Abstract
Background: To date, anal cancer patients are treated with radiotherapy to similar volumes despite a marked difference in risk profile based on tumor location and stage. A more individualized approach to delineation of the elective clinical target volume (CTVe) could potentially provide better oncological outcomes as well as improved quality of life. The aim of the present work was to establish Nordic Anal Cancer (NOAC) group guidelines for delineation of the CTVe in anal cancer.
Methods: First, 12 radiation oncologists reviewed the literature in one of the following four areas: (1) previous delineation guidelines; (2) patterns of recurrence; (3) anatomical studies; (4) common iliac and para-aortic recurrences and delineation guidelines. Second, areas of controversy were identified and discussed with the aim of reaching consensus.
Results: We present consensus-based recommendations for CTVe delineation in anal cancer regarding (a) which regions to include, and (b) how the regions should be delineated. Some of our recommendations deviate from current international guidelines. For instance, the posterolateral part of the inguinal region is excluded, decreasing the volume of irradiated normal tissue. For the external iliac region and the cranial border of the CTVe, we agreed on specifying two different recommendations, both considered acceptable. One of these recommendations is novel and risk-adapted; the external iliac region is omitted for low-risk patients, and several different cranial borders are used depending on the individual level of risk.
Conclusion: We present NOAC consensus guidelines for delineation of the CTVe in anal cancer, including a risk-adapted strategy.
Background
Squamous cell carcinoma of the anal region (anal cancer) is a relatively rare malignancy with an increasing incidence. The main curative treatment is chemoradiotherapy (CRT) [Citation1–4]. The optimal radiotherapy dose is not known but is currently being investigated in the PLATO trial (ISRCTN88455282). Although CRT is an effective treatment, some patients report severe late toxicity and impaired quality of life [Citation5,Citation6].
Over the past decade, intensity modulated radiotherapy (IMRT) has replaced older treatment techniques as the standard of care [Citation7,Citation8]. In the IMRT era, four major international anal cancer target delineation guidelines have been published from the RTOG (2009), AGITG (2012), UK (2016), and ECOG/ACRIN (2022), respectively [Citation9–12]. Even though there is still a shortage of empirical data, the results of a number of studies published over the recent years could inform updated delineation guidelines. The aim of the present work was to review these studies and to establish Nordic Anal Cancer (NOAC) group guidelines for delineation of the elective clinical target volume (CTVe) in anal cancer.
Material and methods
NOAC is a collaborative group of clinicians and researchers that has organized meetings and workshops and conducted clinical trials for >20 years [Citation13,Citation14]. All hospitals treating anal cancer in Denmark, Iceland, Norway, and Sweden are represented in NOAC. Results from a contouring workshop in Oslo 2019 identified the most important differences in delineation approaches. At a NOAC meeting in Aarhus in November 2021, a delineation group was officially established, consisting of experienced radiation oncologists from each country. First, the group was divided into four subgroups, each with the task to review the literature on relevant anal cancer studies, as well as studies in other pelvic malignancies, in one of the following four areas: (1) previous/current delineation guidelines; (2) patterns of recurrence; (3) anatomical studies; (4) common iliac (CI) and para-aortic (PA) recurrences and delineation guidelines. Second, a written report from each subgroup was discussed in the whole delineation group through e-mail and online meetings. Areas of controversy were identified, and – where possible – consensus was reached. Third, a draft for guidelines was presented and discussed at a NOAC meeting in Uppsala in November 2022.
Systematic searches to the PubMed database were done continuously throughout the process. On 1 February 2023, a final systematic search to the PubMed database was done. The first string ((anal cancer[MeSH Terms]) AND (radiotherapy[MeSH Terms])) OR ((anal cancer[MeSH Terms]) AND (recurrence[MeSH Terms])) Filters: Humans, English, from 2006 – 2023 yielded 682 titles. From this search 60 abstracts were selected and read through with the focus on pattern of failure and definition of treatment volumes and finally 23 full manuscripts were selected. The second string (anal cancer[MeSH Terms]) AND delineation Filters: Humans, English, from 2006 – 2023 yielded 40 titles, hereof 5 abstracts were selected leading to inclusion of 4 full manuscripts. The third string (anal cancer[MeSH Terms]) AND pattern of failure Filters: Humans, English, from 2006 – 2023 yielded 31 titles, hereof 13 abstracts were selected leading to inclusion of 1 new manuscript. Lastly, (anal cancer[MeSH Terms]) AND pattern of recurrence Filters: Humans, English, from 2006 – 2023 and (anal cancer[MeSH Terms]) AND extended field Filters: Humans, English, from 2006 – 2023 and (anal cancer[MeSH Terms]) AND paraaortic Filters: Humans, English, from 2006 – 2023 were used, but none of these searches retrieved new manuscripts. Further manuscripts were retrieved from references and similar publications on other pelvic cancers.
