Abstract
Background
We compared the effectiveness of currently available systemic therapies for high-volume metastatic hormone-sensitive prostate cancer (mHSPC) and aimed to establish the optimal treatment regimen.
Material and Methods
We searched multiple databases for randomized controlled trials (RCTs) that evaluated the efficacy of systemic therapy in patients with high-volume mHSPC. Bayesian network meta-analysis was used to indirectly compare overall survival (OS) and progression-free survival (PFS) of various systemic therapies.
Results
Eleven RCTs (6708 participants) finally met the eligibility criteria. Compared with androgen deprivation therapy (ADT) alone, rezvilutamide (REZ) [hazard ratio (HR) = 0.58, 95% confidence interval (CI): 0.44–0.77], abiraterone (ABI) (HR = 0.61, 95% CI: 0.53–0.71), apalutamide (APA) (HR = 0.70, 95% CI: 0.56–0.88), enzalutamide (ENZ) (HR = 0.65, 95% CI: 0.53–0.80), docetaxel (DOC) (HR = 0.72, 95% CI: 0.63–0.84), darolutamide (DAR) + DOC (HR = 0.49, 95% CI: 0.39–0.62), and ABI + DOC (HR = 0.52, 95% CI: 0.38–0.71) significantly improved OS in patients with high-volume mHSPC. Compared with DOC, no advantages were observed for doublet therapies, including REZ, ABI, APA, and ENZ on the basis of ADT, whereas DAR + DOC (HR = 0.68, 95% CI: 0.57–0.82) and ABI + DOC (HR = 0.72, 95% CI: 0.55–0.95) was associated with better OS. The ranking analysis showed that triplet therapy (DAR + DOC + ADT and ABI + DOC + ADT) had the greatest improvement in OS, followed by REZ + ADT. All the regimens showed improved PFS in patients with high-volume mHSPC. Compared with DOC, significant differences were detected for DAR + DOC, ABI + DOC, ENZ + DOC, REZ, and ENZ. According to the ranking analysis, triplet therapy ranked first, followed by ENZ and REZ.
Conclusions
REZ + ADT were the highest ranked doublet therapy for improvement in OS of patients with high-volume mHSPC, second only to triplet therapy (DAR + DOC + ADT and ABI + DOC + ADT).
Introduction
Prostate cancer (PCa) is the second most common malignancy after lung cancer and the fifth leading cause of cancer death in men [Citation1]. Approximately 16% of PCa patients have regional or distant metastases at the time of initial diagnosis [Citation2]. The main treatment methods for PCa are surgical resection and non-surgical treatment, including drug therapy, radiotherapy, chemotherapy, and radionuclide therapy. The 8-year risk of metastases is 3% for patients who undergo radical prostate surgery and 7% for those who receive radiotherapy [Citation3]. For metastatic PCa, ADT with or without a standard nonsteroidal antiandrogen drug (bicalutamide, nilutamide, or flutamide) has been the standard of care (SOC). Although 80% of patients with PCa are significantly better after hormone therapy, the change from hormone-dependent to hormone-insensitive PCa can occur after 12–18 months of treatment and can even lead to death [Citation4].
Approximately 20% of patients with localized PCa progress to metastatic hormone-sensitive PCa (mHSPC) within 5 years; mHSPC has a 5-year survival rate of 30%, and patients with high-volume mHSPC at diagnosis usually have poor survival [Citation5–8]. For mHSPC, ADT using luteinizing hormone-releasing hormone agonists or antagonists, or bilateral orchiectomy is the standard treatment. In-depth research on PCa has resulted in an increase in the number of drugs used for treatment of advanced PCa, such as abiraterone (ABI), enzalutamide (ENZ), apalutamide (APA), and darolutamide. Combination of these drugs, including docetaxel (DOC), with ADT for mHSPC is associated with better survival than with ADT alone [Citation9–14]. For high-volume mHSPC, aggressive treatment is required because of its poor prognosis. The CHART trial (ClinicalTrials.gov, NCT03520478) has shown that rezvilutamide (REZ) also has a good therapeutic effect [Citation15]. However, the effectiveness and safety of these agents in the treatment of high-volume mHSPC are limited, and it is difficult to inform patients about the best treatment options. Therefore, we conducted a systematic review using network meta-analysis (NMA) to analyze current data from RCTs that investigated first-line treatment of high-volume mHSPC to compare the therapeutic effects of these drugs indirectly, to help clinicians and patients select the optimal individualized treatment. The protocol is registered in the International Prospective Register of Systematic Reviews database (PROSPERO: CRD42022359472).
