Abstract
Background
Merkel cell carcinoma (MCC) is a rare, high-grade neuroendocrine neoplasm (NEN) of the skin. Somatostatin receptors (SSTRs) are G protein-linked receptors that regulate cell proliferation and growth. SSTRs are expressed in many NENs; however, scant information is available on their expression in MCCs or their association with clinical parameters and patient outcomes.
Material and methods
This retrospective study was conducted at Helsinki University Hospital and the University of Helsinki. Using a tissue microarray, we investigated SSTR1-5 expression by immunohistochemistry in 99 MCC tissue samples. Samples were collected between 1983 and 2017 and coupled with the patients’ clinical data.
Results
SSTR2-SSTR5 were detected in 69%, 6%, 4%, and 1% of the tumours, respectively. However, SSTR1 expression was not observed. Cytoplasmic SSTR2 positivity was associated with metastatic disease at the time of diagnosis (p = 0.009), but it did not correlate with disease-specificity or overall survival.
Conclusion
SSTR2-5 expression was observed in MCCs. In particular, SSTR2 expression is clinically valid because it is associated with metastatic disease at the time of diagnosis and can thus serve as a prognostic marker. Moreover, SSTR2 overexpression provides a molecular basis for tumour imaging and treatment with somatostatin analogues.
Background
Merkel cell carcinoma (MCC) is a rare, high-grade neuroendocrine skin neoplasm [Citation1,Citation2]. The incidence reported in Europe was 0.13 per 100 000 persons between 1995 and 2002, and in the USA 0.7 per 100 000 persons in 2013 [Citation3,Citation4]. The 5-year survival rate for MCC varies between 23% and 75% depending on the disease spread [Citation5]. MCC was overrepresented in men [Citation6,Citation7]. Men with MCC have a lower 10-year survival rate than women [Citation6].
There are two known pathogenic pathways in MCC. The first one is related to ultraviolet light, and the other involves Merkel cell polyoma virus (MCPyV), which is found in up to 80% of the MCCs [Citation1,Citation3,Citation8,Citation9]. These aetiologies may also overlap. Virus-negative MCCs (MCPyV-) have a larger mutation burden than MCPyV positive (MCPyV+) MCCs [Citation10]. The larger mutation burden of MCPyV-MCCs includes the silencing of tumour suppressor genes, such as tumour protein 53 (TP53) and retinoblastoma protein (RB1) [Citation11].
MCCs are usually located in the skin of the head and limbs; however, MCPyV + tumours are more frequently found in the upper or lower limbs. Patients with MCPyV + tumours have better outcomes, most likely due to a higher antitumour immune response [Citation6,Citation8].
There are five different human somatostatin receptors (SSTR), SSTR1, SSTR2, SSTR3, SSTR4, and SSTR5. They are G-protein-linked transmembrane receptors [Citation12]. SSTRs’ ligands are somatostatins with a broad range of biological actions. Somatostatins exert antitumour activity by inhibiting tumour angiogenesis and the production of tumour growth-inducing factors [Citation13]. SSTRs are expressed in several normal human tissues and their expression is increased in neuroendocrine tumours (NET) [Citation14,Citation15]. This overexpression provides a molecular basis for using somatostatin analogues (SSAs) and peptide receptor radionuclide therapy (PRRT) as therapeutic options and in PET/CT radiography as contrast ligands [Citation16–21].
Few studies have examined SSTR expression in MCCs. These previous studies have focused on in vivo tumour imaging with SSTR-binding agents as contrast agents [Citation16]. There are few reports on the immunohistochemical expression of SSTR2 and SSTR5; however, they present contradictory results [Citation22,Citation23].
Here, we aimed to study the immunohistochemical expression of all five SSTRs in a large cohort of 99 MCC tumours, combined with patient clinical data. Our aim was to evaluate the prognostic and predictive roles of SSTR expression in MCCs.