Results
Which regions should be included in the CTVe?
For patients with small (< 1 cm) T1N0 perianal tumors (not involving the anal canal) we believe that elective nodal irradiation (ENI) probably can be omitted, but data from the ongoing ACT3 trial (ISRCTN88455282) is awaited before any firm recommendations can be made [Citation15–19]. For all other patients, the internal iliac, presacral, mesorectal, superior rectal, and inguinal regions, as well as the entire anal canal, should be included in the CTVe; an exception being patients with squamous cell carcinomas located in the rectum without extension into the anal canal, for whom the inguinal region should be omitted.
External iliac
Previous international guidelines have included the external iliac region for all patients [Citation9–12]. By contrast, current Norwegian guidelines recommend inclusion of the external iliac region only for patients with T3-4 or lymph node (LN) positive disease. In the studies by Bentzen et al. and Slordahl et al. >150 low-risk (T1-2N0) patients were treated with a 2–4 field technique according to Norwegian guidelines and, to the best of our knowledge, no external iliac recurrences were seen [Citation20,Citation21]. Our literature review identified a single study which has reported external iliac recurrences in anal cancer patients treated without external iliac CTVe coverage: Wright et al. found 2 external iliac recurrences in 180 patients treated with a 3-field technique with the anterior border of the lateral fields located behind the lower part of the external iliac vessels [Citation22].
Tumor cells can reach the external iliac LNs through two different routes. The first route is a direct lymphatic spread of tumor cells from the primary tumor site. The second route is a spread of tumor cells from metastatic LNs in the ipsilateral inguinal or, less frequently, from the ipsilateral internal iliac region [Citation23]. In a recent study by Frennered et al. PET-positive LNs at diagnosis in 103 anal cancer patients were mapped on a standard reference CT. External iliac LN metastasis was relatively common (33 patients), but only one patient had external iliac LN metastasis without any LN metastasis in other regions [Citation24]. This observation indicates that isolated external iliac LN metastasis is infrequent, and that the second route is much more common than the first route in anal cancer patients with pathologic external iliac LNs. This is most likely the reason why stage N1b (isolated external iliac LN metastasis) is exceedingly rare; in a study from the UK, only 1 of 385 anal cancer patients had stage N1b [Citation25].
Taken together, there is some emerging evidence suggesting that the external iliac region could be omitted from the CTVe in low-risk node-negative patients. On the other hand, most of the prospective anal cancer studies to date have only included patients with CTVe coverage of the external iliac region, making it a well-established standard of care. Following thorough discussions, we agreed on specifying two alternatives, which were both considered acceptable. ‘Alternative A’ is to include the external iliac region for all patients. ‘Alternative B’ is to omit the external iliac region for patients with T1-2N0 tumors and for patients with T1-2N1a tumors with LN metastasis confined to the mesorectal, superior rectal, and presacral regions; for all other patients, the external iliac region is included. Of note, with our definition of the internal iliac region (anterior limit at the posterior border of the external iliac vein; ), the most commonly involved part of the external iliac region – which is located just behind the vein – will always be included [Citation24].
Table 1. Cranial border of the CTVe according to 'alternative B'.
Table 2. How the regions should be Defined.
Ischiorectal fossa
Two anal cancer studies have mapped pathologic LNs based on diagnostic PET-CTs [Citation24,Citation26]. None of them found any pathologic LNs in the ischiorectal fossa (IRF; sometimes also referred to as the ischioanal fossa). According to UK guidelines, only the part of the IRF located inside the primary tumor CTV (CTVp) is included in the target volume, not the entire IRF [Citation9]. To the best of our knowledge, there have been no reports of any IRF recurrences beyond the CTVp in patients treated according to UK guidelines. Lymphatic vessels travel anteriorly along the ano-inguinal lymphatic drainage (AILD) and laterally along the inferior rectal/internal pudendal artery in the IRF which implies a small risk of in-transit metastasis [Citation27,Citation28]. That risk is probably higher if the tumor extends into the IRF. Consequently, we suggest including the entire IRF if there is radiographic evidence of tumor extension beyond the levator ani muscles or the external sphincter. For patients with tumor extension into, but not beyond, the levator ani muscles or the external sphincter a 2 cm margin into the IRF is considered sufficient. For other patients, no extra margin beyond the 10–15 mm primary tumor gross tumor volume (GTVp) to CTVp margin is needed in the IRF.
