Abstract
Background: The association between statin use and risk of renal cell carcinoma (RCC) has been debated. We aimed to evaluate whether statin use is associated with RCC risk.
Material and methods: We studied 100,195 women in the Nurses’ Health Study (NHS) from 1994 to 2016; 91,427 women in the Nurses’ Health Study II (NHS II) from 1999 to 2015; and 45,433 men in the Health Professionals Follow-up Study (HPFS) from 1990 to 2016. Statins and covariate data were collected at baseline and then biennially. Outcome was measured as incidence of total RCC and clinically relevant disease subgroups. Cox proportional hazards models estimated covariate-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs).
Results: During follow-up, 661 participants developed RCC. There was no significant association between the use of statins and the risk of overall RCC, fatal RCC, or advanced or localized disease. Across cohorts, the adjusted HR for ever vs. never users was 0.97 (95% CI 0.81–1.16). Female ever users of statins were at increased risk of high-grade disease in the NHS only (HR 1.75, 95% CI 1.07–2.85). Among men only, ≥4 years of statin use was associated with an increased risk of clear cell RCC (HR 1.65, 95% CI 1.10–2.47).
Conclusions: Statin use was not associated with the overall risk of RCC. However, it was associated with an increased risk of high-grade disease among women in the NHS cohort and an increased risk of clear cell RCC among men. The reasons for these inconsistent results by sex are unclear.
Introduction
Statins have been shown to reduce cholesterol synthesis and are currently one of the most widely used antihyperlipidemic agents for the prevention of cardiovascular disease. Beyond lipid-lowering properties, statins have been shown to possess anti-inflammatory, anti-proliferative, and anti-angiogenic activities [Citation1] and to modulate signaling pathways involved in cell survival, apoptosis, and immune response [Citation2,Citation3]. As such, their possible antitumor effects, including against kidney cancer, have long been a question of great interest [Citation4].
In the epidemiological literature, the association between statin use and kidney risk has been subject to considerable debate. One study of nearly half a million American veterans found a significantly lower risk of RCC (the most common subtype of kidney cancer) with statin use [Citation5]. However, registry-based studies from Denmark [Citation6] and Taiwan [Citation7], found no such association for RCC and overall kidney cancer, respectively. More recently, a Korean study found an increased risk of kidney cancer among statin users [Citation8]. Such prior studies have not adjusted for known kidney cancer risk factors that may be important confounders, including body mass index (BMI), physical activity, smoking, diabetes, and hypertension.
Previous results from our group using the Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS) indicated that statin use is associated with a reduced risk of RCC among women, but not among men [Citation9]. As evidence for the relationship remains inconclusive, there is a need to investigate the association further, with full consideration of possible confounders. Thus, we conducted an updated analysis of statin use and risk of total and fatal RCC in the NHS and HPFS, incorporating the Nurses’ Health Study II (NHS II), a younger cohort of female nurses in the US. We now have more than twice as many cases as our previous publication and have continued to assess potential confounders during follow-up. We aimed to assess the association between statin use and the incidence of RCC overall, as well as defined by pathologic stage, grade, and histologic subtype.
Material and methods
Study populations
This study is based on three prospective US cohorts: NHS, NHS II, and HPFS. The NHS began in 1976 when 121,700 female nurses aged 30–55 completed a baseline questionnaire regarding their medical history and lifestyle. The NHS II began in 1989 when 116,429 female nurses, aged 25–42, completed a similar questionnaire. The HPFS is a study of 51,529 US male health professionals aged 40–75 when they completed the first questionnaire in 1986. In each cohort, updated information on health status and lifestyle is collected biennially. The questionnaire response rate is roughly 90% in each study. The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required.
Assessment of RCC
Preliminary information on the occurrence of RCC was self-reported on biennial questionnaires. After reporting an RCC diagnosis, participants (or next of kin, in the case of death) were asked for permission to collect medical records, including pathology reports. In the event of death, the last follow-up questionnaire was completed by the family. In the absence of a response, the National Death Index [Citation10] was searched. Follow-up for mortality is over 98% complete [Citation10].
