Abstract
Objective
Most patients receive whole breast radiotherapy in a supine position. However, two randomised trials showed lower acute toxicity in prone position. Furthermore, in most patients, prone positioning reduced doses to the organs at risk. To confirm these findings, we compared toxicity outcomes, photographic assessment, and dosimetry between both positions using REQUITE data.
Methods
REQUITE is an international multi-centre prospective observational study that recruited 2069 breast cancer patients receiving radiotherapy. Data on toxicity, health-related quality of life (HRQoL), and dosimetry were collected, as well as a photographic assessment. A matched case control analysis compared patients treated prone (n = 268) versus supine (n = 493). Exact matching was performed for the use of intensity-modulated radiotherapy, boost, lymph node irradiation, chemotherapy and fractionation, and the nearest neighbour for breast volume. Primary endpoints were dermatitis at the end of radiotherapy, and atrophy and cosmetic outcome by photographic assessment at two years.
Results
At the last treatment fraction, there was no significant difference in dermatitis (p = .28) or any HRQoL domain, but prone positioning increased the risk of breast oedema (p < .001). At 2 years, patients treated in prone position had less atrophy (p = .01), and higher body image (p < .001), and social functioning (p < .001) scores. The photographic assessment showed no difference in cosmesis at 2 years (p = .22). In prone position, mean heart dose (MHD) was significantly lower for left-sided patients (1.29 Gy vs 2.10 Gy, p < .001) and ipsilateral mean lung dose (MLD) was significantly lower for all patients (2.77 Gy vs 5.89 Gy, p < .001).
Conclusions
Prone radiotherapy showed lower MLD and MHD compared to supine position, although the risk of developing breast oedema during radiotherapy was higher. At 2 years the photographic assessment showed no difference in the cosmetic outcome, but less atrophy was seen in prone-treated patients and this seems to have a positive influence on the HRQoL domain of body image.
Introduction
Whole breast irradiation (WBI) after breast-conserving surgery (BCS) results in better overall survival, but the benefit is partly undone by secondary heart disease and lung cancer [Citation1–4]. Several methods to reduce organs-at-risk (OAR) dose have been developed including deep inspiration breath hold (DIBH), prone positioning, and better planning techniques [Citation5]. Usually, WBI is performed in supine position, but several studies have found better dosimetric results when treating in prone position, especially in patients with larger breasts [Citation6–9]. A recent comparison of supine DIBH and prone position with free breathing found prone as the optimal position in 62% of patients, most notably for lung dose [Citation10]. Besides better dosimetry, other advantages have been described, including lower rates of acute and late toxicity [Citation11–14]. Two randomised trials compared the acute toxicity between both positions for large-breasted women and both studies found a reduction in de rate of acute toxicity [Citation12,Citation14]. Of these two trials, one trial also reported a reduction in late toxicity, but no results on quality of life [Citation11].
REQUITE (www.requite.eu) is a large prospective multi-centre cohort study of patients undergoing radiotherapy for breast, prostate, or lung cancer [Citation15]. Over 2000 breast cancer patients were included and prospective data collection was done using standardised case report forms. Very detailed information is available for each individual patient including, but not limited to, fractionation, treatment techniques, and breast volume. To confirm the advantages of prone positioning, we performed a matched case-control analysis using data from the REQUITE breast cohort [Citation15]. Our analysis compares the differences between prone and supine positions for toxicity and patient-reported health-related quality of life (HRQoL), both acute and at 2 years. In addition, a dosimetric comparison was performed.
Materials and methods
Study population
REQUITE is an international multi-centre study using prospective standardised data collection with the aim to validate prediction models for late toxicity. From April 2014 until March 2017, the study recruited 4438 patients in 26 hospitals, of which 2069 were breast cancer patients (99% of target). The inclusion criteria were patients suitable for adjuvant radiotherapy (RT) after breast-conserving surgery including patients receiving (neo-)adjuvant chemotherapy, with the last cycle at least 2 weeks before the start of WBI. All patients had planned potentially curable RT according to the local regimes. The choice of treatment position was based on the local treatment protocol. Exclusion criteria were mastectomy, prior RT in the same region, bilateral breast cancer, male breast cancer, partial breast irradiation, breast implants, and bilateral breast cancer. Follow-up was for at least 24 months, with longer follow-up encouraged. More detailed information on the REQUITE study and the patient characteristics of the breast cancer cohort can be found in a recent publication [Citation15].