Material and methods
Patient cohort
This retrospective study was conducted using the previously described methods [Citation24]. We collected 171 MCC tumours from Finland, from which 99 had enough tissue material for this study. The MCC diagnosis was set between 1983 and 2017. Clinical data of the patients were obtained from the Finnish Cancer Registry (Helsinki, Finland) and patient records were maintained at the Helsinki University Hospital (HUH, Helsinki, Finland). Formalin-fixed paraffin-embedded tissue blocks were retrieved from pathology archives.
The diagnosis of MCC was reconfirmed by a pathologist (TB) with special expertise in MCC, as described earlier [Citation24]. Staging was performed according to the American Joint Committee on Cancer classification of Merkel cell carcinoma (eighth edition) [Citation25]. The epidemiological and clinical data of the patients are presented in . The median survival follow-up time was 2.14 years (range 0.03–33.18 years).
Table 1. The clinicopathological variables, their median, range, and distribution.
The collection of patient data was approved by the Ministry of Health and Social Affairs, and the collection of tissue samples and their analysis were approved by the National Supervisory Authority for Welfare and Health (VALVIRA, Helsinki, Finland) (Code:4942/05.01.00.06/2009), which also waived the need for written informed consent. HUH’s ethics committee approved the study.
Tissue microarray and immunohistochemistry
Tissue microarrays (TMAs) were constructed as described previously [Citation26]. Depending on the tumour material available, one (n = 41), two (n = 47) or 3–6 (n = 11) representative 1.0 mm cores were taken from each formalin-fixed paraffin-embedded (FFPE) tumour tissue block and placed in the recipient TMA block.
SSTR1 and 3–5 staining’s were performed in our research laboratory, and SSTR2 was performed in the diagnostic laboratory of the HUH Diagnostic Centre. All staining protocols were optimised in advance [Citation27]. Normal endocrine pancreas, Langerhans islets and epithelial neuroendocrine cells in small intestine served as positive controls for SSTR1 and SSTR3-5 while exocrine pancreas, acinar cells and epithelial non-neuroendocrine cells in small intestine served as negative controls. Neuroendocrine cells in normal colon tissue were used as a positive control for SSTR2 while non-neuroendocrine cells served as a negative control. Detailed information on the primary antibodies is provided in Supplementary Table 1.
Immunohistochemistry (IHC) was performed as previously described [Citation28,Citation29]. Briefly, 3.5 µm tissue sections were de-paraffinized, heat-induced antigen retrieval was performed, and the slides were incubated with primary antibodies. Antibody binding was visualised using either the OptiView Universal DAB Detection Kit (Ventana Medical System, Inc., Tucson, AZ, USA) for SSTR2 or EnVision Detection Systems (Dako, Agilent Pathology Solutions) for SSTR1 and SSTR3-5.
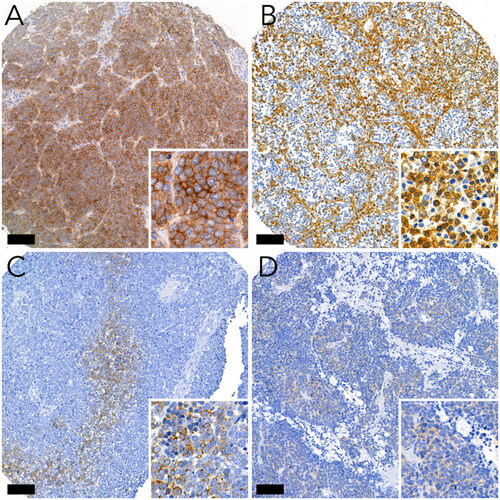
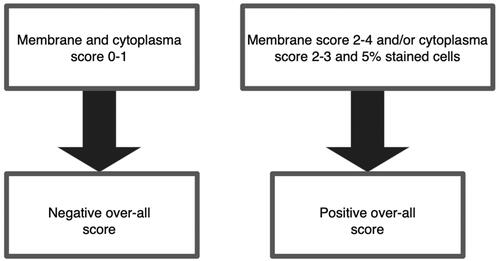
Two researchers (HL and TV) scored the SSTR stainings independently (without knowing the clinicopathological variables), and if there was a discrepancy in the scoring, a consensus was reached via discussion. Membrane staining scores are shown in Supplementary Table 2. The cytoplasmic score was calculated based on the staining intensity (0, negative; 1, weak; 2, intermediate; and 3, strong). Values 1–3 were given only if more than 5% of the tumour cells were stained. Examples of these scores are shown in . The overall score is calculated according to the rules shown in . If the scores varied in parallel TMA cores from the same tumour, the highest score was selected for further analysis.
