Abstract
Background
This study investigates whether inequalities in the utilization of resection and/or ablation for synchronous colorectal liver metastases (SCLM) between patients diagnosed in expert and non-expert hospitals changed since a multi-hospital network started.
Materials and methods
Patients diagnosed with SCLM between 2009 and 2020 were included. The likelihood of receiving ablation and/or resection was analyzed in the prenetwork (2009–2012), startup (2013–2016), and matured-network (2017–2020) periods.
Results
Nationwide, 13.981patients were diagnosed between 2009 and 2020, of whom 1.624 were diagnosed in the network. Of patients diagnosed in the network’s expert hospitals, 36.7% received ablation and/or resection versus 28.3% in nonexpert hospitals (p < 0.01). The odds ratio (OR) of receiving ablation and/or resection for patients diagnosed in expert versus nonexpert hospitals increased from 1.38 (p = 0.581, pre-network), to 1.66 (p = 0.108, startup), to 2.48 (p = 0.090, matured-network). Nationwide, the same trend occurred (respectively OR 1.41, p = 0.011; OR 2.23, p < 0.001; OR 3.20, p < 0.001).
Conclusions
Patients diagnosed in expert hospitals were more likely to receive ablation and/or resection for SCLM than patients diagnosed in non-expert hospitals. This difference increased over time despite the startup of a multi-hospital network. Establishing a multi-hospital network did not have an effect on reducing the existing unequal odds of receiving specialized treatment.
Synopsis
Specialized oncology treatments are increasingly provided through multi-hospital networks. However, scant empirical evidence on the effectiveness of these networks exists. This study analyzes whether a regional multi-hospital network was able to improve equal access to specialized oncology treatments.
Background
With 10% of all cancer diagnoses, colorectal cancer (CRC) is the third most commonly diagnosed cancer worldwide [Citation1]. Approximately 20% of all CRC patients have distant metastases at diagnosis, of whom 83% are located in the liver, most of whom without extrahepatic metastases [Citation2,Citation3]. Patients with liver-only metastases have a five-year overall survival (OS) of 25.2% [Citation4,Citation5].
In recent decades, various specialized treatment options for synchronous colorectal liver metastases (SCLM), such as resection and ablation, have become available [Citation6], which aim to increase local disease control and improve survival [Citation7–10]. Centralization of specialized treatment in expert hospitals improves treatment outcomes [Citation11–16] but has also been associated with unequal access to those treatments [Citation9,Citation17]. Ensuring equal access to these specialized treatments could thus improve disease control and survival for a broader group of patients with SCLM.
Research asserts that collaboration in multi-hospital networks can reduce unequal access to specialized treatments [Citation18] by making use of scarce specialist expertise [Citation19], standardizing care [Citation19], transmitting resources to each other [Citation20], facilitating knowledge transfer [Citation21], and exchanging information [Citation22]. Consequently, specialized treatments are increasingly provided through multi-hospital networks [Citation23–25]. Literature on stroke and community care shows that networks can indeed reduce unequal access to care [Citation18,Citation26,Citation27]. However, scant empirical evidence on the effectiveness of multi-hospital networks on equal access to centralized oncological treatments exists. Therefore, this study aims to analyze whether the start of a multi-hospital network mitigated the already existing unequal odds of receiving specialized treatment, more specifically: resection or ablation for SCLM.
Materials and methods
The Dutch hospital landscape
The Netherlands has 57 general hospitals, 28 teaching hospitals, and eight university medical centers [Citation28]. While SCLM are diagnosed in every hospital, resection and ablation for SCLM are only performed in hospitals that meet specific requirements, leading to centralization since 2011 [Citation29,Citation30]. Hospitals performing resection and ablation for synchronous colorectal liver metastases are referred to as ‘expert hospitals’. In the Netherlands, patients are not required to seek care only within their network. However, in practice, it appears that referrals do often occur within the network. Only 4% of all treated Dutch cancer patients (not limited to SCLM) are referred outside their network [Citation25]. Information on the distribution and characteristics of Dutch hospitals treating CRC has been described elsewhere [Citation31].
