Abstract
Background
Life expectancy for patients diagnosed with metastatic breast cancer (BC) has improved in recent years, especially due to better systemic treatment. This has led to an increased incidence of brain metastases (BM), and BC is now the leading cause of BM in women. Treatment of BM primarily consists of surgery and/or radiotherapy. We aimed to investigate survival time and prognostic factors for BC patients treated with radiotherapy for BM.
Material & methods
During the period 1st of January 2015 to 1st of June 2020, 144 consecutive BC patients treated for BM from one centre were retrospectively analyzed. The patients were either diagnosed with BM as the first metastatic lesion, or developed BM during palliative therapy for distant non-brain metastasis. The study was approved by the Central Denmark Region.
Results
Median age at BM diagnosis was 66 years, and 90% of the patients already had extracranial metastatic disease at BM diagnosis. Median overall survival after diagnosis of BM was 6.1 months. Short survival was observed for patients with poor performance status, leptomeningeal metastasis or more than three solid BM. Several of these factors were overrepresented in patients with estrogen receptor-positive (ER+) tumours who had poorer survival than patients with different receptor status.
Conclusion
The number of metastatic BC patients developing BM is high, and survival following local treatment remains poor. Several prognostic factors appear to influence survival after radiotherapy. Treatment of BC patients with BM should be individualized according to performance status, leptomeningeal disease, number of BM, and receptor status of the disease.
Introduction
The life expectancy for patients diagnosed with metastatic breast cancer (BC) has increased, especially due to improved systemic treatment options [Citation1,Citation2]. The prolonged survival time among metastatic BC patients has led to an increased incidence of brain metastasis (BM) [Citation1,Citation3]. Survival from the first diagnosis of distant metastases depends, among other factors, on the organ(s) involved, with CNS involvement leading to the worst prognosis [Citation4]. BC is now the leading cause of BM in women [Citation5], and up to 30% of BC patients with extracranial metastatic disease eventually develop BM [Citation5,Citation6]. Reports have found that young age at BC diagnosis, estrogen receptor negativity (ER-), overexpression of human epidermal growth factor (HER2+), high histological grade and nodal involvement at primary BC diagnosis were the most important and consistent risk factors for developing BM [Citation7–9].
Even though systemic treatment has prolonged life among BC patients, most systemic treatment options have limited effect on BM, as most drugs seem unable to pass the blood-brain barrier in sufficient amounts for establishing an effective treatment [Citation1].
Local treatment options such as surgery and radiotherapy have become the predominant treatment for BM [Citation10,Citation11]. In recent years, the local treatment approach has gained increased focus due to improved surgical technique and the rising availability of stereotactic radiosurgery (SRS), while the use of whole brain radiation therapy (WBRT) has declined mainly due to a growing awareness of the cognitive side effects following radiation [Citation5,Citation12]. Thus, SRS is the preferred radiotherapy option if patients fulfil the requirements. Recently, even proton therapy has been tested for leptomeningeal disease, where it seems to improve survival based on a recent randomized phase II study [Citation13].
Selection of patients for WBRT or SRS is primarily based on the performance status of the patient, BC subtype, the extent of cranial and extracranial metastatic disease, number and size of the metastatic lesions in the brain, and expected lifetime [Citation1,Citation5,Citation10,Citation14]. Of these factors, the number of brain lesions is of utmost importance. Surgical resection (SR) or SRS is generally preferred if there are few (≤3) and accessible lesions. WBRT is preferred if lesions are excessive in size or numbers, or surgically inaccessible [Citation5,Citation11,Citation15,Citation16]. Even though local treatment might lead to prolonged survival for patients with solitary BM [Citation17], treatment options for both localized, multiple, or inaccessible BM remain palliative [Citation1]. Conclusively, effective life-prolonging treatments for BM remain scarce. Recently, however, trials evaluating new HER2-directed therapies have reported a significant improvement in overall survival compared to standard treatment, in patients with HER2+ disseminated disease and BM [Citation18].