Statistical analysis
The scoring results were analysed against the clinical data. IBM SPSS Statistics version 27 (IBM Corp., Armonk, NY, US) was used for the statistical analysis. Fischer’s exact test and χ2 test were used for the analysing the association between SSTR expression and categorial variables. The Mann–Whitney U test was used to determine the association between SSTR expression, tumour size, and age at diagnosis. Survival rates were analysed using Kaplan–Meier analysis and the log-rank test. To exclude confounding factors in any significant correlations, multivariate logarithmic regression was used with backwards stepwise model selection. Statistical significance was set at p < 0.05 (two tailed) was interpretated as statistically significant.
Results
Expression of SSTRs in MCCs
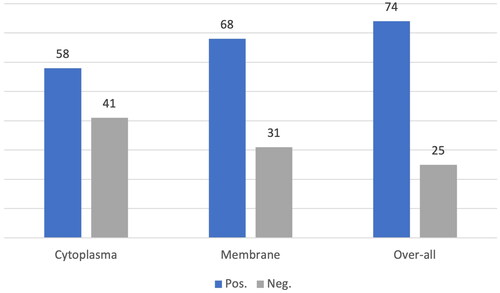
SSTR2 is the most abundant SSTR subtype in MCC. The distribution and staining intensity between different tumours were heterogeneous. Seven tumours were positive for cytoplasmic staining, whereas 17 were exclusively membrane-positive. The distribution of the SSTR2 scores is shown in . SSTR2 membrane score in parallel TMA cores from the same tumour was homogenous in 45 tumours. Heterogeneity between cores within the case was found in 13 cases.
SSTR1 was completely absent, and no cytoplasmic or membranous expression was observed. Five tumours showed cytoplasmic SSTR3 expression, and two showed membranous expression. In addition, only membranous SSTR3 expression was observed in one tumour. Of the three tumours showing SSTR4 cytoplasmic expression, one showed membrane expression. One tumour exhibited only membranous SSTR4 expression. Membranous SSTR5 expression was detected in only one tumour.
SSTR2 expression and clinicopathological data
The association of SSTR2 with clinical parameters requires further analysis. However, the number of positive tumours was too small to draw any conclusions regarding SSTR1 and SSTR3–5.
Positive expression of SSTR2 in the cytoplasm was associated with metastatic disease at the time of diagnosis (p = 0.009). In the cytoplasm, SSTR2 was positive in thirteen out of the 15 metastatic MCCs and 48% of non-metastatic MCCs. Over-all positive SSTR2 or positive membrane expression was not regarded as statistically significant in relation to local or metastatic disease. The statistically significant finding between SSTR2 cytoplasmic positivity and metastatic disease was controlled for confounding factors, such as: Age, Gender, Location, MCPyV expression and tumour size. None of these were confounding.
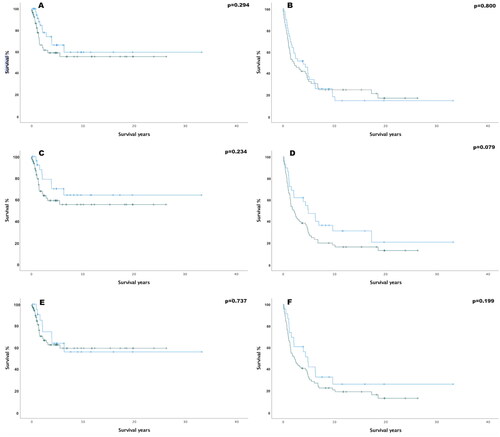
SSTR2 expression did not correlate with disease-specific or overall survival. Patients with tumours showing positive membrane expression of SSTR2 showed a trend towards poorer overall survival (p = 0.079). The MCC-specific survival and overall survival rates are shown in .