Cranial border of the CTVe
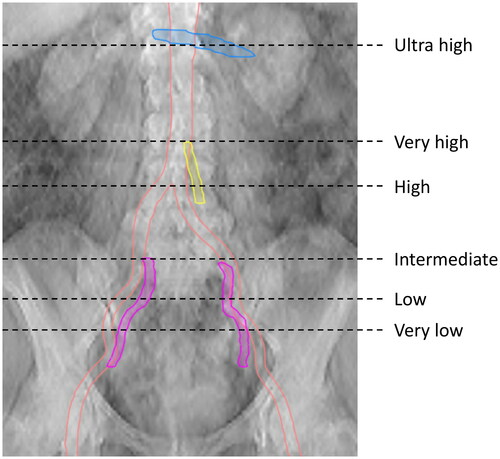
Previous guidelines have recommended the same cranial border for all anal cancer patients, regardless of tumor stage [Citation9–12]. We discussed this previous standard compared to a risk adapted approach and again, we agreed on two different recommendations which were both considered acceptable. ‘Alternative A’ is to use the bifurcation of the common iliac artery as the cranial border for all patients. This is in accordance with the guidelines by the RTOG, AGITG, and ECOG-ACRIN and needs no further explanation. ‘Alternative B’ is to use six different cranial borders depending on the individual level of risk (, ). ‘Alternative B’ is novel, and the rationale and evidence therefore need further explanation, which is provided in the following sections.
Low cranial border in low-risk patients
In a study from 2007, Das et al. reviewed patterns of recurrence in 167 anal cancer patients. Five regional LN recurrences were seen, all in patients with a low superior border located at the bottom of the sacroiliac joint (SIJ). Consequently, the authors recommended that the superior border should be at L5/S1, corresponding to the bifurcation of the common iliac artery [Citation29]. However, there are now several studies which have shown that it is safe to lower the cranial border for low-risk patients [Citation18,Citation20,Citation21,Citation25,Citation30]. In Norway, patients with T1-2N0 tumors without extension into the rectum have for many years been treated with a cranial border at the inferior aspect of the SIJ. Bentzen et al. reported outcomes for 113 such patients, and with a median follow-up of 4.1 years they found no regional LN recurrences above the upper field border [Citation20]. The prospective ANCARAD study showed the same result: no regional LN recurrences above the upper field border [Citation21]. Tomaszewski et al. reported 9 pelvic LN recurrences in 284 anal cancer patients who were treated with a cranial border 1 cm above the bottom of the SIJ or 5 cm above the GTVp, whichever was more cranial [Citation30]. Shakir et al. reported 12 regional LN recurrences in 385 unselected anal cancer patients who were treated according to UK guidelines with the cranial border 2 cm above the inferior aspect of the SIJ [Citation25].
According to ‘Alternative B’ we recommend that the cranial border of the CTVe should be the inferior aspect of the SIJ for patients with T1-2N0 tumors not extending into the rectum. For patients with T1-2N0 tumors extending <1 cm into the rectum we recommend that the cranial border of the CTVe should be 2 cm above the inferior aspect of the SIJ (, ).
High cranial border in high-risk patients
Most anal cancer patients have a low risk of recurrence in the CI and PA regions [Citation28]. However, some tumor or patient characteristics might be predictors of CI/PA recurrence, which could merit inclusion of the CI/PA regions in the CTVe for those patients. Extended field irradiation (EFI) including the CI/PA regions is feasible with modern radiotherapy techniques and could therefore be an option for patients with a good performance status [Citation31–33]. However, reports from anal cancer cohorts on acute and late toxicity are largely missing and needs to be focus for future studies. Careful selection of patients with consideration of the balance between risk of recurrence and late morbidity is mandatory.
