Abstract
Background
Sentinel lymph node biopsy (SLNB) is a critical staging tool for melanoma patients. The optimal number of lymph nodes removed in SLNB remains unclear. In this study, we retrospectively analysed and tested different criteria for selecting sentinel lymph nodes (SLNs) by radiotracer uptake and blue dye, and their impact on nodal staging. We also evaluated the association between SLN tumour burden and radiotracer uptake.
Methods
The study population consisted of melanoma patients undergoing SLNB. During the operation all radioactive and blue nodes were removed and sent for histopathological analysis. The ex vivo radioactive count and presence of blue dye of each node were recorded, and these were correlated with presence and size of metastasis in each SLN.
Results
Altogether 175 patients with clinically occult metastasis presented with one or more positive, i.e. metastatic, SLNs. The mean number of lymph nodes removed was 4.5, and the mean number of positive lymph nodes was 1.5 per patient. The most radioactive or hottest node was negative in 38 patients (22%). By removing the hottest node and all nodes with radioactivity >10% of the hottest node, 97% of patients would have been staged correctly. In five patients, metastasis was found solely in a SLN with radioactivity <10% of the hottest node. Of all 267 positive nodes removed, 125 (47%) contained blue dye. Patients with a negative hottest node were associated with lower SLN tumour burden.
Conclusions
By removing the hottest node and all nodes with radioactivity >10% of the hottest node, 97% of patients with SLN metastases are correctly staged with or without using blue dye.
Introduction
Status of the sentinel lymph node (SLN) is the most accurate predictor of survival in melanoma patients with clinically negative regional lymph nodes. Sentinel lymph node biopsy (SLNB) has an essential role in staging and selecting patients for follow-up and adjuvant therapies [Citation1,Citation2].
Until recently, most patients with a positive, i.e. metastatic, SLN underwent completion lymph node dissection (CLND) [Citation1]. The multicentre trials MSLT-II and DeCOG-SLT failed to show any survival benefit for CLND, and subsequently, CLND has been omitted for most patients with SLN metastases [Citation3–5]. Further, targeted therapies and immunotherapy have evolved rapidly, and SLNB has an essential role in selecting patients for adjuvant therapies and trials [Citation6–8].
SLNB is based on an orderly progression of metastatic cells from the primary tumour site to the lymphatic system [Citation9]. SLN is defined as a node or nodes that receive direct lymphatic drainage from the primary tumour site [Citation10].
The SLNB procedure commonly involves preoperative lymphoscintigraphy with radiotracer, intraoperative use of a gamma-detector and blue dye, and histopathological analysis of SLNs [Citation1,Citation11,Citation12]. Each of these steps contributes to the accuracy of the procedure and should be of high standard to minimise the number of false-negative biopsies [Citation11,Citation12].
The static images of a lymphoscintigram frequently show multiple lymph nodes in a lymph node basin. It may be difficult to differentiate the true sentinel nodes from second-tier nodes, i.e. nodes that receive radio tracer after it has passed through the sentinel node(s), an issue commonly encountered with inguinal/iliac lymph nodes [Citation10,Citation12–14].
The number of nodes that need to be removed must be optimised to balance sensitivity and morbidity of the procedure [Citation10,Citation12]. Several attempts have been made to refine the SLNB procedure by limiting the number of nodes removed without compromising sensitivity [Citation15–19]. The most widely used method is the ‘10% rule’, which states that all blue nodes and nodes that have ex vivo radioactivity of 10% or more of the radioactivity of the most radioactive node, i.e. the hottest node, need to be removed [Citation17].
SLN tumour burden has prognostic value [Citation20–23]. The most employed method to measure tumour burden in SLN is the maximum diameter of the largest metastatic tumour deposit in SLN [Citation22,Citation24,Citation25]. The correlation between SLN tumour burden and radioactivity in patients with more than one positive SLN has not been analysed previously.
In this study, we sought to investigate the correlation of radioactivity with status of SLN to discover which nodes harbour metastases and how many nodes need to be removed to detect SLN metastases. Different criteria for harvesting SLNs were applied to test their impact on nodal staging of patients. In addition, we explored whether the radioactivity correlates with the diameter of the SLN metastasis in patients with more than one positive SLN. The role of blue dye was also evaluated.