Matching
Before matching, fractionation schedules were categorised as normofractionation (above 20 fractions), moderate hypofractionation (10–19 fractions), and strong hypofractionation (1–9 fractions). Each patient treated in a prone position was matched with one or, if possible, two patients treated in supine position, selected by a propensity scoring method without replacement. An exact method was used for lymph node irradiation (LNI), boost, intensity-modulated radiotherapy (IMRT), chemotherapy and fractionation schedule, and a nearest neighbour method for breast volume [Citation16–19].
Data collection
For the analysis, three time-points of interest were chosen: baseline, end of RT (acute toxicity), and 24 months after the end of RT. At baseline, demographics, comorbidity, and treatment data were collected, including dosimetry. The physician assessed toxicity was assessed at all three time-points using the following Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 terms: atrophy, oedema, skin ulceration, telangiectasia (inside and outside tumour bed), skin induration (inside and outside tumour bed), erythema, arm lymphoedema, pain, and skin hyperpigmentation. Patient-reported HRQoL data were collected at all three time-points using two standardised questionnaires from the European Organisation for Research and Treatment of Cancer (EORTC): the EORTC QLQ C30 [22] and breast-specific QLQ BR23 [Citation20] questionnaires. As not all HRQoL questions are relevant in the comparison of prone and supine position, only the following scales were retained for the analysis: Physical Functioning, Social Functioning, Fatigue and Pain (QLQ C30), Body Image, Breast Symptoms, and Arm Symptoms (QLQ BR23). A photographical assessment of breast cosmesis was done before RT and after 2 years using the BCCT.core software [Citation21]. Dosimetry data were collected centrally through standardised operating procedures. The dosimetric analysis contained data on mean heart dose (MHD), mean lung dose (MLD), maximum skin dose, and the skin volume receiving a dose of >107% of the prescribed dose.
Objectives
The goal of the current analysis was to compare prone and supine positioning for three domains: (1) toxicity and cosmesis, (2) HRQoL, and (3) dosimetry. Toxicity and HRQoL were separated into acute (at the end of RT) and late reactions (2 years). Before any analysis, to account for multiple testing, three primary endpoints were chosen: (1) acute dermatitis, (2) atrophy at 2 years, and 3) photographic assessment at 2 years. Desquamation and ulceration were only analysed in the acute setting. Atrophy, telangiectasia, fibrosis, and hyperpigmentation were compared at 24 months. All toxicity measurements were dichotomised in no toxicity versus grade 1 or higher toxicity, except for acute dermatitis which was dichotomised between grade 1 or lower and grade 2 or higher toxicity (because 87% of all patients developed at least grade 1 dermatitis).
Statistical analysis
R studio version 3.2.6 was used for all statistical analyses and data visualisation. To compare acute toxicity, acute and 24-months HRQoL and 24-months photographic assessment, the difference between baseline scores and the score after RT was calculated. A deterioration was defined as a worsening of at least one grade for physician-assessed toxicity or cosmesis, and as a negative change of at least 10 points for HRQoL [Citation22]. For the 24 months toxicity assessment, the baseline was not substracted. Cosmesis and toxicity outcomes were analysed using a Chi-Square test. HRQoL scores and dosimetry were compared using the Mann–Whitney U test. For the primary endpoints, an alpha level of 0.05 was chosen. To avoid type I errors due to the multiple tests, the Bonferroni correction was used for all secondary endpoints and for comparison of the baseline characteristics.