Only two studies have investigated predictors of CI/PA recurrence in anal cancer. Both reported that LN metastasis in ≥ 3 pelvic or inguinal LN regions and external or internal iliac metastasis were associated with > 10-15% risk of CI/PA metastasis [Citation24,Citation28]. The results are broadly in line with the results of studies in other pelvic malignancies, e.g., bladder, cervical, and prostate cancer [Citation34–36].
We identified 5 studies with information on TNM stage at diagnosis for in total 28 anal cancer patients who were treated with pelvic radiotherapy and experienced a metachronous CI/PA recurrence above the upper field border [Citation22,Citation25,Citation28,Citation37,Citation38]. A majority of these patients (23 of 28; 82%) had pelvic/inguinal LN metastasis at diagnosis. Furthermore, we identified 6 studies that have detailed characteristics for 67 anal cancer patients with synchronous CI/PA metastasis; 65 of 67 (97%) also had pelvic/inguinal LN metastasis [Citation24,Citation26,Citation28,Citation32,Citation39,Citation40]. Taken together, skip metastasis to the CI/PA LN stations is a very rare event in anal cancer and EFI should not be considered for patients without pelvic/inguinal LN metastasis. Although it has not formally been studied to date, it seems reasonable to assume that LN metastasis in the upper half of the pelvis is associated with a higher risk of CI recurrence compared to LN metastasis in the lower half of the pelvis, given the step-by-step pattern of LN spread. According to ‘Alternative B’ we therefore open up for inclusion of the CI region for patients with LN metastasis in the upper half of the external/internal iliac regions, and also for patients with LN metastasis in ≥3 pelvic or inguinal LN regions. For patients with LN metastasis in the CI region, we recommend using the inferior mesenteric artery take-off from the aorta as the cranial border of the CTVe (, ). Prospective studies will be highly valuable in this setting.
How should the elective regions be defined?
We recommend that all visible LNs in a region should be included, even if they are located outside the borders presented in .
Margin around pelvic vessels
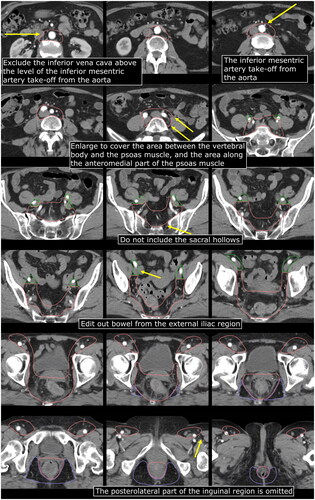
Vilarino-Varela et al. used USPIO-MRI to identify non-metastatic pelvic LNs in patients with gynecologic malignancies and 99.5% of all LNs were within 7 mm from vessels [Citation41]. Shakir et al. evaluated recurrences in 385 anal cancer patients treated according to UK guidelines (7 mm margin) and did not report a single case of margin miss [Citation25]. Similarly, Dapper et al. found that 100% of PET-positive pelvic LNs were within a 7–8 mm margin [Citation26]. Taken together, with a 7–8 mm margin around vessels it seems like 99.5–100% of LNs will be covered. In our opinion, that percentage may probably be too high for an elective volume. We therefore recommend a 5–7 mm margin around pelvic vessels ().
Editing out bowel
LNs along the external iliac vessels are located in the extraperitoneal space and – unless pathologically enlarged – do not extend into the bowel cavity. Consequently, we recommend the large bowel and small bowel to be edited out from the external iliac LN station but not from areas where LNs are located in the same compartment as the bowel, e.g., the superior rectal region and the presacral space.
Inguinal
The posterolateral inguinal area has been included in all previous guidelines. However, 3 anal cancer studies have now shown that no LN metastasis – and probably no benign LNs either – was located there [Citation24,Citation26,Citation42]. We therefore deem it unnecessary to include this area in the CTVe ().
Common iliac
Fontanilla et al. mapped pathologic LNs in cervical cancer. Four CI LNs were not covered by the RTOG guidelines used at that time, which recommended a 7 mm isotropic margin around the CI vessels. Those four LNs were located in front of the psoas muscle or between the vertebral body and the psoas muscle, indicating that an isotropic margin from the blood vessels is not sufficient to cover LNs in those areas [Citation43]. It is reasonable to assume the same applies to anal cancer and therefore we suggest a small expansion of the CTVe to cover LNs in those areas (, ).