Figure 4. Kaplan–Meier curves of SSTR2 (five-year survival-%) (A) cytoplasmic disease specific survival (SSTR2 Pos. 59.2%, SSTR2 Neg. 66.4%, hazard ratio (HR)= 1.22, (95%-confidence interval (95%-CI) = 0.88–1.69). (B) Cytoplasmic overall survival (SSTR2 Pos. 34.2%, SSTR2 Neg. 37%, HR= 1.07 95%-CI= 0.86–1.32) (C) Membranous disease specific survival (SSTR2 Pos. 59.3%, SSTR2 Neg. 70.2%, HR= 1.62, 95%-CI= 0.73–3.64). (D) Membranous overall survival (SSTR2 Pos. 30.1%, SSTR2 Neg. 47.5%, HR= 1.58, 95%-CI 0.94–2.65). (E) Overall positivity disease specific survival (SSTR2 Pos. 62.5%, SSTR2 Neg. 63.9%, HR= 1.15, 95%-CI= 0.51–2.57). (F) Overall positivity in overall survival (SSTR2 Pos. 33.3%, SSTR2 Neg. 42.1%, HR= 1.43, 95%-CI= 0.82–2.50). In all the Kaplan–Meier plots, the blue graph represents negative SSTR2 expression survival rate and the green one represents positive expression survival rate.
There was no correlation between MCPyV positivity and SSTR2 score (data not shown). No correlation was found between positive SSTR2 expression and, tumour size, tumour location, sex, or age at diagnosis (data not shown).
Discussion
Here, we present the case of a large cohort of 99 patients with MCC. We showed that immunohistochemically, SSTR2-5 was present in MCCs, whereas SSTR1 was absent. The most frequently found receptor subtype was SSTR2, and its cytoplasmic expression correlated with the stage of the disease at the time of diagnosis.
In the study’s MCC series, positive SSTR2 staining was observed in the membrane and cytoplasm. The antibodies used in this study were also used in our previous studies on pheochromocytomas, paragangliomas [Citation30], pulmonary carcinoid tumours [Citation29], pancreatic neuroendocrine neoplasia, [Citation28] and parathyroid neoplasms [Citation31]. It was previously suggested that weak cytoplasmic staining could be a false positive; thus, we grouped scores 0–1 as negative [Citation31]. A membranous staining pattern of SSTR2 is usually observed in NETs [Citation27]. In pheochromocytomas, paragangliomas, and parathyroid carcinomas, SSTR2 staining is most commonly membranous [Citation30,Citation31]. We observed that in the MCCs, SSTR2 expression could be in the membrane, cytoplasm, or both.
There are only a few reports on SSTR expression in MCCs, which show contradictory results. Gardair et al. [Citation23] found SSTR2 positivity in 59% of MCC (n = 98); however, they did not find any correlation between SSTR2 and disease severity. Orlova et al. reported SSTR2 positivity in 88% of tumours (n = 32) and suggested that SSTR2 is a prognostic marker for better survival in MCC [Citation22]. Both studies analysed only membranous SSTR2 staining. Another study showed six of nine MCC tumours were SSTR2 positive [Citation2], but the results lacked survival data. We also studied cytoplasmic SSTR2 expression and found an association with the metastatic disease rather than better survival. None of the previous reports have found any correlation between SSTR2 or SSTR5 expression and metastasis at the time of diagnosis.
