Abstract
Background
Cancer treatment frequently results in chemotherapy-induced peripheral neuropathy (CIPN), which is a side effect that is now neither properly preventable nor treatable. Physical therapy has been studied in this patient population and is frequently utilised for neurological rehabilitation after damage.
Purpose
This study set out to thoroughly review randomised controlled trials (RCTs) examining the efficacy of physical therapy for patients with chemotherapy-induced peripheral neuropathy.
Data Sources
From their beginning in January 2017 to January 2023, EMBASE, PubMed, Medline, PEDro, and the Cochrane Library were searched for pertinent RCTs. Additionally, manual search techniques were applied.
Study Selection
On the basis of the inclusion criteria, two reviewers independently determined the study’s eligibility.
Data Extraction
Reviewers evaluated the quality of the studies and took note of their methodologies, designs, interventions, outcomes, and conclusions.
Data Synthesis
Ten RCTs met all inclusion criteria.
Limitations
Overall results are constrained by the variety of interventions and the small sample sizes of the included studies, which also indicate the need for more studies.
Conclusions
Physical therapy has additional benefits for enhancing the quality of life of patients with peripheral neuropathy brought on by chemotherapy.
Introduction
Today, cancer is the leading cause of mortality worldwide. However, due to advances technological advances, the availability of sensitive testing and diagnostic instruments, to diagnose cancer at an early stage are available and thus the use of increasingly powerful medicines, such as chemotherapeutic medications, the number of cancer survivors is increasing. Even though these cancer patients may have recovered from their illness, many of them suffer from poor outcomes as a result of several syndromes that worsen their quality of life as a result of cancer therapy [Citation1]. Chemotherapy drugs are designed to destroy rapidly dividing cancer cells; so they have a variety of targets and modes of action. Unfortunately, these drugs also affect the body’s healthy cells and tissues, which can cause many negative and at times fatal side effects (such as anaemia, diarrhoea, nausea, vomiting, infections, neurological changes, fatigue, hair loss, infertility, pain, and peripheral neuropathy). Chemotherapy regimens may have to be altered or even discontinued as a result, which would lessen the effectiveness of cancer treatment [Citation2].
Chemotherapy Induced Peripheral Neuropathy (CIPN), a sensory-predominant neuropathy, may also include disorders of the motor and autonomic systems. The onset of CIPN symptoms normally occurs within a week after chemotherapy of chemotherapy and is sustained till a few months after the conclusion of the chemotherapy cycle. The severity of these symptoms is proportional to the total dose of the drug, with the exception of paclitaxel and Oxaliplatin, which cause acute neuropathy during or immediately after infusion [Citation2]. Some individuals have paradoxical worsening and/or aggravation of symptoms after stopping their medication, as well as a syndrome known as coasting, in which either mild neuropathy grows worse or new CIPN manifests [Citation3]. Because there are no signs or symptoms that would support reducing the dosage to lessen CIPN symptoms during the chemotherapy cycle, this condition presents a dilemma for oncologists. Pain and sensory issues may last for months or even years after chemotherapy has ended. As a result, cancer treatment can cause debilitating neuropathy in cancer-free persons [Citation4].
Chemotherapy drugs can harm the neural structures and result in CIPN by a number of different pathomechanics, such as DNA damage, microtubule disruption, oxidative stress and mitochondrial damage, altered ion channel activity, myelin sheath damage, and neuroinflammation [Citation5]. Three platinum-based chemotherapeutic agents, Oxaliplatin, Cisplatin, and Carboplatin, are frequently used to treat various types of solid tumours. Small-cell lung cancer, testicular, ovarian, brain, uterine, and bladder cancers should be treated with Cisplatin and Carboplatin, whereas advanced colorectal, oesophageal, stomach, liver, and pancreatic cancers should be treated with Oxaliplatin [Citation6].