Para-aortic
In anal cancer, lymphatic spread of tumor cells can reach the PA region via two different routes. The first route is the ‘lateral route’: internal iliac/externa iliac/presacral → CI → PA. The lateral route is the same as in other pelvic malignancies, e.g., cervical cancer, and studies on those cancers should be informative for anal cancer as well. The second route is the ‘mesorectal route’: mesorectal → superior rectal → inferior mesenteric → PA. At a certain level, these two routes converge into retroperitoneal PA LN stations. Although we have not found any studies providing conclusive evidence, we consider it likely that the routes converge at the level of the inferior mesenteric artery (IMA) take-off from the aorta, and that the LNs below that level follow the IMA rather than the inferior mesenteric vein (IMV). In some patients, the IMV is located relatively far away (anterior/left) from the IMA well into the bowel cavity and in such cases, we do not consider it necessary to include the IMV – only the IMA – in the CTVe. Supporting published evidence in anal cancer has, however, not been identified.
For LNs in the lateral route, there are a number of informative cervical cancer studies. In the largest to date, Wang et al. mapped 344 pathologic PA LNs; 216 (63%) were left para-aortic, 101 (29%) aorto-caval, and 27 (8%) right para-caval [Citation44]. Only 3 LNs were located in the anterolateral part of the right para-caval region, and all of those LNs were below the IMA take-off from the aorta. The authors therefore recommended to exclude the antero-lateral part of the right para-caval region above the IMA take-off. Correlations were described between vascular anatomy and bony anatomy, and the IMA take-off was most commonly located at the level of the L3 vertebral body (56% of the patients) or the L2/L3 interspace (28%) [Citation44].
Studies on rectal cancer could be informative for anal cancer regarding the mesorectal route. Unfortunately, to our knowledge, no mapping studies of IMA/PA LNs in rectal cancer have been published. Overall, only the two relatively small anal cancer studies summarized previously have mapped LNs in the mesorectal route [Citation24,Citation26]. The study by Dapper et al. included 5 patients with pathologic CI/PA LNs. In these patients there were no pathologic LNs located anterior to the aorta or the inferior vena cava (IVC) [Citation26]. In the study by Frennered et al. 44 pathologic PA LNs were mapped; 22 were left para-aortic, 16 were aorto-caval, and 6 were in the right para-caval subregion. All pathologic LNs to the right of the IVC were below the L2/L3 interspace [Citation24]. Also in the study by Nilsson et al. on CI/PA recurrences in anal cancer, all LN recurrences to the right of the IVC were below the L2/L3 interspace [Citation28].
In 2021, updated NRG Oncology/RTOG consensus guidelines for delineation of the CTVe in cervical cancer and endometrial cancer were published [Citation45]. Our anal cancer guidelines are similar to those guidelines regarding how the PA region should be delineated, but we have added information about the IMA (mesorectal route) and have also adopted the CTVe in the right superolateral part with reference to the results of the anal cancer mapping studies as well as the recently published cervical cancer study by Wang et al. [Citation44].
Discussion
We formed a group of clinical experts to review the literature and establish Nordic Anal Cancer (NOAC) group guidelines for delineation of the CTVe in anal cancer. Some of our recommendations are different from previous international guidelines. For instance, the posterolateral part of the inguinal region is excluded, decreasing the volume of normal tissue being irradiated. Although we did not reach a uniform consensus on the inclusion of the external iliac region and the cranial border of the CTVe, we reached consensus on specifying two different recommendations, both considered acceptable, leaving the decision to the treating physician or institution to decide which to use. In some situations, the decision could probably be made together with the patient. ‘Alternative B’ is novel and risk-adapted; the external iliac region is omitted for low-risk patients, and the cranial border varies with the individual level of risk. We point out that ‘Alternative B’ should only be used by institutions with imaging follow-up of their patients, in order to enable early detection of out-of-field recurrences. We also point out that patients eligible for EFI should be individually selected based on the expected benefit versus the risk of toxicity.