SSTR5 expression was reported in 13–45% of MCCs in previous studies [Citation22]. The small number of SSTR5 positivity (1%) may be explained by different scoring criteria. Another possible explanation could be the differences in immunohistochemistry. To our knowledge, no previous reports have been published on the immunohistochemical SSTR1, 3, or 4 expressions.
SSTR1 was the only negative SSTR subtype identified in the present study. Although only a few SSTR3-5 positive MCCs were found in this study, their expression may have clinical relevance in imaging and treatment and thus should be analysed.
Here we report the correlation between cytoplasmic SSTR2 expression and metastatic disease at the time of diagnosis. One possible explanation for the role of cytoplasmic staining of SSTR2 is due to its ability to internalise from the cell surface. The cytoskeletal protein, filamin A (FLNA), plays a role in the internalisation of SSTR2 in growth hormone-secreting pituitary tumours [Citation32,Citation33]. Cytoplasmic FLNA promotes actin polymerisation and metastasis in prostate cancer [Citation34]. The presence of FLNA could explain why cytoplasmic SSTR2 levels correlate with metastatic disease in MCC. No previous clinical reports have directly correlated internalised SSTR2 with metastatic disease. However, the hypothesis of SSTR2 internalisation requires further investigation.
SSTR expression is usually abundant in low-grade tumours, such as NETs, and absent in high-grade tumours, such as neuroendocrine carcinomas. Hence, our finding of SSTR2 expression in MCC is somewhat surprising. SSTR2 correlates with MCPyV-positive status [Citation23]. However, we could not confirm these results. The literature shows up to 80% of the MCC tumours are MCPyV positive [Citation3,Citation8]. In our study, 64.3% of the cases were MCPyV positive, which is in line with the numbers from the literature. In Australia the MCPyV positivity is much lower (24%) [Citation35]. This is thought to be because of the high UV-index and fair skin of the population. As mentioned earlier, the MCPyV relation to SSTR2 expression has showed contradictory results. SSTR2 expression should be studied in different populations and ethnicities to investigate the difference in UV and MCPyV aetiological pathways in MCC.
Moreover, SSTRs are used as therapeutic targets. SSAs bind to SSTRs and inhibit both the proliferation and hormonal secretion of neuroendocrine neoplasms [Citation36]. Regarding MCC, SSA therapy is little studied [Citation2,Citation37]. SSTRs can also be used as therapeutic targets for PRRT [Citation21]. Their function in contrast imaging is another reason to target the somatostatin receptors [Citation28].
Based on a case report presented by Guida et al. PD1/PDL1 treatment together with SSA shows a potential therapeutic option in MCC with high SSTR expression [Citation37]. PD1/PDL1 treatment is also suggested to have a positive effect on over-all survival in malignancies with high SSTR2 expression [Citation38]. The correlation between SSTR2 expression and PD1/PDL1 treatment efficiency, and SSTR2 as a prognostic factor in MCC needs to be evaluated in further prospective studies.
SSTR2 immunohistochemistry is used in routine diagnostic pathology. Its expression correlates with SSTR imaging in e.g. pancreatic neuroendocrine tumours and thus it can also be considered as a surrogate marker for somatostatin analogue treatment [Citation39]. However, the value of SSTR2 in MCC needs further validation.
We found SSTR2 expression in most tumours and a few positive tumours for SSTR3-5. This makes SSTR2 of interest in imaging and as treatment option for MCC. In the era of personalised medicine, this may play a role in treating MCCs.
The strength of our study is the large tumour cohort with clinical data. In addition, all tumours were re-evaluated for histological diagnosis. One limitation could be that we used TMA technology which may not fully represent the whole tumour due to the possibility of sampling error. Previous studies have shown spatial heterogenous SSTR2 expression patterns in other NEN tumours, and this may also be the case of MCC [Citation40,Citation41]. In our material, 76% of the membranous staining’s were homogenous and the cytoplasmic staining were 83% homogenous. Female gender was over-represented in our material (73%) compared to some studies where the male gender was more prominent [Citation6,Citation7]. The female predominance in our material may be because of the increase of MCC incidence at higher age and the higher life expectancy of females in Finland [Citation8].