There is limited data on survival following modern therapy with conventional WBRT, SRS or SR either alone or combined with any radiotherapy [Citation19]. This study aims to investigate overall survival and possible prognostic factors after the diagnosis of BM treated with a local strategy (SRS, SR and/or WBRT). Based on previous reports, several prognostic factors were selected for investigation, as they had been proven to impact survival in other studies [Citation7,Citation12,Citation19]. These factors were age, primary BC receptor status, type of treatment for BM, lesion characteristics (number, location and size), performance status, number of systemic treatment lines received before BM diagnosis, and time interval from diagnosis of metastatic BC to BM development.
Material and methods
This is a single-institution retrospective study. We followed the EQUATOR guidelines for formatting, using the SQUIRE 2.0 checklist for quality improvement studies [Citation20].
Consecutive patients were identified by a retrospective review of patient records at the Department of Oncology at Aarhus University Hospital. Patients were included if they were diagnosed with BC, and were treated with radiotherapy for BM during the period from 1st of January 2015 to 1st of June 2020. The study was approved by the Central Denmark Region.
Details on the radiotherapy delivered were not collected, but the institutional guideline for WBRT was to use 20 Gy/4fr (5 Gy/fr) or 30 Gy/10fr/5 weekly fractions. Most patients had 20 Gy/4fr, whilst 30 Gy/10fr was preferred if the patient was expected to have a relatively long survival (good performance status, several remaining systemic treatment options and HER2+ disease). If SRS was selected, 27 Gy/3fr on nonconsecutive days was used, independently of the size of the largest tumor. Patients with extensive disease, poor performance status and short expected remaining survival time were not offered radiotherapy. For patients with limited BM, the decision on SRS vs WBRT and fractionation was made in a multidisciplinary team with the participation of a radiologist, a neurosurgeon, and an oncologist. Most patients were discussed in a multidisciplinary team, however, patients with leptomeningeal carcinomatosis or 5 or more solid tumours were referred directly for radiotherapy. The date of death and prognostic factors were retrieved from patient records and radiologic reports. The vast majority of patients had an MRI of the brain. In cases where a previous CT-scan had shown multiple metastases (>5) the MRI scan was omitted. In some cases, performance status was not stated explicitly in patient records but could be estimated from descriptions; these assessments were made by the study group. Study data were collected and managed using REDCap (Research Electronic Data Capture).
Statistical analysis
The survival length was defined as the time from BM diagnosis until death and presented as Kaplan Meier curves. Longer-term survival was defined as the percentage of patients surviving for at least 2 years after the diagnosis of BM.
The distribution of the different potential prognostic factors is presented as frequencies.
For each prognostic factor, patients were divided into predefined categories, and Kaplan-Meier curves were used to present survival length. Median survival ratios and Log-rank models were used to compare survival length between different patient groups. A multivariate Cox regression was used to adjust for potentially interacting covariates.
Chi-square tests were used to analyze differences between the different groups.
A level of significance at 0.05 was used throughout the analyses.
Results
In the study period, 144 BC patients were treated for BM. The median waiting time from BM diagnosis to the start of brain RT was 14 days (range 1 to 98 days). Patient characteristics showed that 93 patients had ER+/HER2−, 30 had HER2+ regardless of ER status, and 21 had triple-negative (TNBC) disease, respectively (Supplementary Table 1).
shows patient characteristics at BM diagnosis regarding extracranial metastatic status, performance status, total number of completed and ongoing treatment lines, and BM characteristics. Due to a low number of patients, performance status 3 and 4 were categorized together.
Table 1. Patient characteristics at BM diagnosis.
Median age at BM diagnosis was 66 (range 31 to 90) years. Most patients (90%) had extracranial metastases at BM debut. Bones, lymph nodes, and liver were the most frequent metastatic sites. Concerning BM, 58 patients (40%) had more than 3 solid metastatic lesions, while 22 (15%) and 38 (26%) had 2–3 lesions or 1 lesion, respectively. In total, 26 patients (18%) had leptomeningeal carcinomatosis without the presence of solid brain tumors.