Even though the exact definitions could be debated, we believe that moving away from ‘one size fits all’ should be a priority for anal cancer delineation guidelines. To inform future revisions, detailed and contemporary patterns of recurrence studies are much needed. For instance, predictors of CI/PA recurrence need to be explored further, as well as predictors of recurrence in areas that are not included in our present – or any other – anal cancer guidelines, such as the AILD and the pudenda interna region lateral to the sacrospinous ligament. Future clinical studies should also focus on generating the relevant evidence for the areas of discrepancies, i.e., on the inclusion of the external iliac region and the cranial border of the CTVe. This implies prospective observational cohorts including registration of patient reported outcome measures, and/or randomized clinical trials.
| Abbreviations | ||
| AILD | = | ano-inguinal lymphatic drainage |
| CI | = | common iliac |
| CTVe | = | elective clinical target volume |
| CTVp | = | primary tumor clinical target volume |
| EFI | = | extended field irradiation |
| GTVp | = | primary tumor gross tumor volume |
| IMA | = | inferior mesenteric artery |
| IMRT | = | intensity modulated radiotherapy |
| IMV | = | inferior mesenteric vein |
| IRF | = | ischiorectal fossa |
| IVC | = | inferior vena cava |
| LN | = | lymph node |
| NOAC group | = | : Nordic Anal Cancer group |
| PA | = | para-aortic |
| SIJ | = | sacroiliac joint. |
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Dapper H, Oechsner M, Münch S, et al. Dosimetric comparison of organs at risk using different contouring guidelines for definition of the clinical target volume in anal cancer. Strahlenther Onkol. 2020;196(4):368–375. doi: 10.1007/s00066-020-01587-y.
- Johnson N, Pellino G, Simillis C, et al. Discrepancies between NCCN and ESMO guidelines in the management of anal cancer: a qualitative review. Updates Surg. 2017;69(3):345–349. doi: 10.1007/s13304-017-0470-8.
- Rao S, Guren MG, Khan K, et al. Anal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(9):1087–1100. doi: 10.1016/j.annonc.2021.06.015.
- Rouard N, Peiffert D, Rio E, et al. Intensity-modulated radiation therapy of anal squamous cell carcinoma: relationship between delineation quality and regional recurrence. Radiother Oncol. 2019;131:93–100. doi: 10.1016/j.radonc.2018.10.021.
- Bentzen AG, Guren MG, Vonen B, et al. Faecal incontinence after chemoradiotherapy in anal cancer survivors: long-term results of a national cohort. Radiother Oncol. 2013;108(1):55–60. doi: 10.1016/j.radonc.2013.05.037.
- Sterner A, Derwinger K, Staff C, et al. Quality of life in patients treated for anal carcinoma-a systematic literature review. Int J Colorectal Dis. 2019;34(9):1517–1528. doi: 10.1007/s00384-019-03342-x.
- Kachnic LA, Winter KA, Myerson RJ, et al. Long-term outcomes of NRG oncology/RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-Fluorouracil and mitomycin-C for the reduction of acute morbidity in anal canal cancer. Int J Radiat Oncol Biol Phys. 2022;112(1):146–157. doi: 10.1016/j.ijrobp.2021.08.008.
- Kachnic LA, Winter K, Myerson RJ, et al. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2013;86(1):27–33. doi: 10.1016/j.ijrobp.2012.09.023.
- UK guidelines for IMRT in anal cancer. [Dec 17, 2019]. Available from: http://analimrtguidance.co.uk/national-anal-imrt-guidance-v3.pdf.
- Damico N, Meyer J, Das P, et al. ECOG-ACRIN guideline for contouring and treatment of early stage anal cancer using IMRT/IGRT. Pract Radiat Oncol. 2022;12(4):335–347. doi: 10.1016/j.prro.2022.01.015.
- Myerson RJ, Garofalo MC, El Naqa I, et al. Elective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys. 2009;74(3):824–830. doi: 10.1016/j.ijrobp.2008.08.070.
- Ng M, Leong T, Chander S, et al. Australasian gastrointestinal trials group (AGITG) contouring atlas and planning guidelines for intensity-modulated radiotherapy in anal cancer. Int J Radiat Oncol Biol Phys. 2012;83(5):1455–1462. doi: 10.1016/j.ijrobp.2011.12.058.
- Dahl O, Myklebust MP, Dale JE, et al. Evaluation of the stage classification of anal cancer by the TNM 8th version versus the TNM 7th version. Acta Oncol. 2020;59(9):1016–1023. doi: 10.1080/0284186X.2020.1778180.
- Leon O, Guren MG, Radu C, et al. Phase I study of cetuximab in combination with 5-fluorouracil, mitomycin C and radiotherapy in patients with locally advanced anal cancer. Eur J Cancer. 2015;51(18):2740–2746. doi: 10.1016/j.ejca.2015.08.029.