Here, we demonstrated that SSTR2-5 may play a role in MCCs, and that positive cytoplasmic expression of SSTR2 was associated with metastatic MCCs. Thus, our research supports the use of SSTR2 as a potential prognostic marker. However, SSTR3-5 should also be considered as therapeutic or contrast imaging targets, as individual cases may benefit from them in the future.
Ethical statement
The study protocol was approved by the Ethics Committee of the Helsinki University Hospital. The Ministry of Health and Social Affairs approved the data collection. The National Supervisory Authority for Welfare and Health granted permission to collect tissue samples.
Supplemental Material
Download MS Word (21.7 KB)Acknowledgments
We would like to acknowledge Jenni Niinimäki and Eija Heiliö for excellent technical assistance.
Disclosure statement
The authors report no conflicts of interest.
Data availability statement
Due to the nature of the research, clinical data is not available. The original scores adjoined to the TMA is available at request.
Additional information
Funding
References
- Hodgson NC. Merkel cell carcinoma: changing incidence trends. J Surg Oncol. 2005;89(1):1–4. doi: 10.1002/jso.20167.
- Akaike T, Qazi J, Anderson A, et al. High somatostatin receptor expression and efficacy of somatostatin analogues in patients with metastatic merkel cell carcinoma. Br J Dermatol. 2021;184(2):319–327. doi: 10.1111/bjd.19150.
- Schadendorf D, Lebbé C, Zur Hausen A, et al. Merkel cell carcinoma: epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer. 2017;71:53–69. doi: 10.1016/j.ejca.2016.10.022.
- Paulson KG, Park SY, Vandeven NA, et al. Merkel cell carcinoma: current US incidence and projected increases based on changing demographics. J Am Acad Dermatol. 2018;78(3):457–463.e2. doi: 10.1016/j.jaad.2017.10.028.
- Survival Rates for Merkel Cell Carcinoma [Internet]. 2022 [cited 2022 May 4]. Available from: https://www.cancer.org/cancer/merkel-cell-skin-cancer/detection-diagnosis-staging/survival-rates.html#references.
- Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2010;37(1):20–27. doi: 10.1111/j.1600-0560.2009.01370.x.
- Youlden DR, Soyer HP, Youl PH, et al. Incidence and survival for merkel cell carcinoma in Queensland, Australia, 1993-2010. JAMA Dermatol. 2014;150(8):864–872. doi: 10.1001/jamadermatol.2014.124.
- Sihto H, Kukko H, Koljonen V, et al. Clinical factors associated with merkel cell polyomavirus infection in merkel cell carcinoma. J Natl Cancer Inst. 2009;101(13):938–945. doi: 10.1093/jnci/djp139.
- Zur Hausen A, Rennspiess D, Winnepenninckx V, et al. Early B-Cell differentiation in merkel cell carcinomas: clues to cellular ancestry. Cancer Res. 2013;73(16):4982–4987. doi: 10.1158/0008-5472.CAN-13-0616.
- Moshiri AS, Doumani R, Yelistratova L, et al. Polyomavirus-Negative merkel cell carcinoma: a more aggressive subtype based on analysis of 282 cases using multimodal tumor virus detection. J Invest Dermatol. 2017;137(4):819–827. doi: 10.1016/j.jid.2016.10.028.
- Harms PW, Harms KL, Moore PS, et al. The biology and treatment of merkel cell carcinoma: current understanding and research priorities. Nat Rev Clin Oncol. 2018;15(12):763–776. doi: 10.1038/s41571-018-0103-2.
- Sihto H, Kukko H, Koljonen V, et al. Merkel cell polyomavirus infection, large T antigen, retinoblastoma protein and outcome in merkel cell carcinoma. Clin Cancer Res. 2011;17(14):4806–4813. doi: 10.1158/1078-0432.CCR-10-3363.