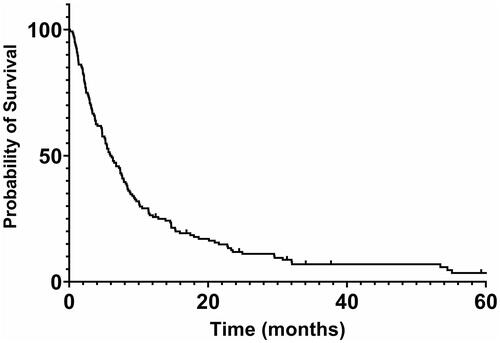
A Kaplan–Meier survival curve for the total patient population is shown in . The median overall survival (OS) after diagnosis of BM for all patients was 6.1 months, with 12% of the patients surviving longer than two years.
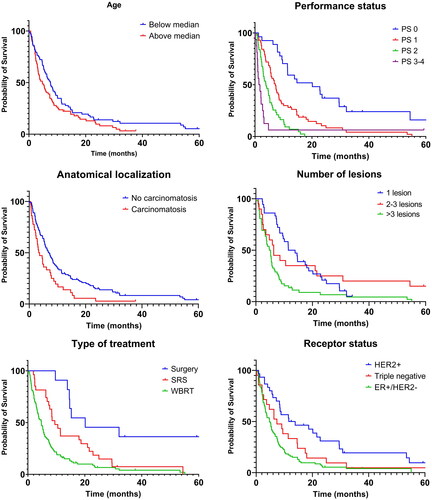
Kaplan–Meier curves with regard to age, performance status, anatomical localization, number of lesions, treatment type and receptor status are shown in . Median OS in months in the entire cohort and subgroups are shown in .
Table 2. Median survival and longer-term survival (all patients) from diagnosis of brain metastasis.
There was a non-significant trend toward longer survival in younger (<66 years) versus older patients: median OS was 7.6 months versus 4.8 months respectively. Long-term survival was 14% and 8%, respectively.
Median OS for patients with performance status 0, 1, 2 and 3-4 was 20.1, 7.2, 3.8 and 1.6 months respectively, p < 0.0001.
Patients with leptomeningeal carcinomatosis had a significantly shorter OS compared with patients with only solid metastatic lesions (3.5 versus 7 months, p = 0.007). Notably, only 3% of patients with leptomeningeal carcinomatosis were alive after 2 years, compared with 15% in the solid tumor group.
For patients with solid brain tumors the median OS was 12.7 months in the presence of only one lesion, which was not significantly longer compared with the 6.3 months in cases with 2–3 tumors present - but significantly longer compared with the 5.0 months in patients with >3 tumors (p = 0.0001).
Survival was shorter for patients receiving SRS (median OS 9.5 months) compared to those having surgery (median OS 20.1 months, p = 0.008), and the WBRT-group had an even shorter survival than the SRS group (median OS 4.7 months, p = 0.009). Furthermore, 2-year survival for those treated with surgery, SRS and WBRT was 45%, 14% and 7%, respectively.
At the time of BM diagnosis, 106 (74%) patients were receiving systemic treatment. Of those, 66 (62%) discontinued their treatment. The median OS for those who continued and discontinued their treatment was 5.9 and 4.7 months, respectively.
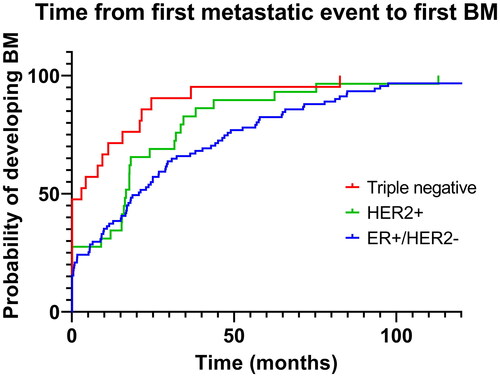
Concerning receptor status, median OS was shortest among patients with ER+/HER2− tumors (4.8 months) compared to both TNBC (7.4 months, non-significant), and especially HER2+ tumors (12.7 months, p = 0.0001). illustrates the distribution of potential prognostic factors based on receptor status. Regarding performance status, it should be noted that only 14% of ER + patients had performance status 0, contrary to 24% in the TNBC and 30% in the HER2+ groups (p < 0.0001). In total, 34 out of the 36 patients with leptomeningeal carcinomatosis had ER+/HER2− BC, whilst two had TNBC (p < 0.0001). The distribution of number of lesions was not related to receptor status. Patients with ER + disease had a significantly longer time interval from the first metastatic event to BM development compared with TNBC patients (median 20.7 vs 3 months, p = 0.001), while no difference was detected when comparing patients with ER + and HER2+ disease (median 20.7 vs 16.7 months, non-significant), , .