Medical records were reviewed to confirm the diagnosis, identify the histologic subtype, and abstract stage, grade, and treatment information. Only individuals diagnosed with RCC based on the International Classification of Diseases for Oncology, 2nd edition [Citation11] (code C64), or the International Classification of Diseases, 9th Revision, Clinical Modification [Citation12] (code 189.0) were included as cases in this study. Upper tract urothelial carcinoma and oncocytoma were excluded from the case definition. Fatal RCC was defined as RCC-specific death before the end of follow-up. Advanced RCC was defined as stage T3 or T4, N1, or M1 disease at diagnosis. Localized disease was defined as stage T2 or lower at diagnosis with no evidence of lymph node involvement or metastatic disease. High-grade disease included Fuhrman grade 3 or 4 or poorly or undifferentiated cancer. Low-grade disease was defined as Fuhrman grade 1 or 2 or well or moderately differentiated cancer.
Assessment of statin use
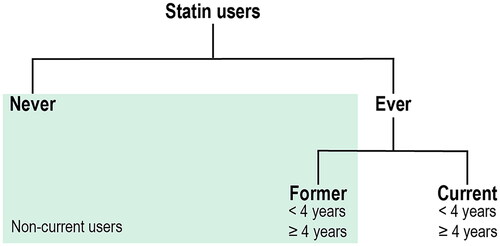
Since 1994 in the NHS and 1990 in the HPFS, participants were asked biennially whether they regularly (≥2 times per week) use any cholesterol-lowering drugs. From 2000 in the NHS and HPFS and 1999 in NHS II, participants were asked specifically about the use of statins and the duration of prior statin use in 2-year categories. For this analysis, we considered ‘cholesterol-lowering drugs‘ to be statins for questionnaires prior to 2000, as statins accounted for 93% and 91% of cholesterol-lowering drugs used in the NHS and HPFS, respectively, by the 2000 questionnaire [Citation9]. We conducted analyses for the following categories of statin users (): ever vs. never, current vs. non-current, former vs. never, current vs. never, total duration < 4 years vs. never, and total duration ≥ 4 years vs. never.
Assessment of covariates
BMI was calculated based on height reported on the baseline questionnaire and body weight updated biennially. History of smoking, diabetes, hypertension, and regular use of nonsteroidal anti-inflammatory drugs (NSAIDs) was updated every 2 years. Physical activity was characterized as the number of metabolic equivalent (MET)-hours per week. Finally, parity, defined as the number of childbirths experienced, was assessed in the NHS and NHS II.
Statistical analysis
Follow-up began in 1994 for the NHS, 1999 for the NHS II, and 1990 for the HPFS, at which times the use of cholesterol-lowering drugs was first assessed in each cohort. We included participants who completed the questionnaire in the baseline year, excluding those with cancer (excepting non-melanoma skin cancer) prior to cohort enrollment. Doing so left 100,195 women in the NHS, 91,427 women in the NHS II, and 45,433 men in the HPFS. Participants contributed person-time from baseline until the date of RCC diagnosis, date of death, or the end of follow-up (June 2016 for the NHS, January 2015 for the NHS II, and January 2016 for the HPFS), whichever came first.
Statin use was treated as a time-dependent variable, with participants considered current users for 2-year periods in which they reported statin use. The duration of use was calculated by summing the number of years of statin use from baseline questionnaire up to a given questionnaire cycle. In the case of study participants who skipped a questionnaire, information was carried forward from the previous questionnaire for one 2-year cycle; participants were censored after missing more than two consecutive questionnaires.
Cox proportional hazards models [Citation13] were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for total, fatal, advanced, localized, high grade, low grade, and clear cell RCC in each cohort. (Only four fatal cases occurred in NHS II, so analysis of fatal RCC is restricted to the HPFS and NHS only.) All models were stratified by age in months and calendar time (2-year intervals). In multivariable models, we additionally adjusted for possible confounding factors and established risk factors for RCC: BMI (<24.9, 25–29.9, 30+ kg/m2), history of diabetes (yes, no), history of hypertension (yes, no), smoking (never, former, current, and pack-years as a continuous variable), physical activity (quintiles), long-term NSAID use (<5 years, ≥5 years), and parity (women only; 0, 1–2, 3, ≥4 children). All covariates were treated as time-varying and updated biennially.
