Abstract
Objective
To compare the incidence of grade ≥2 gastrointestinal (GI) or genitourinary (GU) toxicity for patients undergoing 3DRT versus IMRT in the postoperative setting for endometrial cancer.
Methods
Eligible patients were post-operatively randomly assigned to one of two parallel groups in a 1:1 ratio, to have their RT delivered using either a 3DRT technique or using IMRT. The prescription dose was 45 Gy in 25 fractions over 5 weeks followed by vaginal vault brachytherapy. Toxicity was graded according to National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version (v) 3.0. Fisher’s exact tests were used to test for associations between toxicity and arm. Differences in dosimetric parameters for patients with or without toxicity were tested using Mann–Whitney U-tests.
Results
84 patients with a median age of 62 were evaluable for primary outcome. The median follow-up was 52 months. 14 (35%) participants from the 3DRT arm and 15 (34%) from the IMRT arm experienced acute grade ≥2 GI toxicity with older patients having a statistically higher risk of grade ≥2 acute GI toxicity. 20 (50%) participants from the 3DRT arm and 25 (57%) from the IMRT arm experienced acute grade ≥2 GI or GU toxicity (p = .662). 12 (30%) patients from the 3DRT arm and 17 (39%) from the IMRT arm experienced acute grade ≥2 GU toxicity (p = .493).
Conclusion
Although IMRT can reduce dose to normal tissue, in this study no benefit in acute GI or GU toxicity outcome was seen.
Introduction
Endometrial cancer is the most common female genital tract cancer in the western world [Citation1]. World-wide data from the Global Cancer Observatory reports that there are 417,000 new incidence of cancer per year [Citation2]. In Ireland, cancer registration data reports an annual incident rate of 26.2 per 100,000, accounting for 4.8% of all invasive cancers diagnosed per year [Citation3]. 5 year overall survival rates can be as high as over 70% [Citation4] with more than 600,000 and 6455 survivors of endometrial cancer living in the United States [Citation5] and Ireland [Citation3] respectively, making it a significant contributor to the national and global burden of cancer treatment and survivorship aftercare.
The standard of care for endometrial cancer is surgery followed by a risk stratification approach to rationalise the benefit of adjuvant radiation or chemotherapy [Citation6]. Whole pelvic radiation therapy (WPRT) is recommended for ESGO-ESTRO-ESMO classified high-intermediate risk (lymph node staging not performed) and high-risk endometrial cancer prognostic risk groups [Citation6]. This approach is supported by the landmark PORTEC 1 trial which demonstrated a significant reduction in the rates of locoregional recurrence in patients treated with WPRT versus surgery alone (15-year actuarial locoregional recurrence rate being 6% in the treated WPRT arm compared to 15.5% in the surgery alone arm. (p < .0001) [Citation7]). Several other studies, including a meta-analysis by Kong et al., have also shown reduced local recurrence rates with acceptable toxicity. In this meta-analysis, WPRT resulted in a relative reduction of 0.28 (95% confidence interval (CI) (95% CI = 0.17–0.44) [Citation8].
The addition of adjuvant chemotherapy and its impact on OS was investigated by PORTEC 3, in this trial high-risk patients had a 5% OS benefit and 7% failure-free survival benefit with the addition of two cycles of cisplatin during radiotherapy and four cycles of carboplatin-paclitaxel against radiotherapy alone [Citation9]. However, at present, no trial has shown an improved overall survival benefit (OS) with adjuvant WPRT. Furthermore, PORTEC 1 demonstrated that the majority of local recurrence were vaginal [Citation7]. Against this background, PORTEC 2 and Swedish trial [Citation10,Citation11] demonstrated excellent vaginal control for high intermediate risk, particularly in patients with focal only LVSI positive/LVSI negative endometrial cancer, with vaginal vault brachytherapy (VVBT) alone. Rates of acute grade 1–2 gastrointestinal toxicity were significantly lower with VVBT than WPRT at the completion of radiotherapy (12.6% vs 53.8%) [Citation10]. This implication, that sparing normal tissue reduces acute toxicity, warrants further exploration in patients where adjuvant WPRT is also clinically indicated.