- Rossi V, di Zazzo E, Galasso G, et al. Estrogens modulate somatostatin receptors expression and synergize with the somatostatin analog pasireotide in prostate cells. Front Pharmacol. 2019;10:28. doi: 10.3389/fphar.2019.00028.
- Theodoropoulou M, Stalla GK. Somatostatin receptors: from signaling to clinical practice. Front Neuroendocrinol. 2013;34(3):228–252. doi: 10.1016/j.yfrne.2013.07.005.
- Öberg KE, Reubi J, Kwekkeboom DJ, et al. Role of somatostatins in gastroenteropancreatic neuroendocrine tumor development and therapy. Gastroenterology. 2010;139(3):742–753.e1. doi: 10.1053/j.gastro.2010.07.002.
- Zou YI, Tan H, Zhao Y, et al. Expression and selective activation of somatostatin receptor subtypes induces cell cycle arrest in cancer cells. Oncol Lett. 2019;17(2):1723–1731. doi: 10.3892/ol.2018.9773.
- Buder K, Lapa C, Kreissl MC, et al. Somatostatin receptor expression in merkel cell carcinoma as target for molecular imaging. BMC Cancer. 2014;14(1):268. doi: 10.1186/1471-2407-14-268.
- Giovannini E, Giovacchini G, Borsò E, et al. [68Ga]-dota peptide PET/CT in neuroendocrine tumors: main clinical applications. Curr Radiopharm. 2019;12(1):11–22. doi: 10.2174/1874471012666181212101244.
- Yoo J, Kim SH, Jeon SK, et al. Added value of [68Ga]Ga-DOTA-TOC PET/CT for characterizing pancreatic neuroendocrine neoplasms: a comparison with contrast-enhanced CT and/or MRI in a large study cohort. Eur Radiol. 2021;31(10):7734–7745. doi: 10.1007/s00330-021-07859-0.
- Rinzivillo M, de Felice I, Magi L, et al. Octreotide long-acting release (LAR) in combination with other therapies for treatment of neuroendocrine neoplasia: a systematic review. J Gastrointest Oncol. 2021;12(2):845–855. doi: 10.21037/jgo-20-292.
- Delpassand ES, Tworowska I, Esfandiari R, et al. Targeted α-Emitter therapy with 212Pb-DOTAMTATE for the treatment of metastatic SSTR-Expressing neuroendocrine tumors: first-in-Humans Dose-Escalation clinical trial. J Nucl Med. 2022;63(9):1326–1333. doi: 10.2967/jnumed.121.263230.
- Askari E, Moghadam SZ, Wild D, et al. Peptide receptor radionuclide therapy in merkel cell carcinoma: a comprehensive review. J Nucl Med Technol. 2023;51(1):22–25. doi: 10.2967/jnmt.122.264904.
- Orlova KV, Delektorskaya VV, Vishnevskaya YV, et al. Somatostatin receptor type 2 expression in merkel cell carcinoma as a prognostic factor. J Eur Acad Dermatol Venereol. 2018;32(6):e236–7–e237. doi: 10.1111/jdv.14769.
- Gardair C, Samimi M, Touzé A, et al. Somatostatin receptors 2A and 5 are expressed in merkel cell carcinoma with no association with disease severity. Neuroendocrinology. 2015;101(3):223–235. doi: 10.1159/000381062.
- Sundqvist B, Sihto H, von Willebrand M, et al. LRIG1 is a positive prognostic marker in merkel cell carcinoma and merkel cell carcinoma expresses epithelial stem cell markers. Virchows Arch. 2021;479(6):1197–1207. doi: 10.1007/s00428-021-03158-7.
- Brierley J, Gospodarowicz M, Wittekind C, et al. TNM classification of malignant tumours. Eight edit. ÓSullivan O, Mason M, Asamura H, Lee A, van Eycken E, Denny L editors. Oxford: John Wiley & Sons, Ltd; 2017. p.147–149.
