6]. For patients without oncogenic driver alterations, previous standard treatment strategies in Denmark included a platinum-based doublet chemotherapy in first line followed by subsequent different single chemotherapy regimens at time of progression. However, treatment strategies changed in 2015, when the first immune checkpoint inhibitor (ICI) was approved for use in subsequent treatment lines in patients with advanced/metastatic NSCLC disease. Since then, the ICIs have quickly moved to the first-line setting with Danish approval in 2017. Today monotherapy with an ICI is the preferred systemic treatment choice in first line in patients with a performance status (PS) of 0–1 and a programmed death ligand-1 (PD-L1) tumor proportion score (TPS) ≥50% [Citation7]. Thus, depending on the level of PD-L1 TPS and PS, ICIs are currently used in first line treatment either as monotherapy (those with PD-L1 ≥ 50%) [Citation8], in different combinations with doublet chemotherapy (PD-L1 < 50%) [Citation9,Citation10] or following curative chemo-radiation in locally advanced disease with PD-L1 ≥ 25% as part of a consolidation strategy [Citation11] according to the Danish medicines council [Citation7]. This revolution of the treatment paradigm for NSCLC results from pivotal randomized controlled trials (RCTs) proving the indisputable superiority of ICI over chemotherapy in patients with PS 0-1 in terms of improving progression-free survival (PFS), mOS [Citation12–15] and long-term survival [Citation16,Citation17]. Additionally, ICIs have a highly acceptable toxicity profile compared to chemotherapy [Citation8,Citation12,Citation14,Citation15,Citation18,Citation19]. However, since the RCTs present no data on those with PS 2, current Danish guidelines do not recommend using ICI in patients with PS 2 regardless of PD-L1 TPS. The pivotal ICI RCTs did not include this patient group [Citation8,Citation12–15]. Data on other subgroups including those with cardiopulmonary comorbidities, other cancer diseases and/or autoimmune diseases, and those with brain metastases or age ≥75 years, are also lacking [Citation8,Citation12–15]. In routine clinical practice, these subgroups constitute a substantial part of the total NSCLC population [Citation20,Citation21]. Obtaining long-term survival data for these uninvestigated subgroups in particularly for those with PS 2 is essential to guide clinicians toward optimized treatment strategies for real-world NSCLC patients in the future. Thus, this study presents the first 5-year OS and PFS data for Danish patients with advanced/metastatic NSCLC treated with ICI in either first or subsequent line of therapy in a real-world setting.
Materials and methods
Study design
This was an observational multicenter (Aalborg University Hospital, Vejle Hospital, University Hospital of Southern Denmark, Sønderborg Hospital and Odense University Hospital) retrospective cohort study of patients with advanced/metastatic NSCLC defined by the Tumor-Node-Metastasis (TNM) classification 8th edition [Citation22,Citation23] treated in the daily clinic with palliative ICI monotherapy from September 2015 to October 2018 (n = 566). All consecutive Danish patients at these oncologic departments eligible for ICI monotherapy, who received at least one full treatment of ICI were included. Patients were identified locally using structured methods with automated search for lung cancer diagnosis and ICI drug administration files registered at the hospital’s pharmacy. Additionally, from April 2018 to April 2021, data from one center (Odense University Hospital, n = 163) were prospectively obtained (registered at ClinicalTrials.gov NCT0387064). The retrospective clinical data from these four centers have previously been published with shorter follow-up time as part of a national Danish study [Citation20,Citation21,Citation24].
Patients and ICI administration
The administration of ICI followed recommendations from the Danish Medicines Council at that time [Citation7]. The ICIs included nivolumab (3 mg/kg/every 2nd week), pembrolizumab (2 mg/kg/every 3rd week or 200 mg/every 3rd week) and atezolizumab 1200 mg/every 3rd week. Patients who received combination regimens of ICI and chemotherapy were not included in this study. ICI was administered for a maximum of 2 years and was continued until radiological and/or clinical progression, death, or unacceptable toxicity.