One such strategy to spare normal tissue in patients undergoing WPRT has been to utilise a more advanced form of radiotherapy called Intensity Modulated Radiation Therapy (IMRT). By splitting the monitor units delivered into smaller but more numerous beamlets from different directions, IMRT can conform dose around the 3D shape of the target. In planning studies comparing pelvic IMRT to 3D, the increased dose conformality achieved with pelvic IMRT has been shown to significantly reduce the dose to normal tissue while increasing tumour dose homogeneity [Citation12,Citation13].
At the time of the trial design, 3D conformal radiation was the established standard of care. However, over the past 20 years, the use of IMRT in treating gynaecological cancers has increased popularity [Citation14,Citation15]. This trend has been supported by the reporting of acceptable levels of acute and late toxicity outcomes for the treatment of endometrial and cervical cancer [Citation16,Citation17] without compromise in disease control [Citation16,Citation18–24]. However, the build-up of evidence favouring IMRT was initially mostly from retrospective studies. For example, Ta et al.’s retrospective comparison study of 3DRT with IMRT in the adjuvant endometrial setting showed a reduced trend of acute GI toxicity with the more conformal radiation technique [Citation25], while two retrospective papers by Mundt et al. reported less acute and chronic GI toxicity in gynaecological cases treated with IMRT versus 3D RT.
Further evidence from two prospective trials that examined the acute toxicity effects of IMRT treatment in the gynaecology setting since the inception of CTRIAL-IE 09-06 are worth noting. Firstly, the prospective single-arm RTCMIENDOMETRE trial proved their hypothesis that post-operative IMRT resulted in a low rate (less than 30%) of acute GI grade 2 toxicity in patients with endometrial carcinomas. In addition, the randomised comparative data from the RTOG 1203 phase III trial that compared 4 field radiation to IMRT, showed a significant reduction in patient-reported GI and GU toxicity, in addition to improvement in reported quality of life [Citation26,Citation27].
Purpose
The aim of this randomised trial was to compare the incidence of grade 2 or higher acute GI toxicity in the Control 3-D arm compared to the IMRT arm. Secondary Objectives included acute/late radiation-induced toxicity, loco-regional control, and overall and disease-free survival.
Methods
Study design
This was a parallel-group study conducted across three sites in Ireland. Patients were randomly assigned to one of two parallel groups in a 1:1 ratio, to have their radiotherapy delivered using a 3D-RT or delivered using IMRT. CTRIAL-IE 09-06 was an ICH-GCP-compliant prospective phase II trial. The study was carried out in compliance with the Cancer Trials Ireland protocol, Good Clinical Practice Guidelines, and applicable ethical standards.
Participants
Eligible participants were all adults aged 18 or over-scheduled to receive adjuvant pelvic radiotherapy for histologically confirmed endometrial endometrioid adenocarcinoma/serous carcinoma/papillary serous carcinoma/clear cell/carcinosarcoma/mixed histology with the following AJCC 2009 grade/stage: Grade 2: stage IB (LVSI +/or >60 yrs), Grade 3: stage IA and IB, Grade 1-3: Stage II and IIIA, IIIB and IIIC1 who were staged with MRI or CT, had an ECOG performance status of 0–2, had surgery consisting of total hysterectomy, ± bilateral salpingo-oophorectomy, ± lymph node sampling and provided written informed consent. Exclusion criteria were previous irradiation to the pelvic region, patients in whom concurrent chemotherapy is planned, macroscopic disease in situ, a history of inflammatory bowel disease, previous hip replacement, and previous bowel surgery (excluding appendectomy).
Radiotherapy treatment regimens
Patients were treated with 45 Gy/25# over 5 weeks external beam WPRT followed by 11 Gy in two fractions vaginal vault brachytherapy. WPRT was delivered using a 3-Dimensional (3-D) planning technique in the control arm and using IMRT in the experimental arm. The target volume followed the NRG Oncology/RTOG 2008 consensus guidelines for the delineation of the clinical target volume for intensity-modulated pelvic radiotherapy in the post-operative treatment of endometrial cancer [Citation28].
Patients’ follow-up and assessment
Acute toxicities were assessed weekly during radiotherapy, and at 2- and 4-weeks post-treatment. Late toxicities were assessed at 3, 6, 9-, 12-, 18-, and 24-months post-treatment, and annually to 10 years and graded by the NCI-CTCAE v 3.
Statistical analysis and trial registration
A Fisher’s exact test or a continuity-corrected chi-squared test with a 0.05 two-sided level of significance was deemed to have 80% power (a risk of 20% of failing to detect a change of this magnitude) to detect a difference in ≥ Grade 2 acute GI toxicities between a hypothesised 34% [Citation29] in the control arm (3D) and 13% in the IMRT arm when the sample size in each group is 71.