After testing for heterogeneity, the results were pooled across cohorts using random effects models. All p values calculated based on 2-sided tests were considered statistically significant at the traditional significance level of p < .05. Analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC).
Ethics considerations
The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health and those of participating registries as required.
Data availability statement
The data generated in this study are not publicly available due to information that could compromise patient privacy or consent but are available upon reasonable request from the corresponding author.
Results
Population characteristics
The characteristics of the study population are described in . Current statin users were more likely to be overweight/obese and diagnosed with diabetes and hypertension. They were also more likely to be chronic NSAID users.
Table 1. Age-adjusted characteristics of the study populations by ever statin use in 2000 (HPFS, NHS) and 1999 (NHS II).
Risk of overall RCC, fatal RCC, and RCC by stage
Over the course of follow-up (median of 259 months NHS, 186 months NHS II, and 297 months HPFS), we identified 661 cases of incident RCC (310 in the NHS, 96 in the NHS II, and 255 in the HPFS). Of these, 136 in the NHS and HPFS resulted in RCC-specific mortality before the end of the follow-up period. Models adjusted for age only showed some suggestion of a relationship between ever use of statins and overall RCC risk (Supplementary Table 1). Multivariable models, however, showed no association between ever use of statins and risk of overall RCC, fatal RCC, or advanced or localized disease in any single cohort or in combined analyses (). The combined, covariate-adjusted HR for RCC for ever users versus never users of statins was 0.97 (95% CI 0.81–1.16). Relative to never use, neither <4 years duration of use nor ≥4 years was associated with RCC risk (). In addition, there was no association for current versus non-current (former/never) statin use, or for either former or current use compared to never use (Supplementary Table 2). There was no evidence of heterogeneity across cohorts for any of the abovementioned results.
Table 2. Hazard ratios, and 95% confidence intervals for renal cell carcinoma (RCC) risk according to statin use in the HPFS (1990–2016), NHS (1994–2016), NHS II (1999–2015) cohorts.
Risk of high-grade RCC
In the NHS, ever use of statins was associated with an increased risk of high-grade RCC (adjusted HR: 1.75, 95% CI 1.07–2.85) (). However, there was no clear association with duration of use; women who used statins <4 years had significantly increased risk (HR: 2.18, 95% CI 1.23–3.85) in comparison with never users, while women with ≥4 years’ duration were not at significantly increased risk (HR: 1.44, 95% CI 0.81–2.57). In a pooled analysis combining the cohorts, the elevated risk of high-grade RCC was suggestive for current users compared to both non-current users (HR: 1.36, 95% CI 0.97–1.92, p-heterogeneity 0.72) and never users (HR: 1.41, 95% CI 0.98–2.01, p-heterogeneity 0.47, Supplementary Table 2). However, the combined HR for ever versus never users did not approach statistical significance (HR: 1.32, 95% CI 0.86–2.02, p-heterogeneity 0.25) ().
Risk of low-grade RCC
Ever use of statins in the NHS was associated with a significantly lower risk of low-grade RCC (HR: 0.67, 95% CI 0.47–0.95) (). The risk reduction was similar, though not significant, for those with <4 versus ≥4 years’ duration, compared to never users. In current users, the risk of low-grade RCC was decreased compared to non-current users (HR: 0.63, 95% CI 0.44–0.92) and never users (HR: 0.62, 95% CI 0.42–0.91, Supplementary Table 2). Statins were not associated with low-grade RCC risk in the NHS II, HPFS, or in the combined estimates across cohorts.
Risk of clear cell RCC
In the HPFS, current statin users had a higher risk of clear cell RCC compared to both non-current users (HR: 1.52, 95% CI 1.07–2.17, Supplementary Table 2) and never users (HR: 1.50, 95% CI 1.04–2.16, ). In addition, ≥4 years of statin use compared to never use was associated with an increased risk of clear cell RCC (HR: 1.65, 95% CI 1.10–2.47). In both the NHS and NHS II, statin use was not significantly associated with the risk of clear cell RCC, and all HR estimates were less than 1. Pooled results combining the NHS, NHS II, and HPFS showed significant heterogeneity for associations between all categorizations of exposure and clear cell RCC.