The study including the database was approved by the Danish Data protection agency and the regional ethical committee of Southern Denmark following the declaration of Helsinki [Citation25]. The prospectively included patients completed a written informed consent form according to regulations. Informed consent from the retrospectively included patients was waived by regulations.
Data collection and management
The retrospective data were obtained from each participating hospital’s electronic medical record (EMR) system. Data from Aalborg University Hospital were stored in a local database; the data from the remaining centers were stored in a Research Electronic Data Capture databases (REDcap) assigned to each center [Citation26]. Alignment of variables was performed before data analyses. For statistical analyses, the data were gathered into one dataset with data control performed for each predefined variable. The time for data cutoff was 1 April 2022.
Baseline characteristics at the time of ICI initiation included age, sex, TNM stage, histology, PD-L1 TPS, location of distant metastases, PS, Charlson’s Comorbidity Index (CCI), body mass index (BMI) and smoking status. Tests for driver alterations in the Epidermal Growth Factor Receptor (EGFR) and Anaplastic Lymphoma Kinase (ALK) proteins were routinely performed in patients with non-squamous histology. Baseline blood samples included hemoglobin level, leucocyte- and platelet count. Reasons for ICI discontinuation included progression, death, immune-related adverse events (irAEs), completion of two years of ICI, declining PS, and/or other toxicity.
Endpoints
The primary endpoint was long-term survival specified as 3, 4, and 5-year survival rates according to PD-L1 ≥ 50% and <50% as well as line of treatment (first versus subsequent lines).
Secondary endpoints were PFS rates at 3, 4, and 5 years according to PD-L1 ≥ 50% and <50% as well as line of treatment (first versus subsequent lines).
The date of progression was defined as the date of clinical or radiologically verified progressive disease (PD), which led to the discontinuation of ICI, or death from any cause without verified PD. The index date was the date of the first ICI administration. For patients still alive at the time of data cutoff, the last follow-up date was the date on which the patient was last observed alive in the EMR [Citation27].
Statistical methods
To compare baseline characteristics, summarized descriptive statistics, medians, ranges and quartiles for continuous variables, and frequencies and percentages for categorical variables were performed. The chi-square test was used for comparison of categorical variables and the Wilcoxon rank-sum test or the unpaired t-test for continuous variables. The log-rank test was performed for OS and PFS distribution and stratified by line of treatment. Kaplan–Meier (KM) estimates were used to illustrate survival functions. The inverse KM method was used to estimate median follow-up time. Cox regression analyses were performed to assess hazard ratios (HRs) with 95% confidence intervals (CI) of OS and PFS for each baseline characteristic. A multivariable Cox regression model was constructed based on backward selection after including already known causal factors (PD-L1 TPS, PS, histology, brain-, liver- or bone metastases), and factors in the univariable analyses with a p-value <0.15. Schoenfeld residuals for each variable was used to test the assumption of proportional hazards. All analyses were two-tailed and a p-value <0.05 was considered statistically significant. Analyses were performed in STATA version 17.0 [Citation28].
Results
A total of 729 patients were included in the study with a median age of 69 years (range 35–92). One patient was lost to follow-up in terms of PFS. Median follow-up was 48.7 months (IQR 37.2–54.3). No patients had ALK translocation.
Distribution of baseline characteristics and reasons for ICI discontinuation according to ICI treatment line is illustrated in and according to PD-L1 TPS in supplementary 1.
Table 1. Baseline characteristics according to ICI administered in 1st and ≥2nd line.
Nine percent (67/721) of patients completed a maximum of two-year ICI treatment. Median time to ICI discontinuation was 3.0 months in ≥2nd line and 5.0 months in first line. At end of follow-up 79% (574/729) had died and 88% (643/728) had progressed.