Differences in dosimetric parameters for patients with or without toxicity were tested using Mann–Whitney U-tests. A multivariate logistic regression analysis was performed to assess the impact of all clinical/dosimetric covariates that appeared to be associated with Grade 2 or higher GI or GU toxicity in univariate analysis (covariates with p ≤ .11). Categorical variables were analysed using chi-square and Fisher’s exact.
The Kaplan–Meier method was used to estimate the overall survival, local recurrence-free survival, and disease-free survival rates. Comparisons between the two arms were made using the Log-rank test.
All statistical tests were two-sided and assessed for significance at the 0.05 level. Statistical analyses were carried out using IBM® SPSS® statistical software version 25.
Results
Patient accrual
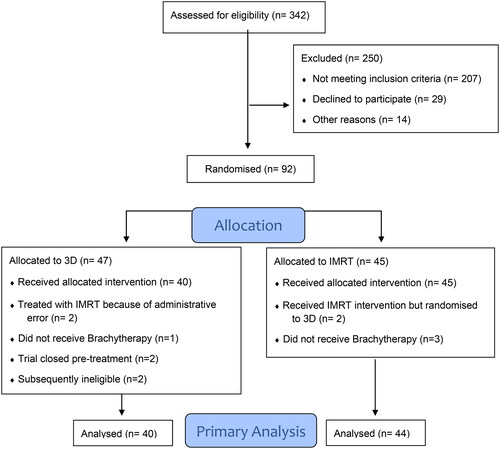
From March 2010 to February 2020, the three St Luke’s Radiation Oncology Network (SLRON) centres accrued 92 patients. is a participant flow diagram that details the reasons 8 patients were excluded in the primary analysis. The median follow-up was 52 months. An interim analysis, undertaken after 40 evaluable patients in the IMRT arm had completed their treatment, demonstrated higher acute ≥ grade 2 GI and GU toxicities in the experimental arm (35% vs. 31% and 30% vs. 18% respectively). The difference in toxicity was not statistically significant but in the opposite direction to that hypothesised. As a result, the study terminated accrual prematurely on 13-Mar-2020. All patients were withdrawn from the trial and were followed up as per the standard of care.
Figure 1. Consort flow diagram 09-06 trial. Flow diagram of a trial of 3D vs IMRT in Endometrial Cancer. The diagram includes detailed information on the excluded participants. RT: radiotherapy
Baseline characteristics
Sample characteristics of the 84 patients are outlined in . The median age was 62 years (range 32–76 years). There was no significant difference in age between treatment groups.
Table 1. Baseline demographic and clinical characteristics of patients who underwent post operative pelvic radiotherapy for endometrial cancer.
Radiotherapy quality assurance
All radiotherapy plans were peer-reviewed at our network’s radiotherapy planning meeting prior to delivery. Both 3-D and IMRT plans were optimised with the ALARA (as low as reasonably achievable) principle applied to normal tissue sparing while ensuring adequate tumour coverage.
Acute toxicity (any)
Twenty (24%) patients had grade 3 acute toxicity, ten in each arm (). 36 (43%) had grade 2 toxicity (GI, GU, infection, fatigue, vaginitis, vaginal discharge/bleeding, radiation dermatitis, other dermatitis, vulval pruritis, pain, low haemoglobin, dehydration, hot flushes, skin breakdown decubitus ulcer, lymphocyte decrease) and 28 had grade 1 toxicity (33%).
Table 2. Acute toxicity: 3D versus IMRT post operative radiotherapy in endometrial patients.
Acute toxicity (GI/GU)
A breakdown of the cumulative incidence of both GI and GU toxicity by subcategory is displayed in and in the supplementary material. 14 (35%) participants from the 3DRT arm and 15 (34%) from the IMRT arm experienced acute grade ≥2 GI toxicity. No episodes of Grade 4 GI toxicity were seen. 9 patients (11%) had grade 3 acute diarrhoea. Diarrhoea was the only grade 3 GI toxicity and was seen in 5 (13%) patients and 4 (9%) patients for 3DRT and IMRT, respectively. There were no statistically significant differences between arms for acute grade ≥2 GI toxicity.