Material and methods
Literature search, study selection and study quality
The objective of this NMA was to compare systemic therapies using next-generation androgen receptor inhibitors (ARIs) and DOC for high-volume mHSPC in patients who had not received another systemic treatment other than ADT (±SOC). PubMed, Embase, Web of Science, Scopus, and Cochrane database were searched to identify all available prospective RCTs before May 2023 on systemic therapy for CHAARTED-defined high-volume mHSPC following the PRISMA guidelines [Citation13,Citation16]. The following keywords were used in our search strategy: (prostate cancer OR prostate carcinoma OR prostatic carcinoma) AND (metastatic OR M1 OR advanced OR high volume) AND (castration sensitive OR castration naive OR hormone sensitive OR hormone naive) AND (randomized). We also reviewed abstracts and presentations from the American Society of Clinical Oncology and the European Society of Medical Oncology. References cited in selected articles, commentaries, editorials, and conference publications of relevant medical societies were manually searched to ensure completeness of our analysis. Following the literature search, all duplicates were excluded.
Studies were considered for inclusion if they investigated high-volume mHSPC in patients who had received systemic therapies compared with those who received ADT, DOC or SOC. High-volume disease was defined as the presence of visceral metastases or ≥4 bone lesions with ≥1 lesion beyond the vertebral bodies and pelvis [Citation13]. Outcomes of interest were overall survival (OS) and progression-free survival (PFS). OS was defined as the time from treatment initiation to death from any cause or to the last follow-up. PFS was defined as the time from treatment initiation to radiological or clinical progression, death or last follow-up. Two authors ascertained whether the publications met the inclusion criteria independently on the basis of the titles and abstracts. Full-text articles were read exhaustively. The Cochrane Collaboration tool was used to assess the risk of bias of the trials [Citation17]. Two authors extracted the data from the included studies independently. Disagreements were resolved by consensus with the coauthors.
Statistical methods
We performed Bayesian NMA to make indirect treatment comparisons using the R software package gemtc. OS and PFS were estimated using the hazard ratio (HR) with 95% confidence interval (CI). The geometry of the treatment network was established using R software. Based on the deviance information criterion, a fixed- or random-effects model was applied to the Bayesian framework model for data analysis. The resulting pooled HRs were graphically depicted in forest plots and a league table. Rank probabilities and surface under the cumulative ranking (SUCRA) scores were calculated to determine the hierarchy of treatments. All statistical analyses were performed using R (version 4.1.1 (R Foundation, Vienna, Austria)). A p value <.05 was adopted to denote statistical significance.
Results
Study selection and characteristics
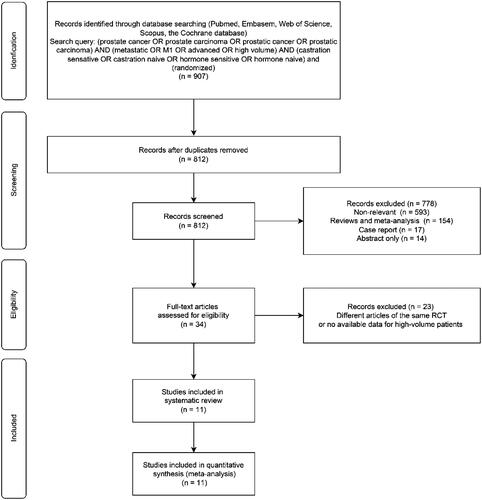
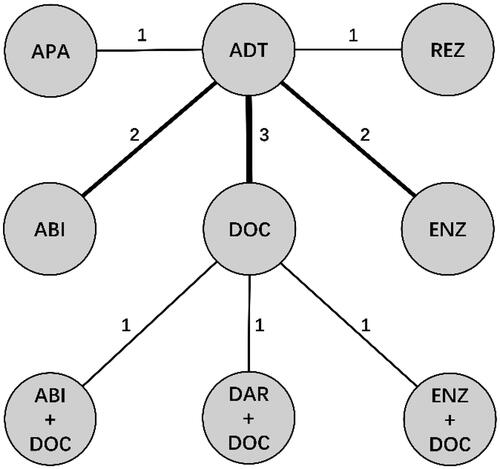
Our initial research identified 907 publications, and 11 RCTs with a total of 6708 high-volume mHSPC patients were included in accordance with the selection criteria () [Citation11,Citation15,Citation18–26]. The characteristics of the enrolled studies are outlined in . ADT (±SOC) alone, doublet therapies, including DOC, ABI, APA, REZ, and ENZ on the basis of ADT, and triplet therapies, including DAR + DOC, ENZ + DOC and ABI + DOC on the basis of ADT, were compared in our study. The treatment network is shown in , in which the thickness of the lines represents the number of comparisons. Based on the deviance information criterion value, a fixed-effect model was adopted for the analysis of OS and PFS.
Table 1. The characteristics of the enrolled studies.