A significant drawback of platinum-based chemotherapy is acute and chronic neurotoxicity, which can lead to extended infusion periods, dose reductions, treatment delays, or even the end of therapy. Cisplatin can potentially cause ototoxicity, myelotoxicity, and nephrotoxicity in addition to peripheral neuropathy. Peripheral neuropathy caused by Cisplatin develops in a dose- and time-dependent way. Chemotherapeutic agents that have neurotoxic effects on the peripheral nerve system are utilised as conventional, regular treatments for the most prevalent cancer types [Citation7]. Six distinct agent groups, which include vinca alkaloids, epothilones, taxanes, paclitaxel, docetaxel, platinum-based antineoplastic (especially Oxaliplatin and Cisplatin), vincristine, and vinblastine, are primarily responsible for the harm caused to the peripheral sensory, motor, and autonomic neurons that results in the development of CIPN. The platinum, taxanes, ixabepilone, thalidomide, and its analogues families of anticancer drugs are the most neurotoxic; other, less neurotoxic, but nevertheless extensively used drugs include bortezomib and vinca alkaloids [Citation8].
Chemotherapeutics can cause CIPN through many different pathomechanisms, including immunological reactions, neuroinflammation, microtubule disruption, mitochondrial damage from oxidative stress, altered ion channel activity, DNA damage, and damage to the myelin sheath. The clinical profile of CIPN and the precise neurotoxicity mechanisms associated with some of the medications most frequently used in cancer chemotherapy, such as vinca alkaloids, taxanes, epothilones, ixabepilone, platinum-based antineoplastic, and proteasome inhibitors (bortezomib), are reviewed in the section that follows [Citation9].
The use of physiotherapy in the management of CIPN has not received significant research. A thorough literature search revealed that the following manual therapy, Neuro-developmental, cardiopulmonary, and electrotherapy modalities, including diathermy, Transcutaneous electrical nerve stimulation, and photobiomodulation, are the most widely used physiotherapy management techniques. Manual therapy techniques include massage. The most popular manual therapy method reportedly used to reduce CIPN-related symptoms is massage. Adults should exercise 150 min per week at a moderate intensity or 75 min at a vigorous intensity [Citation10]. In a study on colorectal cancer patients with CIPN, a relationship between CIPN symptoms and the quantity of physical activity the patients undertook each week was shown to be indirect but linear. The authors also found that patients who did not meet the weekly physical activity requirements had considerably greater CIPN symptoms than those who did, regardless of whether they were receiving chemotherapy or not. Meeting the weekly physical activity criteria led to less severe CIPN symptoms and an improvement in quality of life [Citation11]. The objective of this review is to determine if physical therapy is effective for patients with chronic chemotherapy-induced peripheral neuropathy, by carefully analysing all randomised controlled trials (RCTs).
Method
Data sources and searches
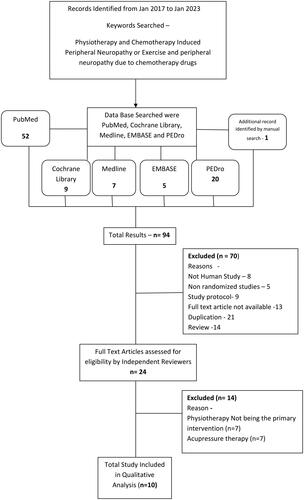
Using the search strategy described by Dickersin et al. [Citation12], a literature search was conducted. There were no restrictions on the publication’s language. EMBASE, PubMed, Physiotherapy Evidence Database (PEDro), and the Cochrane Library were searched for RCTs from their beginning in January 2017 through January 2023. The following medical subject headings were present in the title, abstract, or index word fields: ‘Chemotherapy Induced Peripheral Neuropathy’ AND ‘Physiotherapy’ OR ‘Exercise.’ Two researchers independently assessed the names of the publications located in the databases and, if available, the publications’ abstracts. If either investigator believed that any published publication might have met the inclusion criteria or if there was insufficient data to make a decision, a copy of the article was obtained or the article was requested. Searching for RCTs that may have been overlooked or not included in the databases is part of the second stage of the search technique. This stage involved manual evaluation of the reference lists in all recovered papers and the accessible systematic reviews to identify unpublished or overlooked research. We also browsed websites that contained data on clinical trials, theses, or dissertations. To keep track of which influential authors in the subject were cited the most, citation indexing was used. Local authorities were approached for more information.
Study selection
Regardless of the severity of their condition, studies including cancer patients who are or have received chemotherapy were allowed to be included in this analysis. We did not exclude any study which was based on particular gender or specific age group.