Discussion
Added value of this study
Previous studies evaluating the association between statin use and risk of RCC have yielded inconsistent results. Our analysis of three prospective cohorts with long-term follow-up and assessment of a range of possible confounders over time found no evidence of an association between statins and overall risk of RCC. In addition, statin use was not associated with the risk of fatal, advanced stage, or localized RCC. Though there was some suggestion of an increased risk of high-grade RCC, the absence of a clear dose-response relationship between duration of statin use and grade raises concerns about chance findings. Results for clear cell disease were discordant across cohorts.
Findings in the context of the literature
Our findings are consistent with previous results from the NHS and HPFS, which found no association between ever use of statins or duration of use and overall risk of RCC [Citation9]. The prior publication reported a borderline inverse association between current use and RCC risk among women in the NHS only. With additional follow-up time and cases, we found no suggestion of an association for current users compared to either never users or non-current users, in the NHS or in the other two cohorts. Our results are in line with results from Danish [Citation6] and Taiwanese [Citation7] registry-based studies, which found no association between statins and RCC risk and overall kidney cancer risk, respectively. However, these registry-based studies did not address the possible impact of confounding by smoking and obesity, since such factors are unavailable in population registries [Citation14]. Our results differ from a Korean registry-based study, which found a positive association between statin use and the risk of kidney cancer [Citation8]. Again, based on a registry, the study was unable to adjust for hypertension, BMI, or smoking. The importance of adjustment for such factors is evidenced by the difference between our age- and multivariable-adjusted results. Finally, our results contradict the findings of a study based on 500,000 US veterans [Citation5], which found that statins are protective for RCC. However, the results of this study are difficult to compare to ours without a better understanding of the exposure definition and timing and outcome assessment.
Findings on statins use and risk of RCC by grade and histologic subtype
Our study is the first to assess the relationship between statins and RCC risk based on grade or histologic subtype. Overall, the findings suggest that the association between statin use and RCC risk may vary depending on the subtype of RCC.
In our study, statin use among women in the NHS, albeit not in the NHS II, was associated with an increased risk of high-grade disease and a lower risk of low-grade disease. Notably, our analysis did not demonstrate a consistent dose-response relationship between the duration of statin use and the risk of RCC by grade. This lack of a clear trend raises concerns about the potential influence of random variation.
In our analyses focusing on clear cell renal cell carcinoma (RCC), the findings were inconsistent. We observed an increased risk among men exclusively, indicating a possible sex-specific association. Furthermore, as the duration of statin use increased, the risk showed a consistent pattern of escalation. These results suggest the existence of a potential biological mechanism that is specific to clear cell RCC and may act differently based on sex.
The heterogeneity observed in the results highlights the need for further investigation of factors contributing to divergent associations. Since kidney cancer rates are consistently higher in men than women, across time periods and study populations [Citation15], there is some suggestion that sex plays a role in the development and progression of RCC. Indeed, our group previously found that diabetes is associated with a significantly increased risk of RCC among women, but not men [Citation16]. Our results may thus be explained by some underlying biology that causes statin use to affect RCC risk for women and men differently. For example, statins modulate signaling pathways involved in cell survival, apoptosis, and immune response that may interact with hormonal and biological differences between women and men.
Strengths and limitations of the study
That health professionals tend to have better health literacy and awareness than the general population enhanced the accuracy and reliability of self-reported information. Though the homogeneity of the study population reduced potential confounding factors related to socioeconomic disparities and disparities in healthcare access and improved the study’s internal validity, it also potentially reduced the external validity of our findings. Another major strength of our study was the repeated assessment of a range of known RCC risk factors. In particular, our ability to adjust for BMI, smoking, and hypertension – all of which are correlated with statin use – is critical to disentangling a possible role for statins in RCC. In addition, the combination of three prospective cohorts with long-term follow-up allowed us to study associations with RCC risk based on stage, grade, and histologic subtype, in addition to overall RCC risk.