12 (30%) patients from the 3DRT arm and 17 (39%) from the IMRT arm experienced acute grade ≥2 GU toxicity (p = .493). Rates for grade 3 GU toxicity by treatment arm were 4 (10%) and 4 (9%) for 3DRT and IMRT, respectively. There were no statistically significant differences between arms for acute GU toxicity.
Toxicity based on treatment, patient, and tumour characteristics
A post-hoc analysis was undertaken to evaluate any relationship between dosimetric variables and GI and GU toxicity. A Mann-Whitney U test revealed no statistically significant differences in the Rectum V45%, Rectum D2cc (Gy), Bowel V45% (Gy), Bowel D2cc (Gy), Rectum D50% (Gy) and for Bowel D50% (Gy) and having grade ≥2 or grade ≥3 GI toxicity.
There were also no statistically significant differences in the Bladder V45% (Gy), Bladder D50% (Gy), Bladder D2cc (Gy), or the PTV Homogeneity Index (HI) or PTV Conformity Index (CI) and having Grade ≥2 or Grade ≥3 GU toxicity.
There was a statistically significant difference in patient age and having a grade ≥2 GI toxicity. The median age for those with grade ≥2 GI toxicity was 67 years compared with 60 years for those without such toxicity (p = .024). The strongest predictor of grade ≥2 GU toxicity was having stage 3B disease (n = 6) recording an odds ratio of 15.5 (95% Confidence Interval: 1.1–227.1).
Late toxicity
Late toxicity (and survival) analysis included patients who had EBRT but didn’t have brachytherapy and was available for 87 patients (median follow-up was 55 months).
Late toxicities are displayed in detail in . One patient (IMRT arm) reported late grade 4 constipation. The cumulative incidence of any late grade 3 toxicity was twenty-one (24%) patients (9 in the 3D arm and 12 in the IMRT arm respectively). With regards to grade ≥2 late GI toxicity, 10 (24.4%) patients in the 3D arm and 14 (30.4%) in the IMRT arm reported this. Similarly, with late GU toxicity, 13 (31.7%) patients in the 3D arm and 18 (39.1%) in the IMRT experience grade ≥2 GU toxicity.
Table 3. Late toxicity: 3D versus IMRT post operative radiotherapy in endometrial patients.
Overall survival from consent (OS)
At a median follow-up of 47.2 months, the 5-year OS was 75.5% (95% CI: 65%–86%; n = 88). In the 3D arm the 5-year OS was 84.8% (95% CI: 72%–97%) and 65.9% in the IMRT arm (95% CI: 49%–83%) (Figure S3.1 in supplement).
Disease free survival (DFS)
The 5-year DFS was 54.9% (95% CI: 43%–66%; n = 84). 5-year DFS was 65.7% in the 3D arm (95% CI: 50%–81%) and 44.5% in the IMRT arm (95% CI: 28%–61%) (Figure S3.2 in supplement).
Local recurrence free survival (LRFS)
The 5-years LRFS was 62.1% (95% CI: 50%–74%; n = 84). LRFS was 70.7% in the 3D arm (95% CI: 55%–86%) and 54.0% in the IMRT arm (95% CI: 37%–71%) (Figure S3.3 in supplement).
Discussion
Background
Over the past two decade there has been a clear trend towards the increased utilisation of IMRT in the treatment of gynaecology cancers [Citation14,Citation15]. When this trial was designed, the evidence for adopting this technique was supported by both the demonstrated dosimetric advantages of IMRT-generated treatment plans [Citation14,Citation17] and the growing body retrospective data that was revealing a favourable reduction in toxicity rates without a compromise in tumour control [Citation16,Citation18,Citation21]. Subsequently, prospective and comparative randomised clinical trial with a statistically significant reduction in patient-reported acute and chronic GI toxicity have been published [Citation26,Citation27], further vindicating its use in the post-operative setting for gynaecological malignancies.
Primary outcome: grade ≥2 GI toxicity rates
The trial closed early after 92 of the planned 142 participants had been recruited. In disagreement with postulated outcomes, our acute grade ≥2 GI toxicity rates with both techniques of treatment were slightly higher than expected. Grade ≥2 acute GI toxicity rates were 14/40 (35%) with 3-DCRT and 15/44 (34%) with IMRT. 9 patients in total had a grade 3 acute GI event. In comparison, retrospective reporting on acute GI toxicity rates with IMRT in Chen et al. [Citation22] were rates of acute grade ≥2 GI of 34% with IMRT, similarly Mundt et al. reported grade 2 toxicity rates of 60% with IMRT [Citation16] and Ta et al. reported 30.5% with IMRT [Citation25].