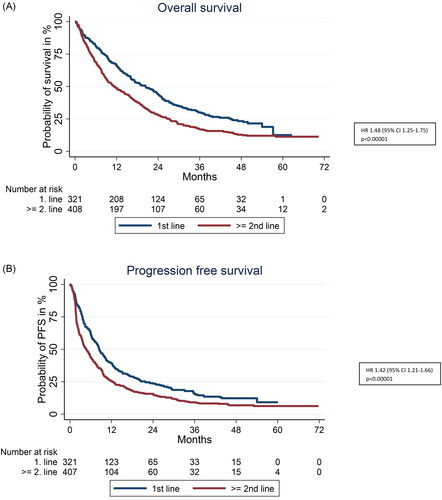
Median OS in ≥2nd line ICI treated patients was 11.4 months (95% CI 9.3–14.3) compared to 20.4 months (95% CI 16.3–23.7) in first line (HR 1.48, 95% CI 1.25–1.75). Estimated probability of survival at 3, 4, and 5 years in ≥2nd line was 17, 13, and 11%, respectively as compared to 30%, 23%, and 13% in first line. Median PFS (mPFS) was 4.8 months (95% CI 3.8–5.8) versus 8.5 months (95% CI 7.4–10.0) in ≥2nd compared to first line (HR 1.42 (1.21–1.66)). The PFS rate at 3, 4, and 5 years was 16, 9, and 7% in ≥2nd line and 24, 15, and 12% in first line, respectively. This is illustrated in .
Figure 1. (A) Overall survival according to line of ICI (≥2nd line ICI compared to 1st line). (B) PFS according to line of ICI administration (≥2nd line compared to 1st line).
Both mOS and mPFS were statistically significantly improved in patients with PD-L1 ≥ 50% versus <50%. The estimated probability of being alive at 2, 3, 4, and 5 years was 20, 14, 11, and 6% versus 44, 29, 23, and 19% for PD-L1 < 50% and PD-L1 ≥ 50%, respectively ( and ). For survival according to distribution of PD-L1 < 1%, PD-L1 ≥ 1 < 50% and PD-L1 ≥ 50% see Supplementary 2.
Figure 2. (A) Overall survival according to PD-L1 ≥ 50% versus PD-L1 < 50%. (B) Progression free survival according to PD-L1 ≥ 50% versus PD-L1 < 50%.
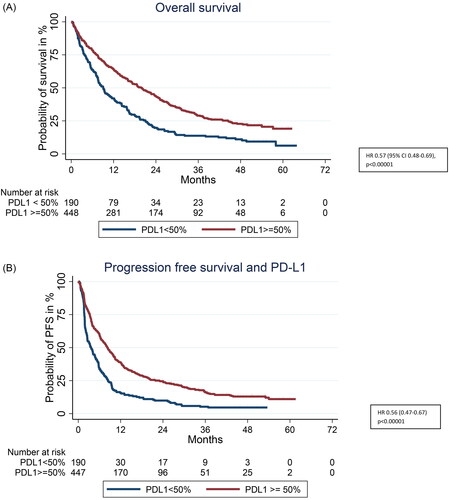
Table 2. Survival at 2, 3, 4 and 5 years for those with PD-L1 < 50% versus PD-L1 ≥ 50%.
Factors significantly associated with overall survival in the uni- and multivariable Cox regression analysis are displayed in .
Table 3. Uni- and multivariate Cox regression analysis of factors associated with overall survival.
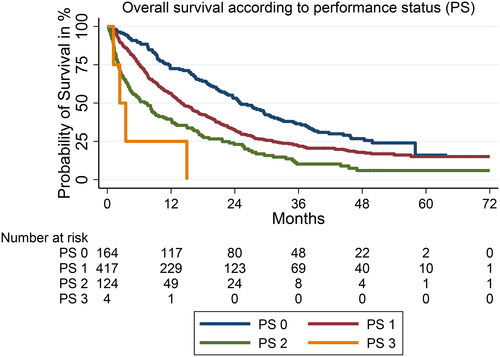
Poor prognostic factors significantly associated with OS in the multivariable analysis were: Age ≥75 years, PS ≥2, low hemoglobin count, leucocyte- or platelet count above the normal upper limit, brain metastasis, liver metastasis and bone metastasis. Positive prognostic factors were female sex and PD-L1 ≥ 50%. Kaplan–Meier estimates for PS 0–3 is illustrated in .