Patients and methods
The Helsinki University Hospital institutional review board approved the study protocol.
Our series consists of 930 consecutive patients who underwent a SLNB procedure during the period of 2001–2008 at the Department of Plastic Surgery, Helsinki University Hospital, Finland. The criteria for SLNB were Breslow thickness of the primary tumour of 1 mm or more or other pathological features suggesting a more aggressive behaviour of melanoma, i.e. ulceration and mitotic activity.
In this study, we included melanoma patients with no clinically evident metastatic disease at the time of surgery, and who presented with one or more positive, i.e. metastatic, SLNs.
Preoperative lymphoscintigraphy was performed on the day before surgery. Technetium-99m–labelled colloidal albumin (Albu-Res and Nanocoll, Nycomed Amersham Sorin s.r.l., Saluggia, Italy) 80 MBq in 0.2 ml was injected intradermally into the primary tumour site on both sides of the excision scar. Static images 30 min and 2 h from injection were obtained. Patent blue dye (Bleu Patenté V, Laboratoire Geuerbet, Aulnay-sous-Bois, France) mean 1 ml was injected intradermally into the site of the primary tumour just prior to surgery. A Gamma detecting probe (Navigator, Tyco Health Care, Norwalk, CT, USA and Neoprobe 2000, Johnson & Johnson Medical, Hamburg, Germany) was used intraoperatively and all blue-stained and/or radioactive nodes were harvested. SLNs were removed until no focal residual activity could be detected. The radioactivity count of the nodes was performed ex vivo and recorded. The radioactivity counts were collected prospectively, and a specific form designed for research and quality-control purposes was filled in for each patient. Lymph nodes that had no radioactivity or blue stain were not considered SLNs. If two or more lymph nodes were removed en bloc and only a single radioactivity count was recorded, they were considered a single SLN in the data analysis.
Each SLN was sent for histopathological analysis. The nodes were embedded in paraffin and serially cut into 1 mm slices and stained with haematoxylin-eosin. Immunohistochemical staining with melanoma-specific antigens S-100, Melan-A, and HMB-45 was performed. The length and width of the SLN metastases were measured. The maximum diameter of the largest tumour deposit and the involved SLN were reported.
The CLND specimen was weighed, and half of each node was taken into histopathological analysis (haematoxylin-eosin). Immunohistochemistry was not used routinely. Metastases were recorded according to size in one dimension and according to the number of positive nodes of all nodes in the basin.
Statistical analysis was performed to test the correlation between patients with a positive hottest SLN and patients with a negative hottest SLN in a subgroup of patients who had more than one positive SLN. The correlation between the status of the hottest node and the maximum diameter of the largest tumour deposit of the SLN was analysed. In addition, the correlation between the radiotracer uptake and whether the SLN harboured the largest metastatic tumour deposit was analysed in patients with more than two positive SLNs. Statistical analysis was performed by Chi-square test for categorical variables and Mann-Whitney U test for continuous variables using SPSS, version 25 (IBM, Armonk, NY, USA). P-values of less than 0.05 were considered significant.
Results
Patients and lymph node basins
The specific inclusion criteria resulted in 175 melanoma patients in whom at least one SLN was positive. A total of 783 SLNs were sampled from 221 lymph node basins. Of the patients, 133 had an operation on just one basin, with a mean number of basins per patient of 1.27 (median 1, range 1–3). Of the 221 basins, 188 (85%) were harbouring one or more metastatic nodes. Of the 175 patients, 163 (93%) had a single positive basin, 11 (6%) had two positive basins, and one (0.6%) had three positive basins. The median number of SLNs removed per patient was four (range 1–15). At least two nodes were removed in 160 cases (91%). Altogether 270 nodes (35%) were positive with the mean number of positive nodes per patient being 1.5. Of all 175 patients, 66 (38%) had more than one positive SLN.
The hottest SLN and relative radioactivity of SLNs
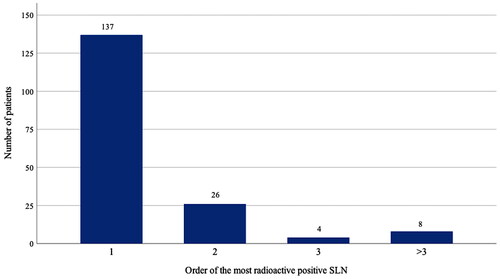
The hottest node – the node with the highest radioactivity count – was positive in 137 patients (78%). When the hottest node was negative, the second hottest node was positive in 26 cases (14%), the third hottest node in four cases (2.3%), and another less active node in eight cases (4.6%) ().