The failure of this trial to prove its primary outcome could be explained by our over-estimation of the magnitude of the benefit of IMRT over 3D, particularly if patients also undergo brachytherapy. We originally postulated that IMRT may reduce Grade ≥2 GI toxicity to 13% compared to 34% in the 3D arm. The Key et al. [Citation29] acute GI toxicity rate of 34% was in the context of 3D post-operative radiotherapy, with no patients undergoing brachytherapy. Indeed, the RTCMIENDOMETRE single-arm prospective trial [Citation17], where 75% of patients also underwent brachytherapy, was designed to test the hypothesis that adjuvant post-op WPRT in endometrial cancer had an incidence of acute grade 2 GI toxicity of less than 30%. Perhaps, our study’s addition of brachytherapy in both arms may have masked any statistically significant benefit of IMRT in the acute setting, and we likely overestimated its postulated benefit in the context of a combined WPRT and brachytherapy treatment protocol.
Our study is not alone in its findings. Ta et al.’s [Citation25] retrospective review also did not show a statistical significant difference in acute toxicity rates between IMRT and 3D treatment arms. Their study reported the incidence of at least one grade 2 toxicity as 30.5% in the IMRT arm, 34% in the 3D (p = .7), with the authors concluding that this represented a reduced trend in GI toxicity favouring IMRT. Of note, only 29% of patients underwent brachytherapy which likely influenced the rate of acute GI toxicity reported. Correspondingly, a study by Cho et al. [Citation30] that prospectively collected GI toxicity in 120 endometrial or cervical cancer patients post hysterectomy who had undergone adjuvant IMRT or 3D radiation, also did not show a statistical significant difference in GI toxicity between the two treatments. Although notably, the median dose in the IMRT arm was 50 Gy in comparison to the 45 Gy median dose in the 3D arm. In terms of randomised data, the highly anticipated NROG RTOG 1203 trial had a similar design to our study. Here, 278 patients with gynaecology malignancies were randomised between IMRT and 3D. Although their study’s primary outcome did show a statistically significant reduction in patient-reported acute and late toxicity with IMRT [Citation26, p.yeung], their physician-reported toxicities, did not demonstrate a statistical benefit- acute grade ≥2 GI rates 26.2% and 22.1% (p = .43) in the IMRT and 3D arms respectively and 16.4% in the IMRT arm with 11% in the 3D arm having an acute grade 3 event. This may suggest that the clinically significant acute difference in toxicity between IMRT and 3D may be unmasked by the use of patient-reported outcomes (PROMs) over physician-reported outcomes [Citation31,Citation32].
Secondary outcomes
The cumulative incidence of grade 2 and 3 late toxicity was 38% and 24% respectively, with no statistical difference between the two treatment arms (median follow-up was 55 months). Reassuringly, there was only one grade 4 late toxicity reported namely and incidence of constipation in the IMRT cohort. We did not report any bowel obstruction (BO) unlike data from Shih et al. [Citation23] who reported a 5-year actuarial rate of BO of close to 5%. Diarrhoea (16%) was the most common grade ≥2 late toxicity reported, and it will be important to discover if this impacts QoL, which we will be reporting on in a future separate paper.
Unlike late GI toxicity, there is less data on GU toxicity with whole pelvic IMRT. Consistent with our GI late toxicity, this present study did not show any statistical difference in either acute or late GU toxicity. 12 (30%) patients from the 3DRT arm and 17 (39%) from the IMRT arm experienced acute grade ≥2 GU toxicity with urinary frequency being the most common grade ≥2 toxicity in both the acute (35%) and late (28%) setting. In contrast to our study, the NRG RTOG 1203 study was able to demonstrate a statistically significant reduced effect on baseline urinary function with IMRT, as assessed using the validated EPIC patient-reported questionnaire, suggesting a meaningful clinical improvement in the acute period with the more conformal radiation technique. A subsequent publication from the RTOG 1203 patient cohort by Yeung et al. showed that there was still a reduction in GU toxicity with IMRT and at 1 and 3 years [Citation33].
When considering other secondary endpoints, the 5-year OS, DFS and LR-free survival was 76%, 52% and 62%, respectively which is consistent with historical data [Citation25,Citation34]. With our study closing early due to futility, we did not have a sample size large enough to power a comparative analysis between the two treatment arms.