The network
Hospitals work together in regional networks to be able to guarantee optimal quality and safety close to the patient’s home [Citation29]. This study focuses on the OncoZON network, consisting of nine autonomous hospitals in the southeast of the Netherlands; one university medical center, four teaching hospitals, and four general hospitals, serving 10–15% of all Dutch cancer patients [Citation32]. The network aims that every patient always receives the best possible cancer care, regardless of the hospital in which the patient enters and thus aims to reduce between-center variation. This regional network is divided into (pathology specific) working groups. In this study, we zoom in on the network’s gastroenterology group. The study period is divided into three network periods; prenetwork (2009–2012), startup (2013–2016), and matured-network (2017–2020). Until 2017, ablation and resection were performed in three of the network’s hospitals, which was further centralized to two hospitals from 2017 onward. Since 2017, delegates from the nine hospitals meet three to four times a year. The network’s working group investigated in this study focuses on gastrointestinal oncology and meetings focus on transmitting resources to each other, facilitating knowledge transfer, and exchanging information, in line with what is suggested by network literature [Citation20–22]. SCLM is one of the topics in the network’s gastroenterology working group. Although the centers performing resection and ablation are clearly defined, no official referral agreements are formalized by the network. Both expert hospitals host expert Hepato–Pancreato–Biliary (HPB) multidisciplinary team meetings (expert-MDT) in which nonexpert hospitals can present their patients to the expert panel. This panel advises whether the patient should be referred for resection or ablation, or whether (first) another treatment would be more appropriate (e.g. chemotherapy). Those other treatments usually take place in the patient’s own hospital.
Data
For this multi-center cohort study, data from the Netherlands Cancer Registry was used [Citation33]. The NCR registers all newly diagnosed malignancies in the Netherlands. Data managers working for the NCR are specially trained and use comprehensive data reporting manuals to extract data on patient, tumor, and treatment characteristics from medical records. De data undergoes regular validation checks. The anatomical site of the primary tumor is defined according to the International Classification of Disease in Oncology (ICD-O) 3rd edition [Citation34]. Staging is based on the International Union Against Cancer (UICC) tumor-node-metastasis-classification (TNM) according to the edition valid at the time of diagnosis based on pathological stage, supplemented with the clinical stage if missing [Citation35]. The year of diagnosis was defined as the year of first histological confirmation. Socioeconomic status (SES) is based on individual fiscal data on the economic value of the home and household income, provided at an aggregated level per postal code. The outcome of interest was whether or not resection and/or ablation of SCLM was received at any time in the patients’ primary treatment journey (yes/no). Given the patient inclusion in this study involves only synchronously metastasized patients, it is expected that if they would have received resection and/or ablation, it would have been in the primary treatment pathway. Resection is defined as curative intent liver resection (i.e., R0 intent), and ablation is defined as microwave ablation, radiofrequency ablation, or cryo-ablation of SCLM. Overall survival (OS) was defined as the interval in months between diagnosis and death, and hospital of diagnosis as the hospital of first contact for possible malignancy, whether outpatient or inpatient. Follow-up on vital status was available up to 31 January 2022.
Patient selection
Adult patients (≥18 years) with stage-IV CRC with SCLM without extrahepatic metastases diagnosed in the Netherlands between 2009 and 2020 were included. Primary tumor localizations included were colon (C18), rectosigmoid transition (C19), and rectum (C20), with the presence of synchronous liver metastases (C22.0), defined as metastases detected before initial treatment was started and/or during surgical exploration. Metastases of the intrahepatic bile ducts (C22.1) were excluded due to their rarity. Patients were also excluded if the primary tumor concerned a neuroendocrine tumor. Only synchronous metastases are included due to data availability. One hospital performed resection and ablation treatment up to 2017 but stopped doing so from 2017 forward. Patients diagnosed in this hospital were assigned to the ‘expert’ group if diagnosed before 2017, and to the ‘nonexpert’ group if diagnosed from 2017 forward.
Statistical analyses
Analyses are based on the hospital of diagnosis. Chi-square tests and Mann–Whitney U-tests were used to detect baseline differences in patient, tumor, and treatment characteristics. Fisher’s exact test was used for discrete variables with <5 observations.
Multilevel logistic regression models were computed to account for the data structure (patients nested in hospitals) for the overall period as well as for three separate time periods. Receiving resection and/or ablation (yes/no) was the dependent variable and the hospital of diagnosis (expert/nonexpert) the variable of interest. Patient and tumor characteristics, listed in , were included to adjust for relevant case-mix factors. Hospital type and other treatments (i.e., not resection or ablation) were not included in the model, given the direct correlations with the hospital of diagnosis and receiving resection or ablation. A Kenward–Roger correction was used to account for the small sample (n = 9) at the hospital level [Citation36,Citation37]. Missing data were included in the analyses coded as ‘unknown’. The same models were created using national data, including all Dutch hospitals except for the network hospitals.