Results
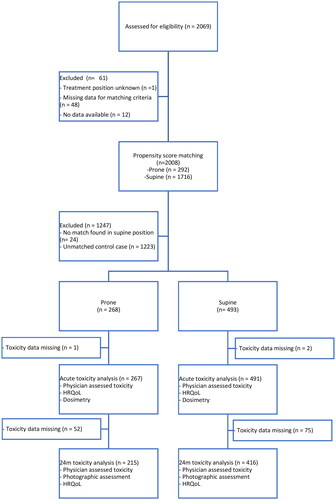
gives an overview of the available data and the matching procedure, data were available for 2069 patients with missing data for one of the matching variables in 61 patients. In total, 2008 patients after BCS were matched, 292 were treated in prone, and 1716 were in supine position. After matching (exactly for LNI, boost, IMRT, chemotherapy and fractionation category, and the nearest neighbour for breast volume) the number of patients was reduced to 761 (268 in prone and 493 in supine position). shows the baseline characteristics before and after matching. Most patients treated in prone position were included in treatment centre A, whereas treatment centres B and C provided 45% of patients treated in supine position. After matching, statistically significant differences remain for age (57 vs 61 year, p < .001), and treatment centre (p < .001).
Table 1. Baseline characteristics of patients treated in prone and supine positions, before and after propensity score matching.
Toxicity and cosmesis
gives an overview of acute and 2-years toxicity. For acute toxicity, the proportion of patients experiencing at least one grade of deterioration is significantly higher for oedema (48% in prone vs 31% in supine, p < .001). The primary endpoint of the proportion of patients with a deterioration (≥2 grades) for dermatitis is not statistically significant (16% vs 20%, p = .28). At 2 years, the proportion of patients experiencing breast atrophy (primary endpoint) is significantly lower: 45% in prone and 56% in supine position (p = .013). For the secondary endpoints, the proportion of patients with at least grade I toxicity is not significantly different between both treatment positions. The photographic assessment, included in , found no difference in the risk of worse cosmesis at 2 years compared to baseline, both for arms on the hips and arms up.
Figure 2. Comparison of physician-assessed toxicity between prone and supine positions. (A) Proportion of patients with a deterioration in toxicity at the end of radiotherapy compared to baseline with one category (oedema, ulceration and breast pain) or two categories for dermatatis. (B) Proportion of patients experiencing grade I or higher toxicity at 2 years after radiotherapy.
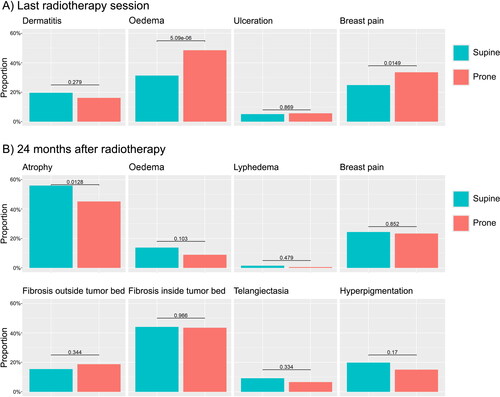
Table 2. Photographic assessment at 24 months of deterioration of cosmesis compared to baseline for the photographs with (A) both arms on the hips and (B) both arms elevated.
Health-related quality of life
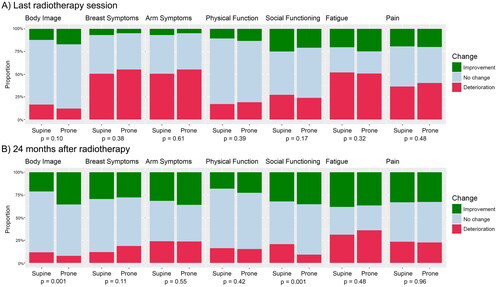
shows improvements or deteriorations of HRQoL from baseline, both acute and after 2 years. After RT, no significant difference in HRQoL between prone and supine positions is found. At 2 years, body image (p = .001) and social functioning (p = .001) are significantly better in patients treated in prone position, with fewer patients experiencing a deterioration and a higher proportion of patients experiencing an improvement. The difference in body image compared to baseline is weakly correlated with the difference in social functioning (Spearman correlation coefficient rs = 0.34).