Multivariate analyses
A post hoc analysis was completed on a broad set of dosimetric, treatment, patient, and tumour variables. We could not find a relationship between the bladder, rectal and bowel DVCs and acute toxicity. Patient’s age was linked with GI toxicity with older patients having a higher risk of grade ≥2 acute GI toxicity. This was also reported by Soisson et al. [Citation35]. Also consistent with Soisson, stage 3B disease (parametrial involvement) was another strong predictor for toxicity, suggesting that patients with more advanced disease have a higher burden of symptoms post-treatment.
Strengths
Our study has several strengths. Our trial design was both prospective and randomised. The two treatment arm groups were balanced in terms of their likelihood of experiencing acute GI toxicity. Both arms had a median follow-up of over 4 years allowing for the prospective capturing late effects, particularly late GI and GU toxicity. There are few randomised trials reporting a direct comparison between IMRT and 3D in the post-operative gynaecological setting- most data has been retrospective, non-randomised and not reported from a registered perspective clinical trial with set time points for formal toxicity data collection.
Weaknesses
Our trial was under-powered by its early closure. An interim analysis concluded that continuing trial recruitment was considered futile due to increased toxicity trend in the IMRT arm. Toxicity outcomes were reported by clinicians only. As with the NRG RTOG 1203 trial, results may have been different with the addition of patient-reported outcomes. Although all delivered radiation treatment plans were peer-reviewed centrally within our hospital network, with contouring and treatment planning based on recommended international guidelines [Citation28], our study protocol did not have an additional radiation treatment quality assurance (RTQA) process to ensure planning consistency. Our trial protocol would have benefitted from a standardised RTQA process or stricter DVCs goals for intermediate bowel and bladder doses.
Wider context and future
This trial, although negative can still contribute valuable data to inform clinical practice [Citation36] and help instruct future clinical trial design for pelvic IMRT studies. Our results are also impactful from a global oncology perspective. With the reassurance that both treatment modalities are effective and safe for endometrial patients, with 3D radiotherapy not proven to be substantially inferior is indeed very relevant to low-income countries where IMRT is not readily available [Citation37].
Conclusion
No statistically significant differences in the incidence of acute GI or GU toxicity were found between patients treated with adjuvant IMRT versus 3DRT radiotherapy for endometrial cancer. Although IMRT can reduce dose to normal tissue, in this study no benefit in acute physician reported GI toxicity outcome was seen. However, prospectively gathered PROMs to further assess the benefit of IMRT would be encouraged.
Author contributions
Guarantor of integrity of the entire study: C. Gilham
Study concepts and design: C Gilham, O McArdle, N. Lavan, O. Salib, M. Dunne
Literature research: K. Nugent D. Browne, M Dunne
Clinical studies: C. Gilham, O. McArdle, O. Salib, N. Lavan, D. Sharma, S. Bradshaw
Experimental studies/data analysis: M. Dunne, A. M Shannon, L. OSullivan
Statistical analyses: M. Dunne
Manuscript preparation: All authors contributed.
Supplemental Material
Download MS Word (117.9 KB)Acknowledgements
Cancer Trials Ireland (formerly All Ireland Cooperative Oncology Research Group) CTRIAL-IE (ICORG) 09-06 trial (NCT01164150)
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Additional information
Funding
References
- Zhang S, Gong T-T, Liu F-H, et al. Global, regional, and national burden of endometrial cancer, 1990–2017: results from the global burden of disease study, 2017. Front Oncol. 2019;9:1440. doi: 10.3389/fonc.2019.01440.
- Endometrial Cancer Statistics, World Cancer Research Fund International [Internet]. WCRF International. 2020 [cited 2022 Aug 1]. Available from: https://www.wcrf.org/cancer-trends/endometrial-cancer-statistics/
- NCRI_AnnualStatisticalReport_2022.pdf. [Internet]. 2022 [cited 2023 Feb 3]. Available from: https://www.ncri.ie/sites/ncri/files/pubs/NCRI_AnnualStatisticalReport_2022.pdf
- Factsheet corpus uteri.pdf [Internet]. 2020 [cited 2022 Aug 1]. Available from: https://www.ncri.ie/sites/ncri/files/factsheets/Factsheet%20corpus%20uteri.pdf.