Table 1. Patient characteristics for stage IV colorectal cancer patients with synchronous liver-only metastases according to hospital of diagnosis being an expert-hospital or a non-expert hospital .
Multilevel Cox Proportional Hazard models were computed to detect differences in survival between patients diagnosed in expert centers versus patients diagnosed in nonexpert centers using national data including the network hospitals, for the same time periods as used in the previous models, using the same independent variables with the addition of receiving resection and/or ablation (yes/no).
Analyses were conducted using SAS®9.4(SAS Institute Cary, USA). The significance level adopted was <0.05. Ethical approval is obtained for a broader project on oncology networks by Maastricht University (FHML-REC/2022/047).
Results
Patient characteristics
Of all 13,981 Dutch patients diagnosed with SCLM without extrahepatic disease between 2009 and 2020, 1.624 (11.6%) were diagnosed in the network. Nonexpert hospitals diagnosed more older patients (p=0.005), with more often well or moderately differentiated tumors (p=0.015). SES and comorbidities significantly differed between expert and non-expert hospitals (p<0.001) ().
Utilization of care
Of patients diagnosed in the network, 83.9% received some form of care (surgery for primary tumor, radiotherapy, chemotherapy, resection of metastases, and/or ablation). Resection and/or ablation of liver metastases was provided to 31.1% of the network’s patients. Of patients diagnosed in the network’s expert hospitals, 36.7% received resection and/or ablation versus 28.3% of patients diagnosed in nonexpert hospitals (p<0.001). From 2009 to 2020, the overall number of patients diagnosed with SCLM per hospital of diagnosis varied from 65 to 413 in the network. The overall number of patients with SCLM that received resection and/or ablation per hospital of diagnosis varied from n = 10 (15.4%) to n = 114 (43.0%) (). Patients diagnosed in expert hospitals received surgery for the primary tumor (p=0.031) and chemotherapy (p=0.049) more often, while they received best supportive care less often (p=0.018).
Figure 1. Distribution of resection and/or ablation for synchronous colorectal liver-only metastases in a regional multi-hospital network (2009–2020).
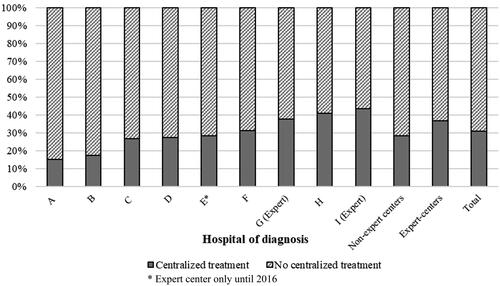
The percentage of patients receiving resection and/or ablation increased over time. Expert hospitals, however, show an increasing trend in the percentage of patients receiving resection and/or ablation, while this percentage appears to be stabilizing in non-expert hospitals ().
Figure 2. Percentage of patients that received resection and/or ablation for SCLM according to hospital of diagnosis being an expert- or non-expert center.
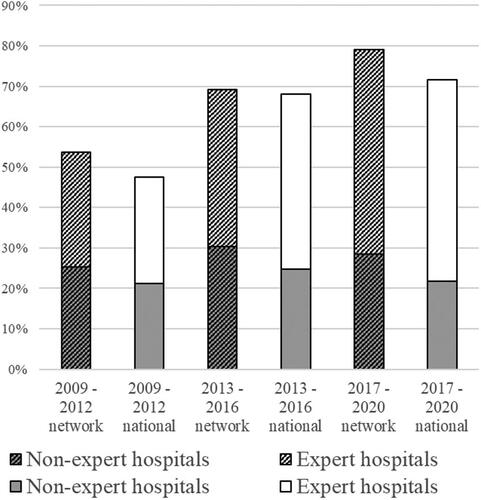
While multilevel-multivariable analyses within the network showed no significant differences in the likelihood of receiving resection and/or ablation in the pre-network period (OR 1.38, p=0.581), startup period (OR 1.66, p=0.108), and matured-network period (OR 2.48, p=0.090), the point estimates seem to increase over time. This implies that the difference in likelihood to receive resection and/or ablation between patients diagnosed in expert versus nonexpert centers increases over time within the network ().
Table 2. Multivariable logistic regression analyses on the likelihood of receiving resection and/or ablation for SCLM within a regional multi-hospital oncology network (n = 1624), over the overall study period and divided into time-intervals.
In nationwide data, the proportion of the total number of patients diagnosed with liver-only SCLM per hospital of diagnosis that received resection and/or ablation varied from 9.1 to 67.6% (mean = 28.8%). A significant difference in likelihood of receiving resection and/or ablation was present at all time periods (prenetwork OR 1.41, p=0.011; startup OR 2.23, p<0.001; matured-network OR 3.20, p<0.001) ().