Dosimetry
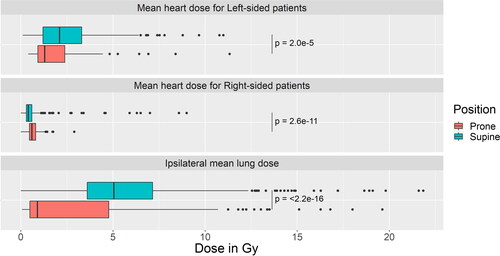
shows the MHD for left- and right-sided patients and the ipsilateral MLD. On the one hand, the median MHD for left-sided patients is 1.29 Gy in prone position and 2.10 Gy in supine position (p < .001). On the other hand, for right-sided patients median MHD is significantly higher in prone (0.60 Gy vs 0.40 Gy, p < .001). A 3.11 Gy lower median MLD is found for prone position, compared to supine position (2.77 Gy vs 5.89 Gy, p < .001).
Discussion
The dosimetric advantages of prone positioning have been known for a long time, yet the application in daily clinical practice remains limited [Citation6–10]. Other potential advantages, such as reduced toxicity and improved HRQoL remain underreported. Only one randomised controlled trial (RCT) has compared acute and late toxicity between prone and supine positions in women with large breasts [Citation11–13]. This RCT showed positive results at all three time points (acute, 2 years, and 5 years). The risk of acute toxicity, measured both at the end of WBI and 1 to 2 weeks thereafter, was lower for prone compared to supine positioning for the following toxicity domains: desquamation (or ulceration), dermatitis, and oedema [Citation12]. Recently a second single-blind RCT confirmed the lower risk of desquamation after WBI in prone position [Citation14]. In contrast, the present analysis of acute toxicity did not find any advantage for prone positioning. On the contrary, prone positioning resulted in a significantly higher risk of oedema. However, acute toxicity was measured only on the last day of irradiation, while it is known that the highest rates of acute toxicity are seen 2 to 8 weeks after irradiation, depending on fractionation [Citation23,Citation24]. Also, the fraction of patients treated in prone positioning is radiotherapy centre-dependent and most prone patients were included from a single institution, hence resulting in a risk of bias due to scoring differences between institutions. Finally, previous RCTs only allowed patients with large breast sizes, which is a risk factor for acute toxicity [Citation6,Citation16,Citation17,Citation19]. In contrast, our analysis included patients of all breast sizes, such as small-breasted patients with a low risk of acute toxicity in both positions. A hypothesis for the increased risk of oedema is the increased gravitational pull in prone position. The higher rate of oedema did not result in any differences in the acute patient-reported outcomes.
In contrast to the acute toxicity results, our 2-year results do confirm the lower risk of breast atrophy (45% vs 56%, p = .013) found in the only RCT reporting late toxicity, despite our analysis including patients with small breasts [Citation11]. However, these findings were not confirmed in the photographical assessment. All RT centres took photographs which were assessed centrally using the BCCT.core software [Citation21]. The HRQoL items' body image and social functioning were significantly better in prone position at 2 years. Better patient satisfaction with their body image could be a result of the lower risk of atrophy. Besides a weak correlation with body image (rs =0.34), the difference in social functioning might be due to other differences. These factors influencing HRQoL include age (supine patients are on average 4 years older), use of hormone therapy, cultures between treatment centres and other factors not used in matching, due to the choice for toxicity as the primary endpoints [Citation25,Citation26].