Figure 1. Order of the most radioactive or hottest positive SLN in the study population of 175 patients according to radiotracer uptake. Number one represents 137 patients with a positive hottest SLN. Numbers two and three represent patients with a negative hottest SLN, who presented with a metastasis in the second or third hottest SLN, respectively. In eight cases, indicated with >3, the three hottest SLNs were negative and a less radioactive node harboured metastasis. SLN: sentinel lymph node.
displays the patient demographics of all 175 patients and the 160 patients with more than one SLN removed stratified by the status of the hottest node. Patients with lower Breslow thickness, smaller diameter of SLN metastasis, and/or fewer positive SLNs were less likely to present with metastasis in the hottest node. Otherwise, we found no significant difference between the subgroups.
Table 1. Clinicopathologic characteristics of all 175 patients and 160 patients with more than one SLN removed stratified by the status of the hottest SLN, i.e. SLN with highest radiotracer uptake.
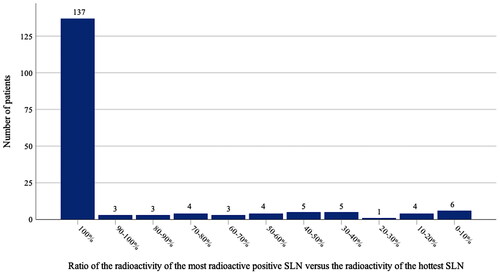
For the 38 patients with a negative hottest node, the median ratio of the radioactivity of the most radioactive positive node versus the radioactivity of the hottest node was 0.46 (range 0.02–0.96). shows the distribution of the relative radioactivity in all 175 patients.
Completion lymph node dissection (CLND)
Of all 175 patients, 151 (86%) underwent CLND and 22 did not for the following reasons: randomised to MSLT-II follow-up group (n = 10), patient refusal (n = 7), poor general health (n = 4), a metastasis found solely in an interval node, i.e. SLN located between the primary tumour site and the lymph node basin (n = 1) [Citation3,Citation26]. For two patients, no CLND or follow-up data were available due to moving abroad. Of the 151 patients who underwent CLND, only 18 (11.9%) had additional metastatic nodes in the CLND specimen.
Blue dye
Of all 783 lymph nodes, 220 (28%), and, of all 270 positive nodes, 125 (46%) contained blue dye. No blue dye in SLNs could be detected in 57 patients (33%). Of 118 patients with any blue dye in SLNs, 104 had blue-stained metastatic SLNs, all of which were also radioactive. In 87 cases, the hottest node was blue and positive. In 15 cases, the hottest node was negative, but the hottest positive node was blue. In three of these 15 patients, the radioactivity of the positive node was less than 10% of the hottest node. There were two patients with a positive hottest node with no blue dye but another less radioactive positive blue node. In 14 cases, blue dye was found only in non-metastatic lymph nodes, and 10 of these patients presented with a blue but negative hottest node.
Various criteria for SLN
displays different criteria applied for a SLN to test their impact on the miss rate of positive nodes, and on nodal and overall staging of patients according to the eighth edition of the AJCC staging manual [Citation2]. For patients operated on for multiple lymph node basins, each rule applies to individual lymph node basins because during the operation the surgeon compares the radioactivity of individual SLNs to the hottest node of the same basin rather than to the hottest node of the patient. By removing the hottest node only, 114 (42%) of all 270 positive nodes would have been missed and 34 (19%) of the 175 patients would have been under-staged. By following the ‘10% rule’, the number of missed positive nodes would have been 14 (5.2%) and the number of under-staged patients two. Following the ‘10% rule’ would have decreased the total number of harvested lymph nodes from an average of 4.5 to 3.4 per patient. However, 18 patients had positive SLNs with radioactivity <10% of the hottest node, and five patients presented with a metastasis solely in such a node. Of these five patients, three had blue dye in the positive SLN.
Table 2. SNB according to various retrospectively applied SLN criteria based on which nodes are removed from a lymph node basin.