Table 3. Multilevel multivariable logistic regression analyses on the likelihood of receiving resection and/or ablation for SCLM in the Netherlands (n = 12.357)* over the overall study period and divided into time-intervals.
Survival analyses
Nationwide, the median overall survival for patients diagnosed in nonexpert centers was 15.8 months (CI 14.3–15.3), which was 20.5 months for patients diagnosed in expert centers (CI 19.5–21.6). Corrected for case-mix differences, receiving resection and/or ablation led to a significantly lower hazard of death compared to not receiving resection and/or ablation for the overall time period (HR 0.32, p>0.001), as well as for the three separate time periods (respectively HR 0.38, HR 0.32, and HR 0.27, all p<0.001). Being diagnosed in an expert hospital, regardless of whether or not resection and/or ablation was received, led to a significantly lower hazard compared to being diagnosed in a non-expert center in the overall time period (HR 0.93, p=0.022), as well as in the startup period (HR 0.90, p=0.031), but not in the pre-network and matured network period. ()
Table 4. Multilevel Cox Proportional Hazard analyses on all patients with SCLM in The Netherlands (n = 13.981) over the overall study period and divided into time intervals.
Discussion
This study sought to understand whether a multi-hospital network can reduce inequalities in receiving specialized treatments. Starting a multi-hospital network was unable to counter the growing disparity in likelihood of getting resection and/or ablation of SCLM. Although there are generally more patients receiving resection and/or ablation, suggesting a positive trend, the between-center disparity in the use of resection and/or ablation has increased, which is nonetheless not desirable. Nationwide, a significant variation in odds of receiving resection and/or ablation between patients diagnosed in experts versus patients diagnosed in nonexpert hospitals existed and even increased over time. The same increasing trend was seen in network data. Methodologically, given the multi-level approach adopted in this study and an n = 9 at level 2 in the network data (hospital level), the lack of significance may be explained by too small a sample size, which is also reflected in the broad CIs. The increasing difference could be explained by developments in the medical field and greater availability of treatment options, in which expert hospitals are often the early adopters. One would expect nonexpert hospitals to, perhaps somewhat delayed, also show an upward trend, but this remained similar (). The differences found in this study are in line with previous studies, which showed an up to 59% lower likelihood of receiving specialized treatment for patients diagnosed in non-expert hospitals [Citation9,Citation15,Citation38]. In , the difference between expert and non-expert centers appears to be smaller in the network than in the rest of the country. However, this study shows that, contrary to what is expected from networks, starting a network was not sufficient to reduce this difference, or even attenuated the increasing trend in difference between both groups.
In secondary analyses, we found a positive effect of receiving resection and/or ablation of SCLM on survival, which is in line with previous studies [Citation7–10]. Also, perhaps more surprising, we found that in the overall period, as well as in the startup period, being diagnosed in an expert hospital was associated with a significantly lower hazard ratio on mortality, regardless of whether resection and/or ablation was performed, which emphases the importance to counter inequality in access to care and continue to explore how to achieve this in networks.
It is noticeable that the number of patients with SCLM in expert centers in 2017–2020 decreased compared to earlier periods. This can be explained by the fact that until 2016, three hospitals performed ablation and surgery for SCLM, which was further centralized to two hospitals in 2017. As a result, if this would have been the only explanation, the number of SCLM diagnoses in non-expert centers in period three would have increased simultaneously compared to the earlier periods. Instead this also slightly decreased, indicating an overall decrease of patients with SCLM compared to previous periods. Possible explanations for this overall decrease are probably the previously described effect of the introduction of nationwide screening, leading to a decrease in overall as well as advanced-stage CRC diagnosis [Citation39], and the fact that the COVID-pandemic covered the last few years of period 3, possibly resulting in delayed visits to general practitioners and/or hospitals [Citation40].
Three possible explanations can be given for the inequalities found in this study. First, patients are referred by nonexpert hospitals but may refuse care at another (i.e., expert) hospital. However, the literature indicates that only 2.6–3.8% of patients refuse cancer treatment [Citation41–43], which suggests that further focus is needed on the collaboration in networks.