- Blinde SE, Schasfoort R, Mens JW, et al. Inguinal lymph node recurrence in the untreated groin of patients with anal carcinoma. Dis Colon Rectum. 2014;57(5):578–584. doi: 10.1097/DCR.0000000000000050.
- Joo JH, Park JH, Yoon SM, et al. Long-term oncologic and complication outcomes in anal cancer patients treated with radiation therapy. J Cancer Res Ther. 2020;16(Supplement):S194–s200. doi: 10.4103/jcrt.JCRT_34_18.
- Ortholan C, Resbeut M, Hannoun-Levi JM, et al. Anal canal cancer: management of inguinal nodes and benefit of prophylactic inguinal irradiation (CORS-03 study). Int J Radiat Oncol Biol Phys. 2012 ;82(5):1988–1995. doi: 10.1016/j.ijrobp.2011.02.010.
- Thompson SR, Lee ISY, Carroll S, et al. Radiotherapy for anal squamous cell carcinoma: must the upper pelvic nodes and the inguinal nodes be treated? ANZ J Surg. 2018;88(9):870–875. doi: 10.1111/ans.14398.
- Zilli T, Betz M, Bieri S, et al. Elective inguinal node irradiation in early-stage T2N0 anal cancer: prognostic impact on locoregional control. Int J Radiat Oncol Biol Phys. 2013;87(1):60–66. doi: 10.1016/j.ijrobp.2013.03.008.
- Bentzen AG, Guren MG, Wanderas EH, et al. Chemoradiotherapy of anal carcinoma: survival and recurrence in an unselected national cohort. Int J Radiat Oncol Biol Phys. 2012 ;83(2):e173-80–e180. doi: 10.1016/j.ijrobp.2011.12.062.
- Slørdahl KS, Klotz D, Olsen J, et al. Treatment outcomes and prognostic factors after chemoradiotherapy for anal cancer. Acta Oncol. 2021;60(7):921–930. doi: 10.1080/0284186X.2021.1918763.
- Wright JL, Patil SM, Temple LK, et al. Squamous cell carcinoma of the anal canal: patterns and predictors of failure and implications for intensity-modulated radiation treatment planning. Int J Radiat Oncol Biol Phys. 2010;78(4):1064–1072. doi: 10.1016/j.ijrobp.2009.09.029.
- Lengelé B, Scalliet P. Anatomical bases for the radiological delineation of lymph node areas. Part III: pelvis and lower limbs. Radiother Oncol. 2009;92(1):22–33. doi: 10.1016/j.radonc.2008.11.007.
- Frennered A, Scherman J, Buchwald P, et al. Patterns of pathologic lymph nodes in anal cancer: a PET-CT-based analysis with implications for radiotherapy treatment volumes. BMC Cancer. 2021;21(1):447. doi: 10.1186/s12885-021-08187-8.
- Shakir R, Adams R, Cooper R, et al. Patterns and predictors of relapse following radical chemoradiation therapy delivered using intensity modulated radiation therapy with a simultaneous integrated boost in anal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2020;106(2):329–339. doi: 10.1016/j.ijrobp.2019.10.016.
- Dapper H, Schiller K, Münch S, et al. Have we achieved adequate recommendations for target volume definitions in anal cancer? A PET imaging based patterns of failure analysis in the context of established contouring guidelines. BMC Cancer. 2019;19(1):742. doi: 10.1186/s12885-019-5970-0.
- Dapper H, Habl G, Hirche C, et al. Dosimetric quantification of the incidental irradiation of the 'true’ (deep) ano-inguinal lymphatic drainage of anal cancer patients not described in conventional contouring guidelines. Acta Oncol. 2018;57(6):825–830. doi: 10.1080/0284186X.2017.1415459.
- Nilsson MP, Nilsson ED, Johnsson A, et al. Patterns of recurrence in anal cancer: a detailed analysis. Radiat Oncol. 2020;15(1):125. doi: 10.1186/s13014-020-01567-7.
- Das P, Bhatia S, Eng C, et al. Predictors and patterns of recurrence after definitive chemoradiation for anal cancer. Int J Radiat Oncol Biol Phys. 2007;68(3):794–800. doi: 10.1016/j.ijrobp.2006.12.052.
- Tomaszewski JM, Link E, Leong T, et al. Twenty-five-year experience with radical chemoradiation for anal cancer. Int J Radiat Oncol Biol Phys. 2012;83(2):552–558. doi: 10.1016/j.ijrobp.2011.07.007.