The current analysis supports the reduced MHD and ipsilateral MLD in prone compared to supine position [Citation6–8,Citation10]. Median MHD for left-sided patients is 39% lower in prone compared to supine position. The 0.81Gy difference in MHD between both positions should lead to a six percent reduction in the increase in the rate of major coronary events after radiotherapy, according to Taylor et al. [Citation2]. In contrast to the MHD reduction for left-sided patient, prone resulted in a 0.2 Gy higher median MHD for right-sided patients. Nevertheless, the median MHD in both positions for right-sided patients is low (0.6 Gy in prone, and 0.4 Gy in supine position). Besides heart disease, a second cause radiation-related mortality in breast cancer patients is secondary lung cancer. A SEER analysis even found that for women treated between 1983 and 1992, there was evidence for secondary lung cancer mortality, but not for cardiac mortality [Citation4]. Taylor et al. found an excess relative risk for lung cancer of 0.11 per Gy [Citation27]. The risk is most prominent after the first decade. In prone-treated patients, the median ipsilateral MLD was more than halved from 5.89 to 2.77 Gy.
A recent analysis comparing prone-free breathing with supine DIBH, found a dosimetric gain for prone position in 62% of patients [Citation10]. The UK HeartSpare Stage IB Study also compared prone-free breathing and supine DIBH, using a cross-over design in patients requiring left-sided WBI with an estimated breast volume of at least 750 cm³ [Citation28]. The authors concluded that supine DIBH resulted in better heart-sparing and higher set-up accuracy, and was preferred by patients. Nevertheless, prone resulted in a 10-fold decrease in ipsilateral MLD (3.73 Gy vs 0.34 Gy, p < .001). Hence, the question becomes: should the focus be on MHD or MLD reduction, in particular for smokers [Citation4,Citation27]? The most promising technique is probably the combination of prone position with DIBH, a combination that has been described to be feasible and of great potential [Citation23,Citation29,Citation30]. Unfortunately, data on DIBH were not collected in the REQUITE study.
Despite the advantages of prone position, implementation of the technique in daily clinical practice remains limited. Only two centres in the REQUITE study used the prone position on a regular basis. Potential reasons for the limited use of the prone position are the superiority of supine DIBH over prone-free breathing for MHD (even though prone DIBH probably is the most optimal technique), the greater set-up errors in prone position resulting in larger PTV margins, the misconception that the benefits of prone position only apply to patients with large breast size and prone positioning being less comfortable for patients. As prone positioning is more complex, training for the technologists is required, but after being accustomed to the technique, treatments can be given in 20 min or less [Citation28,Citation31].
The main limitation of the current analysis is the overrepresentation of patients from one single treatment centre, contributing to 67% of all prone patients, which could have biased the results. The other seven out of nine main participating centres treated only a very limited percentage of patients in a prone position (less than one in ten). This could introduce bias due to differences in target volume contouring, field arrangement, and treatment planning. Furthermore, physician-assessed toxicity is highly susceptible to interobserver variability [Citation32]. Another limitation is age difference, with supine-treated patients being on average 4 years younger. This discrepancy was accepted as the literature does not show a strong connection between age and acute toxicity [Citation17,Citation33]. In contrast, age does impact HRQoL and late toxicity which could influence the results [Citation25]. Nevertheless, scoring was done prospectively using standardised instruments at specific intervals and dichotomised to minimise inter-observer discrepancies [Citation15]. Also, observer-independent measurements were included such as HRQoL and photographic assessment.
Our current findings indicate prone could be superior to supine positioning for late toxicity and dose to the organs at risk: it lowers the risk of atrophy at 2 years, improves body image at 2 years, and lowers ipsilateral MLD and MHD for left-sided patients. Contrary to previous studies that reported lower acute toxicity, the REQUITE data indicate a higher risk of breast oedema at the end of RT. Overall, we endorse the use of prone positioning for WBI.
Disclosure statement
Ghent University owns the patent application entitled Radiotherapy Board and Couch [WO2015144654A1] filed on March 25, 2014 for which LV is listed as inventor. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability statement
Raw data were generated by the REQUITE consortium. Derived data supporting the findings of this study are available from the corresponding author VV on request. External researchers can contact [email protected] for further information on how to apply for access to the full REQUITE data (fees apply).