Types of interventions
If studies were RCTs, they were eligible for inclusion if they compared physical therapy interventions with a placebo condition, a control intervention, or standard care. The World Confederation for Physical Therapy’s policy statement states that experimental physical therapy interventions may consist of a combination of aerobic exercises, strength training, balance drills, basic body awareness exercises, and electrotherapeutic modalities. The main or active part of a physical therapy intervention is considered to be physical therapy, which may be used alone or in conjunction with other interventions. Interventions that included physical therapy in a multi-component weight control plan were ignored because the precise effects of the physical therapy intervention could not be addressed. Additional therapies include pharmacotherapy, psychoeducation, and cognitive-behavioral or motivational techniques relating to exercise behaviour. Standard care was defined as the care that participants would typically receive if they had opted out of the research experiment. Hospitalisation, outpatient therapy, and at-home exercise regimens are some of these services. For an RCT to be included, the duration of the experimental and comparison interventions must be comparable.
Types of outcome
Results were categorised based on assessments of the quality of life of cancer survivors, total neuropathy score pre and post-intervention and patient-rated symptoms of peripheral neuropathy brought on by chemotherapy.
Data extraction and quality evaluation
The quality assessments were done independently by the two evaluators. Disagreements were resolved through discussions. In the event that no consensus could be obtained, a third reviewer made the final decision. Using the previously devised 5-point Jadad scale [Citation13], each study’s level of RCT reporting quality and thoroughness was evaluated, as well as any potential for trial bias. These three internal validity criteria—the quality of randomisation, double-blinding, and withdrawals—are the focus of this widely used measure. This scale is the only published instrument created utilising psychometric principles. Higher scores (between 0 and 5) are awarded. Higher ratings suggest higher trial reporting or behaviour standards. Scores can be between 0 and 5. A trial with at least 3 out of 5 is considered to be of strong quality. A study is considered to have poor methodology if it has a score below 3.
Data synthesis and analysis
A PEDro rating system that Verhagan et al. created was used to evaluate each study [Citation14]. This rating methodology, which was previously applied to physical therapy systematic reviews, offers a complete evaluation of study procedures. The rating system takes into account factors pertinent to physical therapy practice, including participant characteristics, sample size, description of therapies, and the validity and reliability of the selected end measures. Each article was evaluated according to 11 criteria of PEDro Scale, the first of which was eligibility. The eligibility criteria did not receive a score, but the remaining 10 criteria did. Based on the particular criteria of this rating system, the two assessors separately examined each study. There were two possible responses for each criterion: yes (met the criterion) and no (did not satisfy the criterion). No response was also given when the publication didn’t include any details regarding a particular criterion. Each quality criterion was assessed independently. Scores ranging from 0 to 3 are regarded as poor, 4 to 5 as fair, 6 to 8 as good, and 9 to 10 are regarded as excellent. Out of the 10 articles included in the study, 2 are fair, 7 are good, and 1 was excellent.
Results
Study selection
There were 94 articles found in total after the initial electronic database search. One more potentially qualifying article was found through manual reference list searches, web searches, and discussions with subject-matter experts. Ten RCTs were included in after duplicates were eliminated and titles, abstracts, or full texts were scrutinised. In the Figure, the grounds for exclusion are displayed. We determined that there was too much variation in the designs and techniques on the basis of the initial full-text screening to conduct a formal meta-analysis.
Participants
All of the 1047 people who were included in the analysis had cancer diagnoses and had received chemotherapy treatment. Four studies included outpatients, five studies focused exclusively on exercising at home, and only one research included inpatients. One study or another covered each of the 4 stages of cancer. Breast cancer was considered in most studies. Ages 18 and older were included for participation in the study. This method included both people who were receiving chemotherapy and those who had already received it. The participants in the majority of the studies were women. offers comprehensive details on the participant characteristics.
Table 1. A thorough review of various randomised control studies was included in the systematic review.
Methodological quality
The methodological quality of four of the included studies was deemed to be weak. gives more specifics on the research characteristic. Limited sample size and a lack of masking (or ‘blinding’), particularly of participants, were the 2 most prevalent methodological problems.
Table 2. Critical appraisal of each article using PEDro (physiotherapy evidenced database) scale.
Effectiveness of resistance or strength training on chemotherapy-induced peripheral neuropathy
The researchers in 5 studies examined the effectiveness of strength or resistance training in improving neuropathy symptoms, strength, and quality of life of cancer survivors. Out of 5 studies, three studies were considered to be of good methodological quality and 2 studies had poor methodological characteristics. The study by Philipp Zimmer et al. combined resistance training along with balance and endurance training and found that it greatly improved strength and balance ability. According to the study results of Shelly Dhawan et al. exercise group showed significantly lower neuropathic pain scores and improved quality of life compared to the usual care group. Neirman Yukselturk Simsek et al. found that exercises were more effective than cold application. The Jana Muller et al. study added sensorimotor exercise training along with resistance training. The 4 studies out of 5 used the quality of life questionnaire as an outcome measure.