Even with large cohorts and long follow-up, our study was limited by small sample sizes in some analyses. It is also important to acknowledge that the duration variable with two categories (<4 years or 4+ years) may not capture the full spectrum of long-term effects on carcinogenesis. Future studies with extended follow-up durations are warranted to explore the effects of statin use on RCC risk over longer timeframes. Future studies would also benefit from information on the types and doses of statins used, as different statins may have varying pharmacokinetic profiles and mechanisms of action. Without a comprehensive evaluation of dosage, caution should be exercised in drawing definitive conclusions about causality.
Conclusions
In conclusion, statin use was not associated with overall risk of RCC in three large, prospective cohorts of US health professionals. However, it was associated with an increased risk of high-grade disease in the NHS and an increased risk of clear cell RCC in the HPFS. Further work, with even larger sample sizes, is needed to better characterize the sex-stratified relationship between statins and risk of RCC defined by grade and histologic subtype.
Supplemental Material
Download MS Word (19.3 KB)Acknowledgements
We are grateful to Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA. We would also like to thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Apostolova SN, Toshkova RA, Momchilova AB, et al. Statins and alkylphospholipids as new anticancer agents targeting lipid metabolism. Anticancer Agents Med Chem. 2016;16(12):1512–1522.
- Demierre MF, Higgins PD, Gruber SB, et al. Statins and cancer prevention. Nat Rev Cancer. 2005;5(12):930–942. doi:10.1038/nrc1751.
- Brown M, Hart C, Tawadros T, et al. The differential effects of statins on the metastatic behaviour of prostate cancer. Br J Cancer. 2012;106(10):1689–1696. doi:10.1038/bjc.2012.138.
- Bjarnadottir O, Romero Q, Bendahl PO, et al. Targeting HMG-CoA reductase with statins in a window-of-opportunity breast cancer trial. Breast Cancer Res Treat. 2013;138(2):499–508. doi:10.1007/s10549-013-2473-6.
- Khurana V, Caldito G, Ankem M. Statins might reduce risk of renal cell carcinoma in humans: case-control study of 500,000 veterans. Urology. 2008;71(1):118–122. doi:10.1016/j.urology.2007.08.039.
- Pottegard A, Clark P, Friis S, et al. Long-term use of statins and risk of renal cell carcinoma: a population-based case-control study. Eur Urol. 2016;69(5):877–882. doi:10.1016/j.eururo.2015.10.020.
- Chiu HF, Kuo CC, Kuo HW, et al. Statin use and the risk of kidney cancer: a population-based case-control study. Expert Opin Drug Saf. 2012;11(4):543–549. doi:10.1517/14740338.2012.678831.
- Kang M, Ku JH, Kwak C, et al. Effects of aspirin, nonsteroidal anti-inflammatory drugs, statin, and COX2 inhibitor on the developments of urological malignancies: a population-based study with 10-year follow-up data in Korea. Cancer Res Treat. 2018;50(3):984–991. doi:10.4143/crt.2017.248.
- Liu W, Choueiri TK, Cho E. Statin use and the risk of renal cell carcinoma in 2 prospective US cohorts. Cancer. 2012;118(3):797–803. doi:10.1002/cncr.26338.
- Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984;119(5):837–839. doi:10.1093/oxfordjournals.aje.a113804.
- Percy CV, Muir C. International classification of disease for oncology. 2nd ed. Geneva (Switzerland): World Health Organization; 1990.
- Puckett CD. The educational annotation of ICD-9-CM. Reno (NV): Channel Publishing Ltd.; 1986.
- Cox DR. Regression models and life-tables. In: Kotz S, Johnson NL, eds. Breakthroughs in statistics: methodology and distribution. New York (NY): Springer New York; 1992. p. 527–541.
- Rider JR. Trouble in paradise: unmeasured confounding in registry-based studies of etiologic factors. Eur Urol. 2016;69(5):883–884. doi:10.1016/j.eururo.2015.10.044.
- Scelo G, Li P, Chanudet E, et al. Variability of sex disparities in cancer incidence over 30 years: the striking case of kidney cancer. Eur Urol Focus. 2018;4(4):586–590. doi:10.1016/j.euf.2017.01.006.
- Graff RE, Sanchez A, Tobias DK, et al. Type 2 diabetes in relation to the risk of renal cell carcinoma among men and women in two large prospective cohort studies. Diabetes Care. 2018;41(7):1432–1437. doi:10.2337/dc17-2518.