Additional information
Funding
References
- Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. The Lancet. 2011;378(9804):1707–1716.
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–998. doi: 10.1056/NEJMoa1209825.
- Aznar MC, Duane FK, Darby SC, et al. Exposure of the lungs in breast cancer radiotherapy: a systematic review of lung doses published 2010-2015. Radiother Oncol. 2017;126(1):148–154. doi: 10.1016/j.radonc.2017.11.022.
- Henson KE, McGale P, Taylor C, et al. Radiation-related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br J Cancer. 2013;108(1):179–182. doi: 10.1038/bjc.2012.575.
- Taylor CW, Zhe W, Macaulay E, et al. Exposure of the heart in breast cancer radiation therapy: a systematic review of heart doses published during 2003 to 2013. Int J Radiat Oncol Biol Phys. 2015;93(4):845–853. doi: 10.1016/j.ijrobp.2015.07.2292.
- Kirby AM, Evans PM, Donovan EM, et al. Prone versus supine positioning for whole and partial-breast radiotherapy: a comparison of non-target tissue dosimetry. Radiother Oncol. 2010;96(2):178–184. doi: 10.1016/j.radonc.2010.05.014.
- Lymberis SC, De Wyngaert JK, Parhar P, et al. Prospective assessment of optimal individual position (prone versus supine) for breast radiotherapy: volumetric and dosimetric correlations in 100 patients. Int J Radiat Oncol Biol Phys. 2012;84(4):902–909. doi: 10.1016/j.ijrobp.2012.01.040.
- Osa EOO, Dewyngaert K, Roses D, et al. Prone breast intensity modulated radiation therapy: 5-year results. Int J Radiat Oncol Biol Phys. 2014;89(4):899–906. doi: 10.1016/j.ijrobp.2014.03.036.
- Lai J, Zhong F, Deng J, et al. Prone position versus supine position in postoperative radiotherapy for breast cancer: a meta-analysis. Medicine (Baltimore). 2021;100(20):e26000. doi: 10.1097/MD.0000000000026000.
- Wang X, Fargier-Bochaton O, Dipasquale G, et al. Is prone free breathing better than supine deep inspiration breath-hold for left whole-breast radiotherapy? A dosimetric analysis. Strahlenther Onkol. 2021;197(4):317–331. doi: 10.1007/s00066-020-01731-8.
- Veldeman L, Schiettecatte K, De Sutter C, et al. The 2-year cosmetic outcome of a randomized trial comparing prone and supine whole-breast irradiation in large-breasted women. Int J Radiat Oncol Biol Phys. 2016;95(4):1210–1217.
- Mulliez T, Veldeman L, van Greveling A, et al. Hypofractionated whole breast irradiation for patients with large breasts: a randomized trial comparing prone and supine positions. Radiother Oncol. 2013;108(2):203–208. doi: 10.1016/j.radonc.2013.08.040.
- Vakaet V, Van Hulle H, Vergotte M, et al. 5-year outcomes of a randomized trial comparing prone and supine whole breast irradiation in large breasted women. Int J Radiation Oncol Biol Phys. 2021;110(3):766–771. doi: 10.1016/j.ijrobp.2021.01.026.
- Vesprini D, Davidson M, Bosnic S, et al. Effect of supine vs prone breast radiotherapy on acute toxic effects of the skin among women with large breast size: a randomized clinical trial. JAMA Oncol. 2022;8(7):994–1000. doi: 10.1001/jamaoncol.2022.1479.
- Seibold P, Webb A, Aguado-Barrera ME, et al. REQUITE: a prospective multicentre cohort study of patients undergoing radiotherapy for breast, lung or prostate cancer. Radiother Oncol. 2019;138:59–67. doi: 10.1016/j.radonc.2019.04.034.
- De Langhe S, Mulliez T, Veldeman L, et al. Factors modifying the risk for developing acute skin toxicity after whole-breast intensity modulated radiotherapy. BMC Cancer. 2014;14(1):9. doi: 10.1186/1471-2407-14-711.