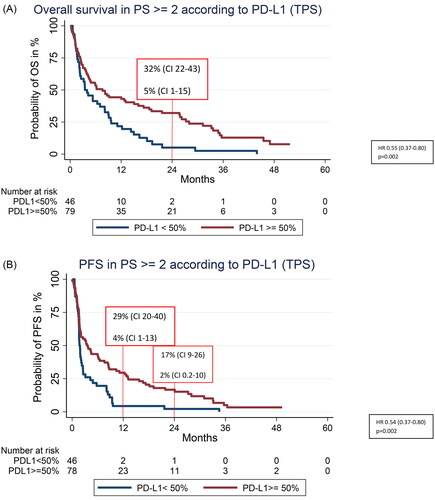
Patients with PS ≥2
For the subgroup with PS ≥2 a significant difference in both PFS and OS was found according to whether they had PD-L1 ≥ 50% or PD-L1 < 50%, illustrated in . Median OS for those with PD-L1 ≥ 50% compared to PD-L1 < 50% was 7.6 months (95% CI 4.20–15.04) and 2.5 months (95% CI 2.25–8.13), (HR 0.55, 95% CI 0.37–0.80, p = 0.002), respectively. Notably the 2-year OS rate was 32% (CI 0.22–0.43) in PD-L1 ≥ 50% compared to 5% (CI 0.01–0.15) in those with PD-L1 < 50% (). Median PFS was 3.5 months (95% CI 1.9–6.5) vs. 1.7 month (95% CI 1.6–2.3) in PD-L1 ≥ 50% and PD-L1 < 50%, (HR 0.54, CI 95% 0.37–0.80, p = 0.002), respectively (). The 1-year PFS rate was 29% (CI 0.20–0.40) in PD-L1 ≥ 50% compared to 4% (CI 0.01–0.13) in those with PD-L1 < 50% (). The same pattern was observed for patients with PS <2 but with higher OS and PFS rates compared to patients with PS 2 (Supplementary 3(A,B)).
Discussion
This study is the first to report 5-year OS and PFS rates in a large Danish real-world population with advanced NSCLC treated with palliative intended ICI monotherapy. Furthermore, we report that ICI is particularly meaningful in the population with PD-L1 ≥ 50% with considerable improvements in both long-term OS and PFS compared to historic rates of chemotherapy. Importantly this also includes the subgroup of patients with PS 2 and PD-L1 ≥ 50%. We report that long-term OS and PFS rates at 3, 4, and 5 years in RW patients treated with ICI in ≥2nd line are comparable to the reported rates from the pivotal RCTs in 2nd line (pooled data from Checkmate 017 and 057) [Citation17]. They report 5-year ICI survival rates of 13% (compared to 11% in our study) with only <3% alive at 5-years in the chemotherapy treated comparative arm [Citation17].
Interestingly, the PFS curve for this NSCLC population treated in ≥2nd line shows the same flattening of the curve as seen in ICI-treated patients with malignant melanoma, where a subset of patients still have not progressed even at 6 years of follow-up [Citation29], indicating that the survival for these patients may be even longer.
This kind of long-term PFS has not been reported for advanced NSCLC patients treated with palliative chemotherapy. Usually, these patients all progress within months and not years which is also reflected in the updated pooled Checkmate 017 and 057, with a 5-year survival rate in the chemotherapy arm of only 2.6% [Citation17]. Some of the long-term survivors in our real-world population treated with ICI may however partly be explained by a high PD-L1 (≥50%), since some of the early patients were treated prior to first line approval in Denmark in 2017. However, patients treated with ICI in ≥2nd line may be somewhat selected as well, since these patients have survived with advanced NSCLC beyond first line therapy.
As previously mentioned historic OS-rates for patients treated with chemotherapy in first line were very poor with a mOS of only 7–10 months and a 5-year OS-rate close to zero.