- American Cancer Society, Cancer Facts & Statistics [Internet]. 2022 [cited 2022 Aug 1]. Available from: http://cancerstatisticscenter.cancer.org/
- Concin N, Matias-Guiu X, Vergote I, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer. 2021;31(1):12–39. doi: 10.1136/ijgc-2020-002230.
- Creutzberg CL, Nout RA, Lybeert MLM, et al. Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int J Radiat Oncol Biol Phys. 2011;81(4):e631–e638. doi: 10.1016/j.ijrobp.2011.04.013.
- Kong A, Simera I, Collingwood M, et al. Adjuvant radiotherapy for stage I endometrial cancer: systematic review and meta-analysis. Ann Oncol. 2007;18(10):1595–1604. doi: 10.1093/annonc/mdm066.
- de Boer SM, Powell ME, Mileshkin L, et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;20(9):1273–1285. doi: 10.1016/S1470-2045(19)30395-X.
- Wortman BG, Creutzberg CL, Putter H, et al. Ten-year results of the PORTEC-2 trial for high-intermediate risk endometrial carcinoma: improving patient selection for adjuvant therapy. Br J Cancer. 2018;119(9):1067–1074. doi: 10.1038/s41416-018-0310-8.
- Sorbe B, Horvath G, Andersson H, et al. External pelvic and vaginal irradiation versus vaginal irradiation alone as postoperative therapy in medium-risk endometrial carcinoma–a prospective randomized study. Int J Radiat Oncol Biol Phys. 2012;82(3):1249–1255. doi: 10.1016/j.ijrobp.2011.04.014.
- Yang R, Xu S, Jiang W, et al. Dosimetric comparison of postoperative whole pelvic radiotherapy for endometrial cancer using three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, and helical tomotherapy. Acta Oncol. 2010;49(2):230–236. doi: 10.3109/02841860903410372.
- Heron DE, Gerszten K, Selvaraj RN, et al. Conventional 3D conformal versus intensity-modulated radiotherapy for the adjuvant treatment of gynecologic malignancies: a comparative dosimetric study of dose-volume histograms. Gynecol Oncol. 2003;91(1):39–45. doi: 10.1016/s0090-8258(03)00461-x.
- Mell LK, Mehrotra AK, Mundt AJ. Intensity-modulated radiation therapy use in the U.S., 2004. Cancer. 2005;104(6):1296–1303. doi: 10.1002/cncr.21284.
- Lee SS, Weil CR, Boyd L, et al. Trends in IMRT utilization for definitive treatment of cervical cancer, 2004-2018. Int J Radiat Oncol Biol Phys. 2022;114(3):e342–e343. doi: 10.1016/j.ijrobp.2022.07.1438.
- Mundt AJ, Lujan AE, Rotmensch J, et al. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2002;52(5):1330–1337. doi: 10.1016/s0360-3016(01)02785-7.
- Barillot I, Tavernier E, Peignaux K, et al. Impact of post operative intensity modulated radiotherapy on acute gastro-intestinal toxicity for patients with endometrial cancer: results of the phase II RTCMIENDOMETRE french multicentre trial. Radiother Oncol. 2014;111(1):138–143. doi: 10.1016/j.radonc.2014.01.018.
- Beriwal S, Jain SK, Heron DE, et al. Clinical outcome with adjuvant treatment of endometrial carcinoma using intensity-modulated radiation therapy. Gynecol Oncol. 2006;102(2):195–199. doi: 10.1016/j.ygyno.2006.01.062.
- Roeske JC, Lujan A, Rotmensch J, et al. Intensity-modulated whole pelvic radiation therapy in patients with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2000;48(5):1613–1621. doi: 10.1016/s0360-3016(00)00771-9.
- Portelance L, Chao KS, Grigsby PW, et al. Intensity-modulated radiation therapy (IMRT) reduces small bowel, rectum, and bladder doses in patients with cervical cancer receiving pelvic and Para-aortic irradiation. Int J Radiat Oncol Biol Phys. 2001;51(1):261–266. doi: 10.1016/s0360-3016(01)01664-9.
- Mundt AJ, Mell LK, Roeske JC. Preliminary analysis of chronic gastrointestinal toxicity in gynecology patients treated with intensity-modulated whole pelvic radiation therapy. Int J Radiat Oncol Biol Phys. 2003;56(5):1354–1360. doi: 10.1016/s0360-3016(03)00325-0.