Table 3. Distribution of prognostic factors by receptor status.
Table 4. Time elapsed between first metastatic event and BM diagnosis.
The results of a multivariate Cox regression comparing performance status, anatomical localization, type of treatment and receptor status can be seen in Supplementary Table 3. In this analysis, performance status and receptor status remain significant prognostic factors, while type of radiotherapy and presence of leptomeningeal carcinomatosis do not seem to impact survival.
Discussion
Several of the investigated prognostic factors, mainly performance status, presence of leptomeningeal carcinomatosis, and number of BM’s, had an impact on survival time. Differences in survival were also related to receptor status, and the findings here were different than what has been found in other studies.
In our study, the median age at BM diagnosis was 66 years, and 90% of patients already had metastatic disease. This number has previously been reported as around 80% [Citation21–23]. The median time elapsed from the first metastatic event to BM development in our study was 17 months, thus more than half of the patients developed CNS disease within 1.5 years from diagnosis of metastatic disease. Other studies report a median interval of between 1–3 years of metastatic disease before developing BM, similar to our findings [Citation5,Citation21,Citation22,Citation24]. WBRT was the most common treatment in our cohort (74%), followed by SRS (19%) and surgery + radiation (7%), reflecting that a large percentage had either >3 lesions (40%) or leptomeningeal carcinomatosis (25%). Other studies have found the presence of carcinomatosis at BM debut between 10-20% [Citation24–27], thus our finding of carcinomatosis in 25% of patients was relatively high, maybe reflecting an increased frequency of patients with carcinomatosis due to improved survival time of patients with metastatic disease [Citation10,Citation25,Citation28]. However, differences in the diagnostic workup could also play a role. In our study, most patients had an MRI scan of the brain, which increases the likelihood of detecting eventual leptomeningeal carcinomatosis. The presence of leptomeningeal carcinomatosis was expected to lead to poor survival, and our findings showed a median OS of only 3.5 months, similar to previous studies (median OS 1.8–4.5 months) [Citation25,Citation29].
Tumor composition by receptor status showed a different pattern than reported in the majority of previous studies. A report by Witzel et al. comprising 1712 BC patients with BM showed that after the introduction of HER2-targeted therapies, the percentage of HER2+ BM patients decreased from 51% (2000-2009), to 44% (2010-2015), while the proportion of luminal A or B (HER2 negative and positive with positive ER, respectively) subtypes significantly rose from 28 to 34% [Citation30]. In another large study (4118 patients) from 20199, 45% were ER+/HER2−, 15% ER+/HER2+, 15% ER-/HER2+, and 25% TNBC. In our study, while using slightly different group definitions, 65% were ER + with negative or unknown HER2 status, 21% were HER2+, and 15% had TNBC. This is in line with the previously reported tendency that due to better systemic HER2-targeted treatment, HER2+ BC patients more rarely develop metastatic disease, more rarely develop BM after developing metastatic disease, and live longer after BM development if treated with HER2-targeted therapy [Citation5,Citation31]. Additionally, our results support the findings in recent years that patients with ER+ tumors constitute a larger part of BC patients developing BM.
Overall, our finding of a median OS of 6.1 is in line with previous reports showing a median OS after BM diagnosis from 4–12 months [Citation14,Citation19,Citation24–27,Citation32–36].
Other studies have identified age as a prognostic factor for survival after BM [Citation14,Citation25,Citation26,Citation37], but we were not able to confirm those findings in our study.
Performance status was an important prognostic factor for survival as also demonstrated earlier [Citation14,Citation37,Citation38]. With worse performance status the median OS declined, and for patients with performance status, 3–4 the median survival of only 1.6 months was barely longer than the duration they spent receiving radiotherapy. Thus, our findings suggest that it should be carefully considered if patients in poor performance status are candidates for radiotherapy, or if they would be better served with medical palliation.