Thus, this study reports that OS-rates on ICI monotherapy for RW patients have improved significantly compared to these rates. Furthermore for RW patients treated with ICI in first line the 3-year (30%) and 4-year (23%) survival rates are significantly better than for patients treated with ICI in subsequent lines (17 and 13% respectively) [Citation17], but somewhat inferior to reports from first line RCTs [Citation16]. The latter report OS rates at 3-years of 44%, 4-years of 36% and 5-years of 32% [Citation16]. Our 5-year OS was only 13% but it is important to remember that our real-world data for patients treated with ICI in first line are more immature at this time of follow-up than for subsequent lines of ICI due to the later approval of ICI in first line. The inferior 3–5 year OS rate in first line observed in this study as compared to RCTs may partly be explained by the inclusion of around 20% of patients with PS ≥2.
Another explanation may rely on risk of PS misclassification when treating patients in routine clinical practice (some patients with PS 2 may be classified as 1, as mentioned in a previous publication [Citation20]. Nevertheless, this real-world study show survival rates, which are much improved compared to the historic survival rates of first line chemotherapy.
When we investigated PD-L1 TPS instead of merely line of ICI, the flattening of the survival curve is evident with 2-, 3-, 4- and 5-year survival rates of 44, 29, 23, and 19% in a heterogenic real-world population. Due to the Danish recommendations with restrictions on ICI use based on PS, only few patients with PS 2 were treated with ICI in this study (n = 128). However, importantly our data on this subgroup indicate that for patients with PS ≥ 2, PD-L1 TPS may guide clinicians toward optimized treatment regimens for this subgroup as well. We found that patients with PS 2 and PD-L1 < 50% did not benefit substantially from ICI with very short mPFS and mOS, whereas the subgroup of patients with PD-L1 ≥ 50% had a significantly improved mPFS and mOS, and importantly a much higher chance of obtaining long-term PFS and OS. At 2-years, 32% of these patients with PD-L1 ≥ 50% were still alive (compared to only 5% in those with PD-L1 < 50%, (95% CI 0.20–0.40) and 17% had not progressed at this point either (95% CI 0.09–0.20). Even though these rates are lower compared to those of patients with PS <2, this indicates that up-front ICI may be considered a better alternative for some patients with PS2 and PD-L1 ≥ 50% instead of chemotherapy. However, it is necessary to investigate this heterogenic group of patients with PS 2 further in larger numbers in future studies.
Factors significantly favoring improved outcome were female sex, normal baseline blood samples, no liver- brain or bone metastasis, PS <2, PD-L1 ≥ 50%, and age <75 years. For baseline blood samples a low hemoglobin count of less than 100 g/L seemed to be the most important factor to predict poor outcome.
These data suggest that when assessing whether a patient is expected to benefit from ICI – baseline blood samples as a supplement in combination with PS and PD-L1 TPS may be helpful and aid in the treatment decision process.
Currently in Denmark, platinum-doublet chemotherapy is recommended to NSCLC patients with PS 2 in first and subsequent lines, but not monotherapy ICI due to lack of RCT data on this subgroup. Studies have shown that especially in the elderly, the toxicity profile of patients with PS 0–1 is in favor of ICI compared to chemotherapy [Citation19]. Thus, current guidelines are paradoxical since ICI induces more long-term survivors as opposed to chemotherapy with a preferable toxicity profile for those with PS 0–1 including the elderly. This may also be the case for patients with PS 2 and PD-L1 ≥ 50%. For this study 29% of patients with PD-L1 ≥ 50% and PS 2 had not progressed at 12 months which is quite high for this group of patients who have a poorer upfront prognosis than patients with PS 0–1. Compared to only 4% for those with PS 2 and PD-L1 < 50%. This illustrates that the group of patients with PS 2 is very heterogenic, and guidelines should include PD-L1 TPS in the clinical treatment decision to avoid that some patients who may benefit from ICI are excluded from the treatment. Thus, we need more data on those with PD-L1 ≥ 50% and PS 2 in the future, since this group of patients may rather benefit from ICI than chemotherapy, which calls for change of current treatment recommendations.