SLN radioactivity and tumour burden
Patients with a negative hottest node presented with lower SLN tumour burden measured by the maximum diameter of the largest tumour deposit of the SLN. The correlation was analysed using the maximum diameter as a continuous variable (p < 0.001) as well as using a cut-off value of 1 mm, which represents the median diameter of SLN metastases (p = 0.004). Of the 67 patients with more than one positive SLN, 45 (67.2%) had the largest tumour deposit in the hottest positive node and 22 (32.8%) in a less active positive node. Altogether 163 positive SLNs were recorded from the 67 patients with more than one positive SLNs. The hottest positive node harboured the largest metastasis in 45 (27.6%), and a less active SLN in 22 (13.5%) of all 163 positive SLNs in patients with more than one positive SLN (p < 0.001) (Supplementary Table 1). Similarly, the higher relative radioactivity of positive SLN vs hottest positive SLN correlated with the presence of the largest metastasis (p < 0.001).
Discussion
In this current study, we retrospectively analysed 175 melanoma patients with at least one positive SLN, to evaluate the criteria of harvesting SLNs and to optimise the staging procedure. We found that in 38 cases (22%), the hottest node was negative and in 12 cases (7%) the two hottest nodes were negative. Of all 175 patients, 66 (38%) had two or more positive SLNs. In cases with a negative hottest node, the radioactivity ratio of the most radioactive positive node to the hottest node was widely distributed. When we compared different criteria of harvesting SLNs, we found that by removing the hottest node and all nodes with radioactivity >10% of the hottest node, 97% of patients would have been staged correctly. By adding blue-stained nodes to the criteria, as in the original ‘10% rule’, only two patients would have been under-staged. In 33% of the cases, no blue dye was detected in SLNs, and only 46% of all positive SLNs were blue-stained. Patients with a negative hottest node were associated with lower tumour burden.
After the large prospective studies MSLT-II and DeCOG-SLT failed to show significant survival benefit of CLND in melanoma patients after a positive SLN, CLND has been largely abandoned [Citation3–5]. The accuracy of lymph node surgery relies on SLNB, and its role in staging and selecting patients for trials, adjuvant therapies and/or follow-up is highlighted [Citation6–8]. In a recently published substudy by the MSLT-II Study Group, more than 80% of patients with a positive SLN who were randomised to nodal observation instead of CLND did not have an in-basin nodal recurrence at 10 years, supporting the therapeutic value of SLNB [Citation27].
The optimal number of nodes that need to be removed is controversial. The number of nodes removed in SLNB is one of the key elements determining the sensitivity of the procedure [Citation10,Citation12]. For staging purposes, it is not merely a question of positive versus negative SLNB but also the number of positive SLNs influences nodal staging. Ideally, all positive nodes in the basin would be removed in SLNB to stage patients precisely – information that we used to get from the CLND specimen. Yet, the original idea of SLNB was to perform a minimally invasive operation with a minimal risk of complications and surgical trauma to the patient [Citation9]. The reported complication rate for SLNB is far less than for CLND [Citation3,Citation11,Citation28,Citation29].
Several studies have investigated the number of nodes needed to determine the SLN status adequately without performing an operation more or less like a CLND [Citation15–17,Citation19]. A few studies suggest that no more than two nodes should be harvested, whereas in many publications a percentage limit relative to the most radioactive node is preferred [Citation16–18,Citation30–32]. Although there is no unanimous agreement on the exact limit, the “10% rule” has been widely accepted and its utility has been tested several times [Citation16,Citation17,Citation19,Citation32–34]. The broad range of relative radioactivity levels displayed in explains why a cut-off at 50% of the hottest node or a predefined number of sentinel nodes represent suboptimal ways to limit the number of harvested nodes. It is advisable to prefer a relative radioactivity cut-off value such as 10% of the hottest node.