As demonstrated in previous studies [Citation24,Citation26,Citation29,Citation39], the presence of leptomeningeal carcinomatosis significantly impacted the median OS of patients, leading to a halving of remaining lifetime expectancy compared to patients with only solid tumors.
The median OS for the groups with 1 vs 2–3 vs >3 lesions was around 12 vs 6 vs 5 months, respectively, similar to previous reports. A study reported the respective median OS in months for these groups as 11.2, 7.7 and 5.7 months [Citation30], while another found a median OS of 13.2 and 6.2 months for patients with 1-3 and >3 solid lesions, respectively [Citation14].
As a type of treatment is partially based on survival time expectancy [Citation12], it was no surprise that survival was better for patients receiving surgery than SRS, and that WBRT was correlated with worse survival than SRS. WBRT is the treatment of choice when lesions are excessive in number or when leptomeningeal carcinomatosis is present, which may explain the difference in survival based on radiation type. These findings strongly support the current algorithms for treatment selection. However, as WBRT resulted in a median OS of only 4.7 months and is expected to cause more severe cognitive impairment than SRS [Citation7], remaining life expectancy should be a crucial factor when deciding between performing or abstaining from treatment since hospital stays can constitute a large part of a short remaining lifetime.
Some unexpected results were observed when comparing receptor status subgroups. Earlier reports found that luminal subtypes (hormone receptor-positive) had a median OS of 5.9–18.9 months; HER2+ (with negative ER) had a median OS of 11.6–17.9 months; and median OS for TNBC was 4.4-4.9 months from BM diagnosis [Citation5,Citation28,Citation30,Citation36,Citation40,Citation41]. Despite our slightly different subgroup definition it was unexpected that median OS was worst among patients with ER+ tumors - a result conflicting with most previous studies, albeit some newer studies are in harmony with our results [Citation9,Citation36,Citation42]. As patients with metastatic ER+ BC have a better prognosis than other groups before developing BM due to - among other factors - multiple systemic treatment options [Citation1,Citation28], they appear to be in a more advanced disease state when developing BM. Our findings suggest that they are of older age, have had metastatic disease for a longer time when developing BM, are (partly as a consequence of these factors) in a worse performance status, and are more likely to develop leptomeningeal carcinomatosis than the other two molecular subgroups. In concordance with previous studies, HER2+ patients had the longest survival [Citation36,Citation40,Citation43,Citation44]. Based on recent data including the HER2CLIMB trial, it is recommended to start with systemic therapy with capecitabine, tucatinib and trastuzumab in patients with HER2-positive disease, as it seems to improve overall survival and offer the possibility to delay local therapy [Citation45]. In addition, several ongoing studies are investigating how treatment with chemotherapy or HER2-targeted treatment in addition to radiotherapy affects patients with BM regarding central nervous system response and survival. Due to a growing awareness of the cognitive side effects following radiation [Citation5,Citation12], cognitive decline following radiotherapy has gained more focus in recent studies and ongoing clinical trials. Thus, several ongoing clinical trials are investigating the survival and cognitive impact of SRS versus WBRT in patients with oligometastatic BM. Recent studies suggest that there is an emphasis for treating patients with memantine to reduce radiation-induced effects on brain tissue [Citation46].
In the multivariate survival analysis, all of the aforementioned prognostic factors but presence of leptomeningeal carcinomatosis and the type of radiotherapy remain significantly impactful. However, due to few events and a small study population, the results of the multivariate analysis should be interpreted with great caution.
Our results underline the need for a more individualized approach to radiotherapy taking factors as receptor status, size and number of brain metastases, performance status, time since diagnoses and metastatic disease into account, as also stated in the recent ASCO guidelines [Citation47,Citation48].
Strengths of this study include that patients were treated relatively recently and within a 5.5-year period, where the institutional treatment guidelines did not change. No patients were excluded due to a lack of data. Finally, the sample included the entire BC-patient population treated for BM in our department.