- Chen C-C, Wang L, Lu C-H, et al. Comparison of clinical outcomes and toxicity in endometrial cancer patients treated with adjuvant intensity-modulated radiation therapy or conventional radiotherapy. J Formos Med Assoc. 2014;113(12):949–955. doi: 10.1016/j.jfma.2013.09.013.
- Shih KK, Hajj C, Kollmeier M, et al. Impact of postoperative intensity-modulated radiation therapy (IMRT) on the rate of bowel obstruction in gynecologic malignancy. Gynecol Oncol. 2016;143(1):18–21. doi: 10.1016/j.ygyno.2016.07.116.
- Jhingran A, Winter K, Portelance L, et al. A phase II study of intensity modulated radiation therapy to the pelvis for postoperative patients with endometrial carcinoma: radiation therapy oncology group trial 0418. Int J Radiat Oncol Biol Phys. 2012;84(1):e23–e28. doi: 10.1016/j.ijrobp.2012.02.044.
- Ta M-H, Schernberg A, Giraud P, et al. Comparison of 3D conformal radiation therapy and intensity-modulated radiation therapy in patients with endometrial cancer: efficacy, safety and prognostic analysis. Acta Oncol. 2019;58(8):1127–1134. doi: 10.1080/0284186X.2019.1599136.
- Klopp AH, Yeung AR, Deshmukh S, et al. Patient-Reported toxicity during pelvic Intensity-Modulated radiation therapy: NRG Oncology-RTOG 1203. J Clin Oncol. 2018;36(24):2538–2544. doi: 10.1200/JCO.2017.77.4273.
- Yeung AR, Pugh SL, Klopp AH, et al. Improvement in Patient-Reported outcomes with Intensity-Modulated radiotherapy (RT) compared with standard RT: a report from the NRG oncology RTOG 1203 study. J Clin Oncol. 2020;38(15):1685–1692. doi: 10.1200/JCO.19.02381.
- Small W, Mell LK, Anderson P, et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy in postoperative treatment of endometrial and cervical cancer. Int J Radiat Oncol Biol Phys. 2008;71(2):428–434. doi: 10.1016/j.ijrobp.2007.09.042.
- Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a gynecologic oncology group study. Gynecol Oncol. 2004;92(3):744–751. doi: 10.1016/j.ygyno.2003.11.048.
- Cho LP, Cheng Z, McNutt TR, et al. Comparison of prospectively-collected genitourinary and gastrointestinal toxicities between post-operative intensity-modulated radiation therapy and non-intensity-modulated radiation therapy patients. Int J Radiat Oncol Biol Phys. 2017;99(2):E286–E287. doi: 10.1016/j.ijrobp.2017.06.1286.
- Atkinson TM, Li Y, Coffey CW, et al. Reliability of adverse symptom event reporting by clinicians. Qual Life Res. 2012;21(7):1159–1164. doi: 10.1007/s11136-011-0031-4.
- Basch E, Jia X, Heller G, et al. Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst. 2009;101(23):1624–1632. doi: 10.1093/jnci/djp386.
- Yeung AR, Pugh S, Klopp AH, et al. IMRT improves late toxicity compared to conventional RT: an update on NRG oncology-RTOG 1203. Int J Radiat Oncol Biol Phys. 2019;105(1):S50. doi: 10.1016/j.ijrobp.2019.06.480.
- He S, Gill BS, Heron DE, et al. Long-term outcomes using adjuvant pelvic intensity modulated radiation therapy (IMRT) for endometrial carcinoma. Pract Radiat Oncol. 2017;7(1):19–25. doi: 10.1016/j.prro.2016.06.005.
- Soisson S, Ganz PA, Gaffney D, et al. Long-term, adverse genitourinary outcomes among endometrial cancer survivors in a large, population-based cohort study. Gynecol Oncol. 2018;148(3):499–506. doi: 10.1016/j.ygyno.2017.12.025.
- Fred Hutch. High impact of positive and negative clinical trial results. [Internet]. 2019 [cited 2023 Mar 24]. Available from: https://www.fredhutch.org/en/news/spotlight/2019/10/phs_unger_jamanetopen.html.
- Laskar SG, Sinha S, Krishnatry R, et al. Access to radiation therapy: from local to global and equality to equity. J Clin Oncol Glob Oncol. 2022;8:e2100358. doi: 10.1200/GO.21.00358.