- Gerum S, Iglseder W, Schmid R, et al. Practice of radiation therapy for anal cancer in Austria-a survey on behalf of the Austrian radiation oncology society gastrointestinal tumor group (ÖGRO-GIT). Strahlenther Onkol. 2021;197(11):953–961. doi: 10.1007/s00066-021-01842-w.
- Holliday EB, Lester SC, Harmsen WS, et al. Extended-field chemoradiation therapy for definitive treatment of anal canal squamous cell carcinoma involving the para-aortic lymph nodes. Int J Radiat Oncol Biol Phys. 2018;102(1):102–108. doi: 10.1016/j.ijrobp.2018.04.076.
- Wang W, Zhou Y, Wang D, et al. Prophylactic extended-field irradiation in patients with cervical cancer: a literature review. Front Oncol. 2020;10:579410. doi: 10.3389/fonc.2020.579410.
- Kim HJ, Chun J, Kim TH, et al. Patterns of locoregional recurrence after radical cystectomy for stage T3-4 bladder cancer: a radiation oncologist’s point of view. Yonsei Med J. 2021;62(7):569–576. doi: 10.3349/ymj.2021.62.7.569.
- Peters M, de Leeuw AAC, Nomden CN, et al. Risk factors for nodal failure after radiochemotherapy and image guided brachytherapy in locally advanced cervical cancer: an EMBRACE analysis. Radiother Oncol. 2021;163:150–158. doi: 10.1016/j.radonc.2021.08.020.
- Spratt DE, Vargas HA, Zumsteg ZS, et al. Patterns of lymph node failure after dose-escalated radiotherapy: implications for extended pelvic lymph node coverage. Eur Urol. 2017;71(1):37–43. doi: 10.1016/j.eururo.2016.07.043.
- de Meric de Bellefon M, Lemanski C, Castan F, et al. Long-term follow-up experience in anal canal cancer treated with intensity-modulated radiation therapy: clinical outcomes, patterns of relapse and predictors of failure. Radiother Oncol. 2020;144:141–147. doi: 10.1016/j.radonc.2019.11.016.
- Sia J, Mou W, Agas RA, et al. Long-term patterns of failure and the value of blood prognostic markers in anal cancers treated with intensity-modulated radiation therapy. Clin Colorectal Cancer. 2022;21(2):e102–e112.
- Hodges JC, Das P, Eng C, et al. Intensity-modulated radiation therapy for the treatment of squamous cell anal cancer with para-aortic nodal involvement. Int J Radiat Oncol Biol Phys. 2009;75(3):791–794. doi: 10.1016/j.ijrobp.2008.11.021.
- Wind KL, Serup-Hansen E, Havelund BM, et al. Definitive therapy for squamous cell carcinoma of the anus with synchronous metastases - a report from the Danish anal cancer group. Acta Oncol. 2022;61(3):321–327. doi: 10.1080/0284186X.2021.1999497.
- Vilarino-Varela MJ, Taylor A, Rockall AG, et al. A verification study of proposed pelvic lymph node localisation guidelines using nanoparticle-enhanced magnetic resonance imaging. Radiother Oncol. 2008;89(2):192–196. doi: 10.1016/j.radonc.2008.07.023.
- Garda AE, Navin PJ, Merrell KW, et al. Patterns of inguinal lymph node metastases in anal canal cancer and recommendations for elective clinical target volume (CTV) delineation. Radiother Oncol. 2020;149:128–133. doi: 10.1016/j.radonc.2020.05.018.
- Fontanilla HP, Klopp AH, Lindberg ME, et al. Anatomic distribution of [(18)F] fluorodeoxyglucose-avid lymph nodes in patients with cervical cancer. Pract Radiat Oncol. 2013;3(1):45–53. doi: 10.1016/j.prro.2012.02.003.
- Wang D, Wang W, Liu X, et al. A modified delineation method of para-aortic nodal clinical target volume in patients with locally advanced cervical cancer. Cancer Med. 2022;11(1):28–39. doi: 10.1002/cam4.4418.
- Small W, Jr., Bosch WR, Harkenrider MM, et al. NRG oncology/RTOG consensus guidelines for delineation of clinical target volume for intensity modulated pelvic radiation therapy in postoperative treatment of endometrial and cervical cancer: an update. Int J Radiat Oncol Biol Phys. 2021;109(2):413–424. doi: 10.1016/j.ijrobp.2020.08.061.