Effectiveness of endurance training on chemotherapy-induced peripheral neuropathy
Two out of three studies have poor methodological quality. In one study stationary cycle was used to improve endurance and the other two studies stated walking as an intervention out of which one study included gymnastics training along with walking. All three studies speculated that balance training improved patients’ functional status. Two studies used patient-reported CIPN scores as an outcome measure whereas the other study used a brief pain inventory score.
Effectiveness of Electrostimulation on chemotherapy-induced peripheral neuropathy
Only one study with good methodological quality used Electrostimulation which showed no differences in Neuropathy Rating Scores.
Effectiveness of nerve glide on chemotherapy-induced peripheral neuropathy
Only one study used home-based nerve glide exercises. It had good methodological quality. The Intervention group demonstrated significant improvements in grip dynamometry and pain pressure thresholds.
Adverse events
Out of 10 studies, 4 studies have stated the adverse events. The three studies stated that there were no adverse events noted. But in the study by Si Yeon Song et al. [Citation23] they reported minor adverse events were seen in 19 participants that included diarrhoea, lymphedema, edoema of limb etc. The limb edoema was resolved in 10 days.
Discussion
General findings
This systematic review explored that Comparative to the control group, the physical therapy treatment group experienced statistically significant increases in pain pressure thresholds, grip strength, and clinically meaningful improvements for CIPN pain on the NPRS. Patients reported pain types changed over the course of the trial and have been previously documented. In general, neuropathic symptoms increased throughout chemotherapy and gradually subsided after treatment. Entrapment, neuropathic pain, nerve healing, and regeneration are all well-established outcomes of physical therapy treatment for nerve problems. Improved pain and function, restoration of lost sensibility, and normalisation of hypersensitivity/allodynia are all goals of treatment for nerve compression injuries, crush injuries, and nerve lacerations [Citation18].
The review identified maximum studies on resistance or strength training in improving chemotherapy-induced peripheral neuropathy symptoms. The balance was found to be improved as a result of increased strength with no adverse events noted. Philipp Zimmer et al., in a palliative situation, this study was the first to demonstrate the beneficial effects of a multimodal exercise programme on CIPN, balance, and strength in patients with metastatic colorectal cancer. The findings confirm past findings that balancing has a beneficial effect on chemotherapy-induced peripheral neuropathy symptoms [Citation22].
Ian R. Kleckner et al. (2018), in their Exercise Group, prescribed walking with 60 to 85% Heart Rate Reserve and Strengthening using theraband. Patients undergoing treatment based on taxanes, platinum, or vinca alkaloids appear to experience fewer CIPN symptoms after exercising. Clinicians ought to think about advising these patients to exercise. Three RCT’s found endurance training to be effective in improving Chemotherapy-induced peripheral neuropathy symptoms. Walking was among the most common [Citation21].
In one study, both groups were engaged in endurance training on stationary bicycles for up to 30 min at a moderate intensity below their own anaerobic thresholds (IAT). Additionally, for 30 min, the IG practised balance. Speculated that balance training improved patients’ functional status, and endurance training also caused a decrease in sensory problems in both groups. This added functional impact may be a result of the IG's improved performance on the CIPN20 motor score. Both activities offer CIPN sufferers a distinct and useful advantage. Overall, the analysis found that physical therapy as an adjuvant therapy may help cancer survivors experience a better quality of life, less pain, and fewer CIPN symptoms.
Electrostimulation is being less researched; we found only one study by Si Yeon Song et al. (2020) [Citation23], interestingly the low-frequency Electrostimulation device with the pharmaceutical intervention was not substantially more effective than a placebo, yet a therapeutic impact was feasible. To our knowledge, the present review is the first to offer evidence for the effectiveness of resistance and strength exercises in improving quality of life and reducing chemotherapy-induced peripheral neuropathy symptoms.