Limitations of this study include the limited generalizability of our subgroup analyses due to the low number of patients. Subgroup definitions used in this study were not exactly similar to those used in previous analyses (i.e. subgroup definitions by receptor status), limiting comparability. The inclusion criteria aimed at patients treated with RT, thus BC patients with BM not considered eligible for RT are not accounted for in this analysis. No information on blood tests was available. As in all retrospective studies, a selection bias may be present.
Conclusion
The number of metastatic BC patients developing BM appears to increase, likely due to prolonged survival time for patients with metastatic BC caused by more effective systemic therapy of extracranial metastatic disease. It appears that HER2+ patients constitute a still smaller fraction of metastatic BC patients due to improved HER2-targeted treatment. The median survival time for all patients in this study was 6.1 months. Worse survival was observed for patients of poor performance status, presence of leptomeningeal carcinomatosis or more than 3 solid BM. Patients with ER + tumors had surprisingly poor survival and appeared to be in a more advanced disease state when developing BM, especially regarding performance status, presence of leptomeningeal carcinomatosis, and time between primary metastatic disease and BM development.
Supplemental Material
Download MS Word (18.7 KB)Disclosure statement
This study is exclusively a retrospective study addressing the survival length and prognostic factors of importance regarding the patient group, aiming to improve future treatment strategies. No patients were involved during the study. The authors report no conflict of interest regarding the materials or methods used in this paper.
Data availability statement
Raw data were generated at Aarhus University Hospital. Derived data supporting the findings of this study are available from the corresponding author MKS on request.
Additional information
Funding
References
- Kim JS, Kim IA. Evolving treatment strategies of brain metastases from breast cancer: current status and future direction. Ther Adv Med Oncol. 2020;12:1758835920936117. doi: 10.1177/1758835920936117.
- Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020;31(12):1623–1649. doi: 10.1016/j.annonc.2020.09.010.
- Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22(17):3608–3617. doi: 10.1200/JCO.2004.01.175.
- Wang R, Zhu Y, Liu X, et al. The clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer. 2019;19(1):1091. doi: 10.1186/s12885-019-6311-z.
- Meattini I, Andratschke N, Kirby AM, et al. Challenges in the treatment of breast cancer brain metastases: evidence, unresolved questions, and a practical algorithm. Clin Transl Oncol. 2020;22(10):1698–1709. Oct doi: 10.1007/s12094-020-02333-7.
- Tabouret E, Chinot O, Metellus P, et al. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res. 2012;32(11):4655–4662.
- Koniali L, Hadjisavvas A, Constantinidou A, et al. Risk factors for breast cancer brain metastases: a systematic review. Oncotarget. 2020;11(6):650–669. doi: 10.18632/oncotarget.27453.
- Cagney DN, Lamba N, Montoya S, et al. Breast cancer subtype and intracranial recurrence patterns after brain-directed radiation for brain metastases. Breast Cancer Res Treat. 2019;176(1):171–179. doi: 10.1007/s10549-019-05236-6.
- Darlix A, Louvel G, Fraisse J, et al. Impact of breast cancer molecular subtypes on the incidence, kinetics and prognosis of Central nervous system metastases in a large multicentre real-life cohort. Br J Cancer. 2019;121(12):991–1000. doi: 10.1038/s41416-019-0619-y.
- Witzel I, Oliveira-Ferrer L, Pantel K, et al. Breast cancer brain metastases: biology and new clinical perspectives. Breast Cancer Res. 2016;18(1):8. Jan 19 doi: 10.1186/s13058-015-0665-1.
- Chao ST, De Salles A, Hayashi M, et al. Stereotactic radiosurgery in the management of limited (1-4) brain metasteses: systematic review and international stereotactic radiosurgery society practice guideline. Neurosurgery. 2018;83(3):345–353. doi: 10.1093/neuros/nyx522.
- Corti C, Antonarelli G, Criscitiello C, et al. Targeting brain metastases in breast cancer. Cancer Treat Rev. 2022;103:102324. Feb doi: 10.1016/j.ctrv.2021.102324.
- Yang JT, Wijetunga NA, Pentsova E, et al. Randomized phase II trial of proton craniospinal irradiation Versus photon Involved-Field radiotherapy for patients with solid tumor leptomeningeal metastasis. J Clin Oncol. 2022;40(33):3858–3867.