The IPSOS study was a global multicenter, open-label randomized phase 3 study of atezolizumab versus single agent chemotherapy which included patients with PS ≥2 who were ineligible for platinum-doublet chemotherapy [Citation30]. On paper, this population was, however, less fit than many of the patients encountered in our daily clinic with PS 2, who end up receiving platinum-based doublet chemotherapy. In IPSOS, patients were included regardless of PD-L1 TPS, and they found a significantly improved OS in favor of ICI vs chemotherapy (HR 0.78, 95% CI 0.63–0.97, p = 0.028). The toxicity profile was likewise in favor of ICI with half the amount of treatment related adverse events compared to the chemotherapy arm. However, this interesting study leaves us with some unanswered questions, since the comparative arm did not include our standard platinum-based chemotherapy doublet. Thus, it is clinically relevant to investigate ICI against a platinum-doublet regimen administered to patients with PS 2 as well.
Strengths of our current study include the large number of patients included and the multicenter design with structured inclusion of patients treated in daily clinical practice. Since the Danish Healthcare System provides free and equal access to otherwise expensive oncologic therapies such as ICI, the included patient population is as unselected as can be within the framework of national guidelines. This minimizes the risk of selection bias. The completeness and structured capture of the clinical data is another strength with few missing data or patients lost to follow-up.
Weaknesses include the overall retrospective design of the study. It would have been preferable if this update was performed on a national basis as for the prior Danish studies which had shorter follow-up time [Citation20,Citation21]. This was unfortunately not feasible at this time point. Furthermore, the choice of pooling both retrospectively and prospectively gathered data can be discussed. However, comparison of results based on these different methods was not part of this article’s scope. It’s a strength that prospective data on real-world patients were obtained since these are currently lacking. However, these data were obtained in a different time period with shorter follow-up time and may therefore differ from the retrospectively gathered data. Thus, these data as for those patients treated in first line should be reassessed again in the future (preferable on a national basis) and compared to those real-world patients who have received combination therapy (ICI and chemotherapy). Currently, we have initialized collaboration on another Danish national study capturing data for patients treated with combination regimens as well.
Hopefully, these future data may help us navigate better within this heterogenic population where some subgroups may benefit from monotherapy and others from combination therapy.
Conclusion
ICI monotherapy significantly improves survival in real-world populations with advanced NSCLC compared to historic data on chemotherapy regardless of treatment line. Importantly, a subset of patients obtain durable long-lasting progression-free responses. Especially in real-world patients with PD-L1 ≥ 50% a substantial long-term survival benefit was observed with 4- and 5-year survival rates of 23 and 19%, respectively. Compared to RCTs the 5-year OS and PFS rate in real-world patients treated in ≥2nd line are fully comparable but in first line long-term OS and PFS rates are inferior to RCT rates. This could rely on real-world populations being more heterogenic including patients not eligible for RCTs. Importantly, our data indicate that even for the subgroup of patients with PS 2, ICI may be a better alternative to palliative chemotherapy in those with high PD-L1 (≥50%), since 32% of these patients were alive at 2-years and 17% had not progressed at 2-years. However, further studies are needed to evaluate whether standard platinum-based chemotherapy doublet or ICI is the preferred choice in the subgroup of patients with PS 2.
Supplemental Material
Download MS Word (35 KB)Supplemental Material
Download MS Word (13 KB)Supplemental Material
Download MS Word (16.3 KB)Acknowlegdements
A special thanks to clinical research nurse Joan Lassen for data capture at Sygehus Sønderjylland.
Disclosure statement
The funding sources were not involved in the study design, collection, analysis and interpretation or in the writing or decision on publication of the study. MP Honoratia for lecture and consultancy from: AstraZeneca, BMS, MSD, Pfizer, Roche; Amgen, but no conflict of interests with the discussed content. No potential conflict of interest was reported by the author(s).
Data availability statement
The study data may be available on request from the corresponding author, Birgitte Bjørnhart. However the data are not publicly available due to the General Data Protection Regulation.
Additional information
Funding
References
- The Danish Cancer Society. Report: Kræft i Danmark. The Danish Cancer Society; 2022. Available from: http://www.cancer.dk.
- DLCG/DOLG. Annual report of lung cancer in the Danish population 2021 (DLCG. Årsrapport 2021); 2022. Available from: http://www.lungecancer.dk/Lungecancer_-aarsrapport-2021offentlig.pdf.