- Rattay T, Seibold P, Aguado-Barrera ME, et al. External validation of a predictive model for acute skin radiation toxicity in the REQUITE breast cohort. Front Oncol. 2020;10:575909. doi: 10.3389/fonc.2020.575909.
- De Santis MC, Bonfantini F, Di Salvo F, et al. Factors influencing acute and late toxicity in the era of adjuvant hypofractionated breast radiotherapy. Breast. 2016;29:90–95. doi: 10.1016/j.breast.2016.07.013.
- Pignol JP, Olivotto I, Rakovitch E, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26(13):2085–2092. doi: 10.1200/JCO.2007.15.2488.
- Sprangers MA, Groenvold M, Arraras JI, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol. 1996;14(10):2756–2768. doi: 10.1200/JCO.1996.14.10.2756.
- Cardoso MJ, Cardoso J, Amaral N, et al. Turning subjective into objective: the BCCT.core software for evaluation of cosmetic results in breast cancer conservative treatment. Breast. 2007;16(5):456–461. doi: 10.1016/j.breast.2007.05.002.
- Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–144. doi: 10.1200/JCO.1998.16.1.139.
- Paelinck L, Gulyban A, Lakosi F, et al. Does an integrated boost increase acute toxicity in prone hypofractionated breast irradiation? A randomized controlled trial. Radiother Oncol. 2017;122(1):30–36. doi: 10.1016/j.radonc.2016.12.023.
- Brunt AM, Wheatley D, Yarnold J, et al. Acute skin toxicity associated with a 1-week schedule of whole breast radiotherapy compared with a standard 3-week regimen delivered in the UK FAST-Forward trial. Radiother Oncol. 2016;120(1):114–118. doi: 10.1016/j.radonc.2016.02.027.
- Wöckel A, Schwentner L, Krockenberger M, et al. Predictors of the course of quality of life during therapy in women with primary breast cancer. Qual Life Res. 2017;26(8):2201–2208. doi: 10.1007/s11136-017-1570-0.
- Urzúa A, Miranda-Castillo C, Caqueo-Urízar A, et al. Do cultural values affect quality of life evaluation? Soc Indic Res. 2013;114(3):1295–1313. doi: 10.1007/s11205-012-0203-9.
- Taylor C, Duane FK, Dodwell D, et al. Estimating the risks of breast cancer radiotherapy: evidence from modern radiation doses to the lungs and heart and from previous randomized trials. J Clin Oncol. 2017;35(15):1641–1649. doi: 10.1200/JCO.2016.72.0722.
- Bartlett FR, Colgan RM, Donovan EM, et al. The UK HeartSpare study (stage IB): randomised comparison of a voluntary breath-hold technique and prone radiotherapy after breast conserving surgery. Radiother Oncol. 2015;114(1):66–72. doi: 10.1016/j.radonc.2014.11.018.
- Mulliez T, Veldeman L, Speleers B, et al. Heart dose reduction by prone deep inspiration breath hold in left-sided breast irradiation. Radiother Oncol. 2015;114(1):79–84. doi: 10.1016/j.radonc.2014.11.038.
- Saini AS, Hwang CS, Biagioli MC, et al. Evaluation of sparing organs at risk (OARs) in left-breast irradiation in the supine and prone positions and with deep inspiration breath-hold. J Appl Clin Med Phys. 2018;19(4):195–204. doi: 10.1002/acm2.12382.
- Kirby AM, Evans PM, Helyer SJ, et al. A randomised trial of supine versus prone breast radiotherapy (SuPr study): comparing set-up errors and respiratory motion. Radiother Oncol. 2011;100(2):221–226. doi: 10.1016/j.radonc.2010.11.005.
- Bentzen SM, Dörr W, Anscher MS, et al. Normal tissue effects: reporting and analysis. Semin Radiat Oncol. 2003;13(3):189–202. doi: 10.1016/S1053-4296(03)00036-5.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-Life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365.