- Lee SS, Ahn JH, Kim MK, et al. Brain metastases in breast cancer: prognostic factors and management. Breast Cancer Res Treat. 2008;111(3):523–530.
- Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American society for radiation oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210–225. doi: 10.1016/j.prro.2011.12.004.
- Arvold ND, Lee EQ, Mehta MP, et al. Updates in the management of brain metastases. Neuro Oncol. 2016;18(8):1043–1065. doi: 10.1093/neuonc/now127.
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494–500.
- Bhargava P, Rathnasamy N, Shenoy R, et al. Clinical profile and outcome of patients with human epidermal growth factor receptor 2-positive breast cancer with brain metastases: real-world experience. J Clin Oncol Glob Oncol. 2022;8:e2200126.
- Subbiah IM, Lei X, Weinberg JS, et al. Validation and development of a modified breast graded prognostic assessment as a tool for survival in patients with breast cancer and brain metastases. J Clin Oncol. 2015;33(20):2239–2245. doi: 10.1200/JCO.2014.58.8517.
- Ogrinc G, Armstrong GE, Dolansky MA, et al. SQUIRE-EDU (standards for QUality improvement reporting excellence in education): publication guidelines for educational improvement. Acad Med. 2019;94(10):1461–1470. doi: 10.1097/ACM.0000000000002750.
- Graesslin O, Abdulkarim BS, Coutant C, et al. Nomogram to predict subsequent brain metastasis in patients with metastatic breast cancer. J Clin Oncol. 2010;28(12):2032–2037. doi: 10.1200/JCO.2009.24.6314.
- Fox BD, Cheung VJ, Patel AJ, et al. Epidemiology of metastatic brain tumors. Neurosurg Clin N Am. 2011;22(1):1–6, v. doi: 10.1016/j.nec.2010.08.007.
- Martin AM, Cagney DN, Catalano PJ, et al. Brain metastases in newly diagnosed breast cancer: a Population-Based study. JAMA Oncol. 2017;3(8):1069–1077. doi: 10.1001/jamaoncol.2017.0001.
- Altundag K, Bondy ML, Mirza NQ, et al. Clinicopathologic characteristics and prognostic factors in 420 metastatic breast cancer patients with Central nervous system metastasis. Cancer. 2007;110(12):2640–2647. doi: 10.1002/cncr.23088.
- Quigley MR, Fukui O, Chew B, et al. The shifting landscape of metastatic breast cancer to the CNS. Neurosurg Rev. 2013;36(3):377–382. doi: 10.1007/s10143-012-0446-6.
- Harputluoglu H, Dizdar O, Aksoy S, et al. Characteristics of breast cancer patients with Central nervous system metastases: a single-center experience. J Natl Med Assoc. 2008;100(5):521–526.
- Kim HJ, Im SA, Keam B, et al. Clinical outcome of Central nervous system metastases from breast cancer: differences in survival depending on systemic treatment. J Neurooncol. 2012;106(2):303–313. doi: 10.1007/s11060-011-0664-8.
- Jeon W, Jang BS, Jeon SH, et al. Analysis of survival outcomes based on molecular subtypes in breast cancer brain metastases: a single institutional cohort. Breast J. 2018;24(6):920–926. doi: 10.1111/tbj.13111.
- Gauthier H, Guilhaume MN, Bidard FC, et al. Survival of breast cancer patients with meningeal carcinomatosis. Ann Oncol. 2010;21(11):2183–2187. doi: 10.1093/annonc/mdq232.
- Witzel I, Laakmann E, Weide R, et al. Treatment and outcomes of patients in the brain metastases in breast cancer network registry. Eur J Cancer. 2018;102:1–9. doi: 10.1016/j.ejca.2018.07.004.
- Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies–improving the management of early breast cancer: st gallen international expert consensus on the primary therapy of early breast cancer 2015. Ann Oncol. 2015;26(8):1533–1546. doi: 10.1093/annonc/mdv221.