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. doi: 10.1056/NEJMoa011954.
- Scagliotti GV, Parikh P, Von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543–3551. doi: 10.1200/JCO.2007.15.0375.
- Edwards BK, Noone AM, Mariotto AB, et al. Annual report to the nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120(9):1290–1314. doi: 10.1002/cncr.28509.
- Howlader N, Noone AH, Krapcho M, et al. SEER cancer statistics review, 1975–2018. Bethesda, MD: National Cancer Institute; 2021. Available from: https://seer.cancer.gov/csr/1975_2018/
- The Danish Medicines Council. [Ikke-småcellet lungekræft - 1. linje (medicinraadet.dk)]. [cited 2023 May]. Danish. Available from: https://medicinraadet.dk/anbefalinger-og-vejledninger/.
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774.
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi: 10.1056/NEJMoa1810865.
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005.
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi: 10.1056/NEJMoa1709937.
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X.
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627.
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643.
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi: 10.1016/S0140-6736(15)01281-7.
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥50. J Clin Oncol. 2021;39(21):2339–2349. doi: 10.1200/JCO.21.00174.
- Borghaei H, Gettinger S, Vokes EE, et al. Five-year outcomes from the randomized, phase III trials Checkmate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol. 2021;39(7):723–733. doi: 10.1200/JCO.20.01605.
- Brahmer JR, Rodriguez-Abreu D, Robinson AG, et al. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): a multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol. 2017;18(12):1600–1609. doi: 10.1016/S1470-2045(17)30690-3.
- Nosaki K, Saka H, Hosomi Y, et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1-positive advanced non-small-cell lung cancer: pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer. 2019;135:188–195. doi: 10.1016/j.lungcan.2019.07.004.
- Mouritzen MT, Carus A, Ladekarl M, et al. Nationwide survival benefit after implementation of first-line immunotherapy for patients with advanced NSCLC-real world efficacy. Cancers. 2021;13(19):4846. doi: 10.3390/cancers13194846.
- Mouritzen MT, Junker KF, Carus A, et al. Clinical features affecting efficacy of immune checkpoint inhibitors in pretreated patients with advanced NSCLC: a Danish nationwide real-world study. Acta Oncol. 2022;61(4):409–416.
- Kari Chansky MS, Nicholson AG, Rusch V, IASLC Staging and Prognostic Factors Committee AB, and Participating Institutions, et al. The IASLC lung cancer staging project: external validation of the revision of the TNM stage groupings in the eighth edition of the TNM classification of lung cancer. J Thorac Oncol. 2017;12(7):1109–1121. doi: 10.1016/j.jtho.2017.04.011.
- Brierley JG, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. 8th ed. John Wiley & Sons, Inc; 2017.
- Bjørnhart B, Hansen KH, Jørgensen TL, et al. Efficacy and safety of immune checkpoint inhibitors in a Danish real life non-small cell lung cancer population: a retrospective cohort study. Acta Oncol. 2019;58(7):953–961. doi: 10.1080/0284186X.2019.1615636.
- World Health organization. World Medical Association declaration of Helsinki. Ethical principles for medical research involving human subjects. Bullet World Health Org. 2001;79:373–374. https://apps.who.int/iris/handle/10665/268312.
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010.
- Reck M, Remon J, Hellmann MD. First-line immunotherapy for non-small-cell lung cancer. J Clin Oncol. 2022;40(6):586–597. doi: 10.1200/JCO.21.01497.
- StataCorp LLC. Stata statistical software: release 17. 17.0 Edition. College Station, TX: statacorp LLC; 2021.
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol. 2022;40(2):127–137. doi: 10.1200/JCO.21.02229.
- Lee SM, Prabhash K, Han B, et al. LBA11 – IPSOS: results from a phase III study of first-line (1L) atezolizumab (atezo) vs. single-agent chemotherapy (chemo) in patients (pts) with NSCLC not eligible for a platinum-containing regimen. Ann Oncol. 2022;33:S1418–S1419. doi: 10.1016/j.annonc.2022.08.052.