Second, the nonexpert hospitals seek consultation from an HBP-expert team, but their patients are still less likely to receive surgery and/or ablation for SCLM. This may be because they are rejected from resection and/or ablation more often than patients diagnosed in an expert center and are thus treated differently compared to the expert hospitals’ own patients. This could have many causes, like technical reasons (e.g., reduced quality of images due to data sharing complicating clinical assessment), time constraints (e.g., reviewing the images last-minute during the expert-MDT, rather than in preparation for the expert-MDT), or a physician (team)’s cognitive bias, which is known to be an important factor in medical decision making [Citation44]. Also, nonexpert hospitals could seek consultation from an HPB-expert team in which it is decided that the patient will be first treated at the non-expert hospital for the primary tumor, after which the patients later will be referred to an expert center for subsequent treatment by surgery or ablation. The possibility is that progression has occurred due to elapsed time, making surgery and/or ablation no longer possible.
As a third possible explanation, patients from non-expert centers are not referred to expert centers. This third explanation could be further distinct in patients who are discussed with specialists form the expert center in a consultation and from whom a joint decision is taken not to refer them, and patients from non-expert centers not referred to expert centers that are not discussed between non-expert and expert centers, leaving the option for resection and/or ablation for SCLM unaddressed. In general, physicians experience a high level of professional autonomy and referrals are often based on habits and routine practice, making behavioral change and guideline adherence concerning patient referral difficult to achieve [Citation45,Citation46]. Moreover, physician barriers could stand in the way of adherence [Citation47], for example, referrals take up a physicians’ valuable time and good technology to share patient information is lacking [Citation48]. Many factors described as barriers are beyond physician control but could be compensated for by providing adequate resources [Citation47]. For instance, MDTs take place in every hospital, but input from physicians of expert hospitals is not yet standard practice. The two expert hospitals affiliated with the network do offer opportunities for non-expert centers to present their patients, like in an expert-MDT, but this may not yet be used structurally. Participating in these (expert)-MDTs costs nonbillable time. Funding for network care is therefore desirable to lower the participating physician’s burden by reimbursing network care like participation in regional MDTs, but also to invest in resources for digital data exchange.
Another barrier that is mentioned in the literature is the lack of a reminder system [Citation47]. It may help for nonexpert hospitals to schedule expert-MDTs as a permanent feature of their calendars instead of something that has to be done in the spur of the moment. It may also help to make one person responsible for attending the structural expert-MDT. This person responsible may, but need not necessarily, be a physician, nurse practitioner, or physician assistant. This way, possible cases that could be discussed are pooled, which can be especially time efficient for hospitals that diagnose relatively few patients with SCLM. This person could put out reminders to all CRC physicians when another expert-MDT for SCLM is scheduled, automatically introducing a reminder system.
Finally, literature shows that clinical pathways are able to standardize practice patterns within large networks, leading to more uniform and cost-effective treatments [Citation49], and agreements and protocols are shown to be able to change practices [Citation50]. It is therefore recommended to create service level agreements, defining which patients with SCLM should be referred to an expert-MDT and when. To perform quality control in the future and to pinpoint which of these possible explanations is the biggest driver for these disparities, it is important to properly report which patients are or are not discussed in expert-MDTs.
Limitations
This study has some limitations. First, due to data availability, we used a somewhat narrow definition of synchronous metastases of CRC, as opposed to some literature that suggests up to twelve months after initial diagnosis of CRC, thereby focusing on only a limited patients selection [Citation51]. Another limitation could be the interference of clinical trials at time of this study, like the CAIRO5 study [Citation52], which could ensure that the number of surgical treatments increases. A previous study showed that a lack of a research environment was a key barrier for enrollment in clinical trials, which is most common in small hospitals [Citation53]. Another limitation of this study is the absence of data on patients referred to an expert-MDT for consultation with an expert HPB surgeon. As a result, we cannot pinpoint which of the possible explanations as described in the discussion section explains the between-center variation as found in this study (i.e., nonexpert hospitals not seeking consultation with an HPB-expert center, or the HPB-expert centers treating their own patients differently compared to patients from nonexpert centers). However, both possible explanations suggest an insufficiency in the collaboration and that the network in its current form was ineffective in reducing the between-center variation in the utilization of resection and/or ablation for SCLM. As described, patients diagnosed in nonexpert hospitals could either be treated differently by the expert hospitals or could not even be referred to expert hospitals at all. Due to the absence of information on whether or not consultation with an expert-HPB team took place, it is not possible to pinpoint the origins of the growing disparities. We therefore advocate follow-up research by in-depth analysis of medical records of patients diagnosed in non-expert centers versus patients diagnosed in expert centers, possibly supplemented by qualitative research to verify these results and identify the reason of the differences. Finally, comparing networks across regions within the country is impossible given the diversity of these networks in, among other things, their structure, governance, formality and startup years.