- Pasquier D, Darlix A, Louvel G, et al. Treatment and outcomes in patients with central nervous system metastases from breast cancer in the real-life ESME MBC cohort. Eur J Cancer. 2020;125:22–30. doi: 10.1016/j.ejca.2019.11.001.
- Griguolo G, Jacot W, Kantelhardt E, et al. External validation of modified breast graded prognostic assessment for breast cancer patients with brain metastases: a multicentric european experience. Breast. 2018;37:36–41. doi: 10.1016/j.breast.2017.10.006.
- Fabregat-Franco C, Stradella A, Navarro V, et al. Validation and comparison of breast graded prognostic assessment scores in patients with breast cancer and brain metastases. Clin Transl Oncol. 2021;23(9):1761–1768. doi: 10.1007/s12094-021-02577-x.
- Kim JS, Kim K, Jung W, et al. Survival outcomes of breast cancer patients with brain metastases: a multicenter retrospective study in Korea (KROG 16-12). Breast. 2020;49:41–47. doi: 10.1016/j.breast.2019.10.007.
- Gao YK, Kuksis M, Id Said B, et al. Treatment patterns and outcomes of women with symptomatic and asymptomatic breast cancer brain metastases: a single-center retrospective study. Oncologist. 2021;26(11):e1951–e1961. doi: 10.1002/onco.13965.
- Mahmoud-Ahmed AS, Suh JH, Lee SY, et al. Results of whole brain radiotherapy in patients with brain metastases from breast cancer: a retrospective study. Int J Radiat Oncol Biol Phys. 2002;54(3):810–817.
- Gullhaug A, Hjermstad MJ, Yri O, et al. Use of radiotherapy in breast cancer patients with brain metastases: a retrospective 11-year single center study. J Med Imaging Radiat Sci. 2021;52(2):214–222. doi: 10.1016/j.jmir.2021.01.002.
- Watanabe J, Mitsuya K, Nakamoto S, et al. Leptomeningeal metastasis in ER + HER2- Advanced breast cancer patients: a review of the cases in a single institute Over a 15-year period. Breast Cancer Res Treat. 2021;189(1):225–236. doi: 10.1007/s10549-021-06246-z.
- Sperduto PW, Kased N, Roberge D, et al. The effect of tumor subtype on the time from primary diagnosis to development of brain metastases and survival in patients with breast cancer. J Neurooncol. 2013;112(3):467–472. doi: 10.1007/s11060-013-1083-9.
- Haque W, Verma V, Adeberg S, et al. Outcomes following stereotactic radiosurgery or whole brain radiation therapy by molecular subtype of metastatic breast cancer. Rep Pract Oncol Radiother. 2021;26(3):341–351. doi: 10.5603/RPOR.a2021.0045.
- Chew S, Carroll HK, Darwish W, et al. Characterization of treatments and disease course for women with breast cancer brain metastases: 5-Year retrospective single institution experience. Cancer Manag Res. 2021;13:8191–8198. doi: 10.2147/CMAR.S330829.
- Eichler AF, Kuter I, Ryan P, et al. Survival in patients with brain metastases from breast cancer: the importance of HER-2 status. Cancer. 2008;112(11):2359–2367. doi: 10.1002/cncr.23468.
- Stavrou E, Winer EP, Lin NU. How we treat HER2-positive brain metastases. ESMO Open. 2021;6(5):100256. doi: 10.1016/j.esmoop.2021.100256.
- Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-Positive metastatic breast cancer. N Engl J Med. 2020;382(7):597–609. doi: 10.1056/NEJMoa1914609.
- Chilukuri S, Burela N. Memantine for prevention of brain irradiation-induced cognitive toxicity: a tale of an underappreciated and underused intervention. J Clin Oncol Glob Oncol. 2020;6:1384–1388.
- Vogelbaum MA, Brown PD, Messersmith H, et al. Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol. 2022;40(5):492–516. doi: 10.1200/JCO.21.02314.
- Schiff D, Messersmith H, Brastianos PK, et al. Radiation therapy for brain metastases: ASCO guideline endorsement of ASTRO guideline. J Clin Oncol. 2022;40(20):2271–2276. doi: 10.1200/JCO.22.00333.