- Jaatinen J, Veija T, Salmikangas M, et al. ALK is frequently phosphorylated in merkel cell carcinoma and associates with longer survival. PLoS One. 2021;16(5):e0252099. doi: 10.1371/journal.pone.0252099.
- Remes SM, Leijon HL, Vesterinen TJ, et al. Immunohistochemical expression of somatostatin receptor subtypes in a panel of neuroendocrine neoplasias. J Histochem Cytochem. 2019;67(10):735–743. doi: 10.1369/0022155419856900.
- Majala S, Vesterinen T, Seppänen H, et al. Correlation of somatostatin receptor 1–5 expression, [68ga]ga-dotanoc, [18f]f-fdg pet/ct and clinical outcome in a prospective cohort of pancreatic neuroendocrine neoplasms. Cancers. 2022;14(1):162. doi: 10.3390/cancers14010162.
- Vesterinen T, Leijon H, Mustonen H, et al. Somatostatin receptor expression is associated with metastasis and patient outcome in pulmonary carcinoid tumors. J Clin Endocrinol Metab. 2019;104(6):2083–2093. doi: 10.1210/jc.2018-01931.
- Leijon H, Remes S, Hagström J, et al. Variable somatostatin receptor subtype expression in 151 primary pheochromocytomas and paragangliomas. Hum Pathol. 2019;86:66–75. doi: 10.1016/j.humpath.2018.11.020.
- Storvall S, Leijon H, Ryhänen E, et al. Somatostatin receptor expression in parathyroid neoplasms. Endocr Connect. 2019;8(8):1213–1223. doi: 10.1530/EC-19-0260.
- Treppiedi D, Jobin ML, Peverelli E, et al. Single-Molecule microscopy reveals dynamic FLNA interactions governing SSTR2 clustering and internalization. Endocrinology. 2018;159(8):2953–2965. doi: 10.1210/en.2018-00368.
- Mantovani G, Treppiedi D, Giardino E, et al. Cytoskeleton actin-binding proteins in clinical behavior of pituitary tumors. Endocr Relat Cancer. 2019;26(2):R95–R108. doi: 10.1530/ERC-18-0442.
- Bedolla RG, Wang Y, Asuncion A, et al. Nuclear versus cytoplasmic localization of filamin a in prostate cancer: immunohistochemical correlation with metastases. Clin Cancer Res. 2009;15(3):788–796. doi: 10.1158/1078-0432.CCR-08-1402.
- Garneski KM, Warcola AH, Feng Q, et al. Merkel cell polyomavirus is more frequently present in North American than Australian merkel cell carcinoma tumors. J Invest Dermatol. 2009;129(1):246–248. doi: 10.1038/jid.2008.229.
- Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev. 2003;24(4):389–427. doi: 10.1210/er.2002-0007.
- Guida M, D’Alò A, Mangia A, et al. Somatostatin receptors in Merkel-Cell carcinoma: a therapeutic opportunity using somatostatin analog alone or in association with checkpoint inhibitors immunotherapy. A case report. Front Oncol. 2020;10:1073. doi: 10.3389/fonc.2020.01073.
- Majala S, Seppänen H, Kemppainen J, et al. Prediction of the aggressiveness of non-functional pancreatic neuroendocrine tumors based on the dual-tracer PET/CT. EJNMMI Res. 2019;9(1):116. doi: 10.1186/s13550-019-0585-7.
- Wang A, Yuan Y, Chu H, et al. Somatostatin receptor 2: a potential predictive biomarker for immune checkpoint inhibitor treatment. Pathol Oncol Res. 2022;28:1610196. doi: 10.3389/pore.2022.1610196.
- Fotouhi O, Zedenius J, Höög A, et al. Regional differences in somatostatin receptor 2 (SSTR2) immunoreactivity is coupled to level of bowel invasion in small intestinal neuroendocrine tumors. Neuro Endocrinol Lett. 2018;39(4):305–309.