Conclusions
Variation in the utilization of resection and/or ablation for SCLM between patient diagnosed in expert hospitals and patients diagnosed in nonexpert hospitals exist, potentially causing patients to miss out on life-extending or even curative treatments. Patients diagnosed in expert hospitals have a significantly higher likelihood of receiving resection and/or ablation for SCLM compared to patients diagnosed in nonexpert hospitals. Our study shows that this difference increased over time. Moreover, contrary to what is expected, starting a multi-hospital network based on meeting frequently, getting to know each other, facilitating knowledge transfer, and exchanging information does not seem to be (sufficiently) effective in reducing this disparity in likelihood of receiving resection and/or ablation. To find out if more targeted network strategies are effective, it is important to understand how the mechanisms in networks work to be able to steer network activities differently toward greater effectiveness.
Acknowledgements
The authors thank the OncoZON consortium, composed of the Anna Hospital, Catharina Hospital, Elkerliek Hospital, Laurentius Hospital, Maastricht University Medical Center+, Maastro Clinic, Máxima Medical Center, Sint Jans Gasthuis, VieCuri Medical Center, and Zuyderland, for making this work possible. Further, the authors extend their thanks to Niels Hameleers and Dr. Maarten Bijlsma for their valuable input concerning the statistical analyses adopted in this paper.
Disclosure statement
R.G.F.M. van der Ven receives partial salary support from the OncoZON consortium for a broader project on network research. All other authors have no disclosures to report. The consortium had no involvement in the study design, collection, analyses and interpretation of data, writing of the report, and in the decision to submit the article for publication. No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the Netherlands Cancer Registry (NCR), maintained by the Netherlands Comprehensive Cancer Organization. Restrictions apply to the availability of these data, which were used under license for this study. Data are available with the permission of the Netherlands Comprehensive Cancer Organization.
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660.
- Susanne SC, Ito K, Raoof M, et al. Cytoreduction for colorectal metastases: liver, lung, peritoneum, lymph nodes, bone, brain. When does it palliate, prolong survival, and potentially cure? Curr Probl Surg. 2018;55(9):330–379. doi: 10.1067/j.cpsurg.2018.08.004.
- Riihimäki M, Hemminki A, Sundquist J, et al. Patterns of metastasis in colon and rectal cancer. Sci Rep. 2016;6:29765. doi: 10.1038/srep29765.
- Ito K, Govindarajan A, Ito H, et al. Surgical treatment of hepatic colorectal metastasis: evolving role in the setting of improving systemic therapies and ablative treatments in the 21st century. Cancer J. 2010;16(2):103–110. doi: 10.1097/PPO.0b013e3181d7e8e5.
- Engstrand J, Nilsson H, Strömberg C, et al. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. 2018;18(1):78–89. doi: 10.1186/s12885-017-3925-x.
- Birrer DL, Tschuor C, Reiner CS, et al. Multimodal treatment strategies for colorectal. Swiss Med Wkly. 2021;151:w20390. doi: 10.4414/smw.2021.20390.
- Petrelli F, Comito T, Barni S, et al. Stereotactic body radiotherapy for colorectal cancer liver metastases: a systematic review. Radiother Oncol. 2018;129(3):427–434. doi: 10.1016/j.radonc.2018.06.035.
- Lee J, Shin I-S, Yoon WS, et al. Comparisons between radiofrequency ablation and stereotactic body radiotherapy for liver malignancies: meta-analyses and a systematic review. Radiother Oncol. 2020;145:63–70. doi: 10.1016/j.radonc.2019.12.004.
- Lam-Boer J ,Al Ali C, Verhoeven RH, et al. Large variation in the utilization of liver resections in stage IV colorectal cancer patients with metastases confined to the liver. Eur J Surg Oncol. 2015;41(9):1217–1225.
- Pawlik TM, Abdalla EK, Ellis LM, et al. Debunking dogma: surgery for four or more colorectal liver metastases is justified. J Gastrointest Surg. 2006;10(2):240–248. doi: 10.1016/j.gassur.2005.07.027.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346(15):1128–1137. doi: 10.1056/NEJMsa012337.
- Ho V, Heslin MJ, Yun H, et al. Trends in hospital and surgeon volume and operative mortality for cancer surgery. Ann Surg Oncol. 2006;13(6):851–858. doi: 10.1245/ASO.2006.07.021.
- Stitzenberg KB, Meropol NJ. Trends in centralization of cancer surgery. Ann Surg Oncol. 2010; 17(11):2824–2831. doi: 10.1245/s10434-010-1159-0.