The present review showed that resistance training, endurance training and neural glide can cause increase progressive muscle strength, and reduction in pain; improving chemotherapy-induced peripheral neuropathy symptoms and overall quality of life in cancer survivors suffering from chemotherapy-induced peripheral neuropathy.
Limitations
The review does have some limitations that need to be noted; despite the fact that we think it is the first to examine the effectiveness of various physical therapy approaches in patients with chemotherapy-induced peripheral neuropathy. First, there is a chance for selection bias in any systematic review; nonetheless, we used a thorough search method. The research data was also examined by two independent reviewers, and the explanations for any study exclusions were made explicit. Second, performance bias might constrain our results. Only one of the included studies was double-blinded. Therefore, estimations of treatment effects may be inflated by the reported outcomes. Every effort should be made to gather study data in a masked manner, even though it may not always be possible for researchers to conceal subjects to physical therapy procedures in order to eliminate the possibility of performance bias. Only 3 of the research articles included in the current evaluation were single-blind studies. Third, the current review was complicated by the heterogeneity across the RCTs, notably in terms of the frequency and length of the experimental treatment and the selected control or comparison intervention. The small number of participants and other errors in methodology in several of the included studies, along with this variability, precluded general findings and emphasised the need for more research.
Future implication
There is a definite need for effective randomised controlled trials that look at rehabilitation therapies as a supplemental form of care for persons taking chemotherapy medications. The size, power, and inclusion of accurate and reliable outcome measures are key requirements for clinically significant trials. Additionally, efforts should be taken to hide information about participants from raters, therapists, and outcome measures, as well as to hide raters’ knowledge of a person’s clinical status, group assignment, and treatment condition. When planning trials, researchers should take the conclusions of this systematic review into account. They should also make an effort to address the shortcomings of the RCTs that were presented. The question of whether short-term advantages lead to long-term changes is still largely unsolved because the majority of the RCTs retrieved in this evaluation lacked longitudinal follow-up to assess if the improvements seen after physical therapy were maintained over time. Long-term studies are therefore required to expand our understanding of how to provide physical therapy to those with chemotherapy-induced peripheral neuropathy.
Future studies should specifically outline the precise nature of a physical therapy programme, paying close attention to the length, regularity, and severity of any reported interventions. Adherence, participant characteristics (such as age, sex, length of illness, and cancer stage and type), and adverse events should all be fully disclosed. Symptoms of chemotherapy-induced peripheral neuropathy should be measured, as should broader clinical outcomes such as hospital admissions, rate of chemotherapeutic dosage, and health-related quality of life. Future research, for instance, could look at the early impacts of implementing self-managed workouts and a home-based programme to reduce the symptoms of peripheral neuropathy brought on by chemotherapy. To improve the quality of life for cancer survivors, intensive research is required to investigate novel management strategies for peripheral neuropathy brought on by chemotherapy.
Conclusion
This systematic review showed that particular physical therapy interventions, such as strength and resistance training exercises, endurance training, and nerve glide exercises, had positive effects on quality of life, balance, pain, and muscle strength in patients with chemotherapy-induced peripheral neuropathy. The effectiveness of physical therapy for cancer survivors who have chemotherapy-induced peripheral neuropathy may be supported by further study into specific aspects of physical therapy interventions, such as adapting therapies to the requirements of patients with CIPN.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly accessed and available in PubMed, Cochrane Library, Embase and Pedro
References
- Pulumati A, Pulumati A, Dwarakanath BS, et al. Technological advancements in cancer diagnostics: improvements and limitations. Cancer Rep. 2023;6(2):e1764.
- Zajączkowska R, Kocot-Kępska M, Leppert W, et al. Mechanisms of chemotherapy-induced peripheral neuropathy. Int J Mol Sci. 2019;20(6):1451. doi: 10.3390/ijms20061451.
- Staff NP, Grisold A, Grisold W, et al. Chemotherapy-induced peripheral neuropathy: a current review. Ann Neurol. 2017;81(6):772–781. doi: 10.1002/ana.24951.
- Colvin LA. Chemotherapy-induced peripheral neuropathy (CIPN): where are we now? Pain . 2019;160 (Suppl 1): S1–S10. doi: 10.1097/j.pain.0000000000001540.
- Areti A, Yerra VG, Naidu VGM, et al. Oxidative stress and nerve damage: role in chemotherapy induced peripheral neuropathy. Redox Biol. 2014;2:289–295. doi: 10.1016/j.redox.2014.01.006.
- McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 2009;8(1):10–16. doi: 10.1158/1535-7163.MCT-08-0840.
- Starobova H, Vetter I. Pathophysiology of chemotherapy-induced peripheral neuropathy. Front Mol Neurosci. 2017;10:174. doi: 10.3389/fnmol.2017.00174.
- Park SB, Cetinkaya-Fisgin A, Argyriou AA, et al. Axonal degeneration in chemotherapy-induced peripheral neurotoxicity: clinical and experimental evidence. J Neurol Neurosurg Psychiatry. 2023; doi: 10.1136/jnnp-2021-328323.
- Han Y, Smith MTP. Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN). Front Pharmacol. 2013;4:156. doi: 10.3389/fphar.2013.00156.
- Niemand EA, Cochrane ME, Eksteen CA. Physiotherapy management of chemotherapy-induced peripheral neuropathy in pretoria, South Africa. South Afr J Physiother. 2020;76(1):1482.
- Whalen LB, Wright WZ, Kundur P, et al. Beneficial effects of exercise on chemotherapy-induced peripheral neuropathy and sleep disturbance: a review of literature and proposed mechanisms. Gynecol Oncol Rep. 2022;39:100927.
- Dickersin K, Chan S, Chalmers TC, et al. Publication bias and clinical trials. Control Clin Trials. 1987;8(4):343–353.
- Berger VW, Alperson SY. A general framework for the evaluation of clinical trial quality. Rev Recent Clin Trials. 2009;4(2):79–88. doi: 10.2174/157488709788186021.
- Paci M, Bianchini C, Baccini M. Reliability of the PEDro scale: comparison between trials published in predatory and non-predatory journals. Arch Physiother. 2022;12(1):10. doi: 10.1186/s40945-022-00133-6.
- Şimşek NY, Demir A. Cold application and exercise on development of peripheral neuropathy during taxane chemotherapy in breast cancer patients: a randomized controlled trial. Asia Pac J Oncol Nurs. 2021;824(3):255–266. doi: 10.4103/apjon.apjon-2075.
- PA A, KV B, MA G, et al. The effect of photobiomodulation on chemotherapy-induced peripheral neuropathy: a randomized, sham-controlled clinical trial. Gynecol Oncol. 2017;144(1):159–166.
- Andersen Hammond E, Pitz M, Steinfeld K, et al. An exploratory randomized trial of physical therapy for the treatment of chemotherapy-induced peripheral neuropathy. Neurorehabil Neural Repair. 2020;34(3):235–246.
- Dhawan S, Andrews R, Kumar L, et al. A randomized controlled trial to assess the effectiveness of muscle strengthening and balancing exercises on chemotherapy-induced peripheral neuropathic pain and quality of life among cancer patients. Cancer Nurs. 2020;43(0 ):269–280.
- Bland KA, Kirkham AA, Bovard J, et al. Effect of exercise on taxane chemotherapy-induced peripheral neuropathy in women with breast cancer: a randomized controlled trial. Clin Breast Cancer. 2019;19( 6):411–422.
- Kneis S, Wehrle A, Müller J, et al. It’s never too late – balance and endurance training improves functional performance, quality of life, and alleviates neuropathic symptoms in cancer survivors suffering from chemotherapy-induced peripheral neuropathy: results of a randomized controlled trial. BMC Cancer. 2019;19( 1):414.
- Kleckner IR, Kamen C, Gewandter JS, et al. Effects of exercise during chemotherapy on chemotherapy-induced peripheral neuropathy: a multicenter, randomized controlled trial. Support Care Cancer. 2018; 26( 4):1019–1028.
- Zimmer P, Trebing S, Timmers-Trebing U, et al. Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: a randomized controlled trial. Support Care Cancer. 2018;26( 2):615–624.
- Song S-Y, Park J-H, Lee JS, et al. A randomized, placebo-controlled trial evaluating changes in peripheral neuropathy and quality of life by using low-frequency electrostimulation on breast cancer patients treated with chemotherapy. Integr Cancer Ther. 2020;19:1534735420925519. doi: 10.1177/1534735420925519.
- Müller J, Weiler M, Schneeweiss A, et al. Preventive effect of sensorimotor exercise and resistance training on chemotherapy-induced peripheral neuropathy: a randomised-controlled trial. Br J Cancer. 2021;125(7):955–965.