SLNB became a standard procedure in our hospital in 2000. From the very beginning we set out to determine the optimal number of nodes and decided to aim at a very low residual radioactivity of the lymph node basin rather than following the “10% rule”, while carefully recording the radioactivity count of every SLN removed. In previous studies on the optimal number of removed nodes, the average number per patient ranges from two to three [Citation16,Citation18,Citation19,Citation30,Citation32]. In our study, the mean number of SLNs was 4.5 per patient. This is partly because of the protocol described above, but also the tracer plays a role in the harvest. We use Technetium-99 m–labelled colloidal albumin, which, compared with sulphur colloid yields higher numbers of nodes [Citation35]. In our study, removing the hottest node and all nodes with radioactivity >10% of the hottest node yielded an average of 3.4 SLNs per patient, which still represents a rather high number of lymph nodes. However, there is currently no better way of limiting the number of harvested nodes intraoperatively. Today, single-photon emission computed tomography/computed tomography (SPECT/CT) is in routine use in our hospital and helps to locate the SLNs, but has less value in limiting their number, i.e. selecting the true SLNs from second-tier nodes [Citation36–38]. Dynamic imaging and discussion between the nuclear medicine physician and the surgeon are paramount. New imaging techniques should further help to solve this issue, but currently lymphoscintigraphy remains the gold standard.
Blue dye has been used routinely since the early years of the procedure but has lost popularity in recent years due to allergic reactions [Citation39,Citation40]. In addition, although it is considered helpful in visualising SLNs and lymph vessels, the use of blue dye does not significantly increase the accuracy of SLNB [Citation16,Citation19]. In our study, in three out of five cases where the radioactivity count was less than 10% of that of the hottest node the positive node was stained blue, suggesting a positive impact on the sensitivity of the procedure. However, only 46% of all positive SLNs were stained blue. Considering the possible risks of blue dye, it is no longer in routine use in our hospital.
Low Breslow thickness of the primary melanoma tumour and small diameter of SLN metastasis were associated with a higher likelihood of a negative hottest SLN, with at least one of the less radioactive SLNs harbouring metastasis. This may be due to minimal deposits of melanoma cells in the hottest SLN that were not detected in histopathological analysis [Citation41]. To our knowledge, there are no similar studies on the association between SLN radioactivity and tumour burden. Carlson et al. [Citation15] reported a higher SLN tumour burden in patients with a low radioactivity count ratio of SLN versus nodal bed.
Even though the radioactivity counts of SLNs were recorded prospectively, our study has limitations due to its retrospective setting. Therefore, different stages of the procedure – lymphoscintigraphy, surgery, and histopathology – may have been more susceptive to subjectivity. However, the same guidelines for SLNB applied to all patients and most patients underwent CLND according to the previous paradigm. A similar prospective analysis is therefore no longer possible as CLND has been rightfully abandoned.
Conclusions
A threshold based on the relative radioactivity count of SLNs should be preferred to removing only one to three of the hottest nodes in SLNB. The number of SLNs needed to be harvested for histopathology depends on their intraoperative radioactivity count. SLNs with low radioactivity counts may harbour metastases. By removing the hottest node and all nodes with radioactivity >10% of the hottest node, more than 97% of patients with SLN metastases were discovered. Blue dye added to the accuracy of SLNB but was not essential.
Supplemental Material
Download MS Word (13.2 KB)Disclosure statement
Each author declares having no financial conflicts of interest regarding the data presented in this manuscript. The Kurt and Doris Palander Foundation for Medical Research awarded the first author a scholarship. Apart from this, the funding for this article was from departmental sources only. Preliminary results have been presented in part at the Nordic Melanoma Meeting, Oslo, Norway, during 6–8 September 2012, and at the Joint International Oncology Congress, San Francisco, California, USA during 27–29 May 2013.
Data availability statement
The participants of this study did not give written consent for their data to be shared publicly, so due to the sensitive nature of the research supporting data is not available.
References
- Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370(7):599–609. doi: 10.1056/NEJMoa1310460.
- Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472–492. doi: 10.3322/caac.21409.
- Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for Sentinel-Node metastasis in melanoma. N Engl J Med. 2017;376(23):2211–2222. doi: 10.1056/NEJMoa1613210.
- Leiter U, Stadler R, Mauch C, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17(6):757–767. doi: 10.1016/S1470-2045(16)00141-8.
- Wong SL, Faries MB, Kennedy EB, et al. Sentinel lymph node biopsy and management of regional lymph nodes in melanoma: american society of clinical oncology and society of surgical oncology clinical practice guideline update. Ann Surg Oncol. 2018;25(2):356–377. doi: 10.1245/s10434-017-6267-7.
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845–1855. doi: 10.1056/NEJMoa1611299.
- Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-Mutated melanoma. N Engl J Med. 2017;377(19):1813–1823. doi: 10.1056/NEJMoa1708539.
- Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824–1835. doi: 10.1056/NEJMoa1709030.
- Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127(4):392–399. doi: 10.1001/archsurg.1992.01420040034005.
- Thompson JF, Uren RF. What is a ‘sentinel’ lymph node? Eur J Surg Oncol. 2000;26(2):103–104. doi: 10.1053/ejso.1999.0752.
- Morton DL, Cochran AJ, Thompson JF, et al. Sentinel node biopsy for early-stage melanoma: accuracy and morbidity in MSLT-I, an international multicenter trial. Ann Surg. 2005;242(3):302–311. doi: 10.1097/01.sla.0000181092.50141.fa.
- Nieweg OE, Jansen L, Kroon BB. Technique of lymphatic mapping and sentinel node biopsy for melanoma. Eur J Surg Oncol. 1998;24(6):520–524. doi: 10.1016/s0748-7983(98)93428-x.
- Uren RF, Howman-Giles R, Thompson JF. Patterns of lymphatic drainage from the skin in patients with melanoma. J Nuc Med. 2003;44(4):570–582.
- Vuoristo M, Muhonen T, Koljonen V, et al. Pelvic sentinel lymph nodes have minimal impact on survival in melanoma patients. BJS Open. 2021;5(6):zrab128. doi: 10.1093/bjsopen/zrab128.
- Carlson GW, Murray DR, Thourani V, et al. The definition of the sentinel lymph node in melanoma based on radioactive counts. Ann Surg Oncol. 2002;9(9):929–933. doi: 10.1007/BF02557533.
- Liu LC, Parrett BM, Jenkins T, et al. Selective sentinel lymph node dissection for melanoma: importance of harvesting nodes with lower radioactive counts without the need for blue dye. Ann Surg Oncol. 2011;18(10):2919–2924. doi: 10.1245/s10434-011-1689-0.
- McMasters KM, Reintgen DS, Ross MI, et al. Sentinel lymph node biopsy for melanoma: how many radioactive nodes should be removed? Ann Surg Oncol. 2001;8(3):192–197. doi: 10.1007/s10434-001-0192-4.
- Porter GA, Ross MI, Berman RS, et al. How many lymph nodes are enough during sentinel lymphadenectomy for primary melanoma? Surgery. 2000;128(2):306–311. doi: 10.1067/msy.2000.107418.
- Ranson JM, Pantelides NM, Pandit DG, et al. Sentinel lymph node biopsy in melanoma: which hot nodes should be harvested and is blue dye really necessary? J Plast Reconstr Aesthet Surg. 2018;71(9):1269–1273. doi: 10.1016/j.bjps.2018.04.020.
- Gershenwald JE, Andtbacka RH, Prieto VG, et al. Microscopic tumor burden in sentinel lymph nodes predicts synchronous nonsentinel lymph node involvement in patients with melanoma. J Clin Oncol. 2008;26(26):4296–4303. doi: 10.1200/JCO.2007.15.4179.
- Satzger I, Leiter U, Grager N, et al. Melanoma-specific survival in patients with positive sentinel lymph nodes: relevance of sentinel tumor burden. Eur J Cancer. 2019;123:83–91. doi: 10.1016/j.ejca.2019.07.004.
- van Akkooi AC, de Wilt JH, Verhoef C, et al. Clinical relevance of melanoma micrometastases (<0.1 mm) in sentinel nodes: are these nodes to be considered negative? Ann Oncol. 2006;17(10):1578–1585. doi: 10.1093/annonc/mdl176.
- Vuoristo M, Muhonen T, Koljonen V, et al. Long-term prognostic value of sentinel lymph node tumor burden in survival of melanoma patients. Acta Oncol. 2021;60(6):803–807. doi: 10.1080/0284186X.2021.1892820.
- Egger ME, Bower MR, Czyszczon IA, et al. Comparison of sentinel lymph node micrometastatic tumor burden measurements in melanoma. J Am Coll Surg. 2014;218(4):519–528. doi: 10.1016/j.jamcollsurg.2013.12.014.