- Torzilli G, Viganò L, Giuliante F, et al. Liver surgery in Italy. Criteria to identify the hospital units and the tertiary referral centers entitled to perform it. Updates Surg. 2016;68(2):135–142. doi: 10.1007/s13304-016-0373-0.
- Vallance AE, VanderMeulen J, Kuryba A, et al. Impact of hepatobiliary service centralization on treatment and outcomes in patients with colorectal cancer and liver metastases. Br J Surg. 2017;104(7):918–925. doi: 10.1002/bjs.10501.
- Weledji EP. Centralization of liver cancer surgery and impact on multidisciplinary teams working on stage IV colorectal cancer. Oncol Rev. 2017;11(2):331. doi: 10.4081/oncol.2017.331.
- Huguet M. Centralization of care in high volume hospitals and inequalities in access to care. Social Science & Medicine. 2020;260:113177. doi: 10.1016/j.socscimed.2020.113177.
- Cadilhac DA, Moodie ML, Lalor EE, et al. Improving access to evidence-based acute stroke services: development and evaluation of a health system model to address equity of access issues. Aust Health Review. 2006;30(1):109–118. doi: 10.1071/AH060109.
- Addicott R, McGivern G, Ferlie E. The distortion of a managerial technique? The case of clinical networks in UK health care. Br J Management. 2007;18(1):93–105. doi: 10.1111/j.1467-8551.2006.00494.x.
- Balkundi P, Harrison DA. Ties, leaders, and time in teams: strong inference about network structure’s effects on team viability and performance. AMJ. 2006;49(1):49–68. doi: 10.5465/amj.2006.20785500.
- Inkpen AC, Tsang EWK. Social capital, networks, and knowledge transfer. AMR. 2005;30(1):146–165. doi: 10.5465/amr.2005.15281445.
- Koka BR, Prescott JE. Strategic alliances as social capital: a multidimensional view. Strat Mgmt J. 2002;23(9):795–816. doi: 10.1002/smj.252.
- Nolte E, Pitchforth E, Miani C. The changing hospital landscape: an exploration of international experiences. RAND Health Quarterly. 2014;4(3):14–20.
- Van de Voorde C, Van den Heede K, Beguin C, et al. Required hospital capacity in 2025 and criteria for rationalisation of complex cancer surgery, radiotherapy and maternity services. Brussels: Federatie Kennisgeving voor de Gezondheidszorg; 2017.
- Netherlands Comprehensive Cancer Organisation, Citrienprogramma Naar regionale oncologienetwerken, Dutch Federation of Cancer Patient Organisations & SONCOS Federation of Medical Specialists, on behalf of Taskforce Oncologie. Regionale Oncologienetwerken in Beeld [Internet]. 2022 [cited 2023 Apr]. Available from: https://oncologienetwerken.nl/sites/default/files/2022-04/Rapport_Oncologienetwerken-in-beeld_2022-april_1.pdf
- Kelaher M, Sabanovic H, La Brooy C, et al. Does more equitable governance lead to more equitable health care? A case study based on the implementation of health reform in aboriginal health Australia. Soc Sci Med. 2014;123:278–286. doi: 10.1016/j.socscimed.2014.07.032.
- Bambra C, Garthwaite K, Hunter D. All things being equal: does it matter for equity how you organize and pay for health care? A review of the international evidence. Int J Health Serv. 2014;44(3):457–477. doi: 10.2190/HS.44.3.c.
- Kroneman M, Boerma W, van den Berg M, et al. The Netherlands health system review. 2nd ed. Health Syst Trans. 2016;18:1–240.
- Foundation For Oncological Cooperation (SONCOS). Multidisciplinary standards for oncological are in the Netherlands. 2021.
- Nederlandse Vereniging voor Heelkunde (NVvH). Normering Chirurgische Behandeling v1.0. 2011.
- Kolfschoten NE, Marang van de Mheen PJ, Gooiker GA, et al. Variation in case-mix between hospitals treating colorectal cancer patients in The Netherlands. Eur J Surg Oncol. 2011;37(11):956–963. doi: 10.1016/j.ejso.2011.08.137.
- Oncologisch Netwerk Zuidoost-Nederland. OncoZON [Internet]. 2017 [updated 2020 Dec 3]. Available from. https://www.oncozon.nl/.
- Netherlands Comprehensive Cancer Organisation (IKNL). Netherlands Cancer. Registry (NCR). Available from https://iknl.nl/en/ncr.
- Fritz A, Percy C, Jack A, et al. International classification of diseases for oncology (ICD-O). 3rd ed. 1st revision. Geneva: World Health Organisation. [Internet]. 2013. Available from: https://apps.who.int/iris/handle/10665/42344
- Sobin LH, Gospodarowicz MK, Wittekind CH, Union for International Cancer Control (UICC). The TNM classification of malignant tumours. 7th ed. Chichester: Wiley-Blackwell; 2009.
- Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53(3):983–997. doi: 10.2307/2533558.
- G KM, H RJ. An improved approximation to the precision of fixed effects from restricted maximum likelihood. Computat Statist Data Anal. 2009;53:2583–2595.
- Tiernan J, Briggs CD, Irving GRB, et al. Evaluation of the introduction of a standardised protocol for the staging and follow-up of colorectal cancer on resection rates for liver metastases. Ann R Coll Surg Engl. 2010;92(3):225–230. doi: 10.1308/003588410X12628812458419.
- Breekveldt ECH, Lansdorp-Vogelaar I, Toes-Zoutendijk E, et al. Colorectal cancer incidence, mortality, tumour characteristics, and treatment before and after introduction of the faecal immunochemical testing-based screening programme in The Netherlands: a population-based study. Lancet Gastroenterol Hepatol. 2022;7(1):60–68. doi: 10.1016/S2468-1253(21)00368-X.
- Dinmohamed AG, Visser O, Verhoeven RHA, et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21(6):750–751. doi: 10.1016/S1470-2045(20)30265-5.
- Fields AC, Lu PW, Yoo J, et al. Treatment of stage I-III rectal cancer: who is refusing surgery? J Surg Oncol. 2020;121(6):990–1000. doi: 10.1002/jso.25873.
- Rahouma M, Harrison S, Kamel M, et al. Consequences of refusing surgery for esophageal cancer: a national cancer database analysis. Ann Thorac Surg. 2018;106(5):1476–1483. doi: 10.1016/j.athoracsur.2018.06.030.
- Coffman A, Torgeson A, Lloyd SC. Correlates of refusal of surgery in the treatment of non-metastatic pancreatic adenocarcinoma. Ann Surg Oncol. 2019;26(1):98–108. doi: 10.1245/s10434-018-6708-y.
- Pines JM, Strong A. Cognitive biases in emergency physicians: a pilot study. J Emerg Med. 2019;57(2):168–172. doi: 10.1016/j.jemermed.2019.03.048.
- Kongstvedt PR. Essentials of managed health care. (4th edition). Sudbury, Massachusetts: Jones and Bartlett Publishers; 2000. (Chapter 19: physician Behavior Change in Managed Health Care).
- Davis R, Campbell R, Hildon Z, et al. Theories of behaviour and behaviour change across the social and behavioural sciences: a scoping review. Health Psychol Rev. 2015;9(3):323–344. doi: 10.1080/17437199.2014.941722.
- Reinertsen JL. Algorithms, guidelines, and protocols: can they really improve what we do? Transfusion. 1994;34(4):281–282. doi: 10.1046/j.1537-2995.1994.34494233573.x.
- Henderson J, Valenti LA, Britt HC, et al. Estimating non-billable time in Australian general practice. Med J Aust. 2016;205(2):79–83. doi: 10.5694/mja16.00287.
- Gebhardt B, Rajagopalan M, Gill B, et al. Impact of dynamic changes to a bone metastases pathway in a large, integrated, national cancer institute-designated comprehensive cancer center network. Pract Radiat Oncol. 2015;5(6):398–405. doi: 10.1016/j.prro.2015.06.013.
- Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–1465. doi: 10.1001/jama.282.15.1458.
- Mekenkamp LJM, Koopman M, Teerenstra S, et al. Clinicopathological features and outcome in advanced colorectal cancer patients with synchronous vs metachronous metastases. Br J Cancer. 2010;103(2):159–164. doi: 10.1038/sj.bjc.6605737.
- Punt CJA, Swijnenburg RJ, van Grieken NCT, et al. Treatment strategies in colorectal cancer patients with initially unresectable liver-only metastases CAIRO5 a randomised phase 3 study of the Dutch Colorectal Cancer Group (DCCG) – study protocol v12 [Study protocol]. 2022 Feb 17. Available from: https://dccg.nl/trial/cairo5
- Wang M, Dolovich L, Holbrook A, et al. Factors that influence community hospital involvement in clinical trials: a qualitative discriptive study. J Eval Clin Pract. 2022;28(1):79–85. doi: 10.1111/jep.13583.