- van Akkooi AC, Nowecki ZI, Voit C, et al. Sentinel node tumor burden according to the rotterdam criteria is the most important prognostic factor for survival in melanoma patients: a multicenter study in 388 patients with positive sentinel nodes. Ann Surg. 2008;248(6):949–955. doi: 10.1097/SLA.0b013e31818fefe0.
- Verwer N, Scolyer RA, Uren RF, et al. Treatment and prognostic significance of positive interval sentinel nodes in patients with primary cutaneous melanoma. Ann Surg Oncol. 2011;18(12):3292–3299. doi: 10.1245/s10434-011-1988-5.
- Crystal JS, Thompson JF, Hyngstrom J, et al. Therapeutic value of sentinel lymph node biopsy in patients with melanoma: a randomized clinical trial. JAMA Surg. 2022;157(9):835–842.
- Wrightson WR, Wong SL, Edwards MJ, et al. Complications associated with sentinel lymph node biopsy for melanoma. Ann Surg Oncol. 2003;10(6):676–680. doi: 10.1245/aso.2003.10.001.
- Kretschmer L, Thoms KM, Peeters S, et al. Postoperative morbidity of lymph node excision for cutaneous melanoma-sentinel lymphonodectomy versus complete regional lymph node dissection. Melanoma Res. 2008;18(1):16–21. doi: 10.1097/CMR.0b013e3282f2017d.
- Abou-Nukta F, Ariyan S. Sentinel lymph node biopsies in melanoma: how many nodes do we really need? Ann Plast Surg. 2008;60(4):416–419. doi: 10.1097/SAP.0b013e31812c65e2.
- Chagpar AB, Scoggins CR, Martin RC, II, et al. Are 3 sentinel nodes sufficient? Arch Surg. 2007;142(5):456–459. doi: 10.1001/archsurg.142.5.456.
- Murphy AD, Britten A, Powell B. Hot or not? The 10% rule in sentinel lymph node biopsy for malignant melanoma revisited. J Plas Reconstruct Aesthet Surg. 2014;67(3):316–319. doi: 10.1016/j.bjps.2013.11.008.
- Emery RE, Stevens JS, Nance RW, et al. Sentinel node staging of primary melanoma by the “10% rule”: pathology and clinical outcomes. Am J Surg. 2007;193(5):618–622. doi: 10.1016/j.amjsurg.2007.01.001.
- Kroon HM, Lowe L, Wong S, et al. What is a sentinel node? Re-evaluating the 10% rule for sentinel lymph node biopsy in melanoma. J Surg Oncol. 2007;95(8):623–628. doi: 10.1002/jso.20729.
- Pijpers R, Borgstein PJ, Meijer S, et al. Transport and retention of colloidal tracers in regional lymphoscintigraphy in melanoma: influence on lymphatic mapping and sentinel node biopsy. Melanoma Res. 1998;8(5):413–418. doi: 10.1097/00008390-199810000-00005.
- Even-Sapir E, Lerman H, Lievshitz G, et al. Lymphoscintigraphy for sentinel node mapping using a hybrid SPECT/CT system. J Nucl Med. 2003;44(9):1413–1420.
- Uren RF. SPECT/CT lymphoscintigraphy to locate the sentinel lymph node in patients with melanoma. Ann Surg Oncol. 2009;16(6):1459–1460. doi: 10.1245/s10434-009-0463-z.
- Moncrieff M, Pywell S, Snelling A, et al. Effectiveness of SPECT/CT imaging for sentinel node biopsy staging of primary cutaneous melanoma and patient outcomes. Ann Surg Oncol. 2022;29(2):767–775. doi: 10.1245/s10434-021-10911-4.
- Hunting AS, Nopp A, Johansson SG, et al. Anaphylaxis to patent blue V. I. Clinical aspects. Allergy. 2010;65(1):117–123. doi: 10.1111/j.1398-9995.2009.02192.x.
- Leong SP, Donegan E, Heffernon W, et al. Adverse reactions to isosulfan blue during selective sentinel lymph node dissection in melanoma. Ann Surg Oncol. 2000;7(5):361–366. doi: 10.1007/s10434-000-0361-x.
- Karim RZ, Scolyer RA, Li W, et al. False negative sentinel lymph node biopsies in melanoma may result from deficiencies in nuclear medicine, surgery, or pathology. Ann Surg. 2008;247(6):1003–1010. doi: 10.1097/SLA.0b013e3181724f5e.