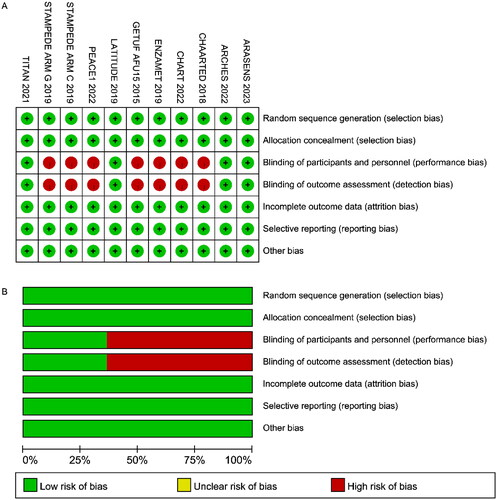
The quality of the studies was evaluated using the Cochrane risk of bias tool (). ARCHES, LATITUDE, and TITAN were double-blind RCTs, and the rest were open-label trials and had high risks of performance and detection bias. All other aspects of the included RCTs were determined to be of high quality.
OS in patients with high-volume mHSPC
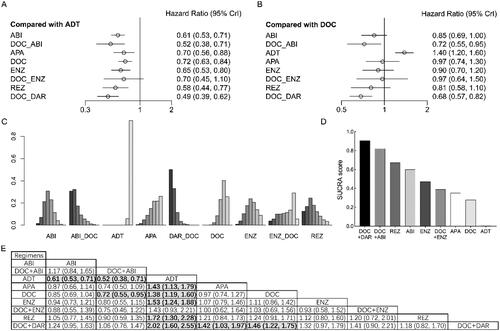
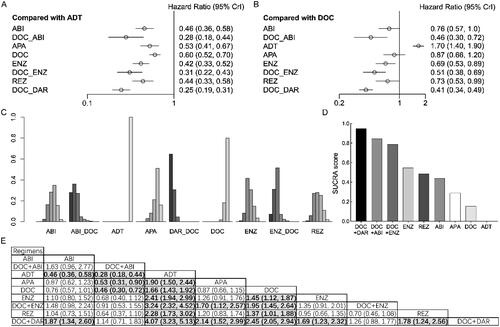
Compared with ADT, ABI (HR = 0.61, 95% CI: 0.53–0.71), APA (HR = 0.70, 95% CI: 0.56–0.88), DOC (HR = 0.72, 95% CI: 0.63–0.84), ENZ (HR = 0.65, 95% CI: 0.53–0.80), REZ (HR = 0.58, 95% CI: 0.44–0.77), DAR + DOC (HR = 0.49, 95% CI: 0.39–0.62), and ABI + DOC (HR = 0.52, 95% CI: 0.38–0.71) significantly improved OS in patients with high-volume mHSPC (). However, we failed to detect a significant difference with ENZ + DOC (HR = 0.70, 95% CI: 0.45–1.10). Compared with DOC, no advantages were observed for doublet therapies, whereas DAR + DOC (HR = 0.68, 95% CI: 0.57–0.82) and ABI + DOC (HR = 0.72, 95% CI: 0.55–0.95) was associated with better OS. According to the ranking analysis, DAR + DOC (SUCRA score: 0.907) and ABI + DOC (SUCRA score: 0.822) had the best performance for OS, followed by REZ (SUCRA score: 0.673), ABI (SUCRA score: 0.600) and ENZ (SUCRA score: 0.472).
Figure 4. Overall survival (OS) in patients with high-volume metastatic hormone-sensitive prostate cancer (mHSPC). (A) OS for each intervention compared with androgen deprivation therapy (ADT). (B) OS for each intervention compared with docetaxel (DOC). (C) Ranking plot based on the probabilities of interventions. (D) Surface under the cumulative ranking (SUCRA) score for each intervention. (E) League table of OS.
PFS in patients with high-volume mHSPC
Compared with ADT, all the regimens showed improved PFS in patients with high-volume mHSPC: ABI (HR = 0.46, 95% CI: 0.36–0.58), APA (HR = 0.53, 95% CI: 0.41–0.67), DOC (HR = 0.60, 95% CI: 0.52–0.70), ENZ (HR = 0.42, 95% CI: 0.33–0.52), REZ (HR = 0.44, 95% CI: 0.33–0.58), DAR + DOC (HR = 0.25, 95% CI: 0.19–0.31), ABI + DOC (HR = 0.28, 95% CI: 0.18–0.44), and ENZ + DOC (HR = 0.31 95% CI: 0.22–0.43) (). Compared with DOC, significant differences were detected for REZ (HR = 0.73, 95% CI: 0.53–0.99), ENZ (HR = 0.69, 95% CI: 0.53–0.89), DAR + DOC (HR = 0.41, 95% CI: 0.34–0.49), ABI + DOC (HR = 0.46, 95% CI: 0.30–0.72), and ENZ + DOC (HR = 0.51, 95% CI: 0.38–0.69). According to the ranking analysis, DAR + DOC (SUCRA score: 0.950) had the best performance for PFS, followed by ABI + DOC (SUCRA score: 0.846), ENZ + DOC (SUCRA score: 0.788), ENZ (SUCRA score: 0.548), REZ (SUCRA score: 0.486), and ABI (SUCRA score: 0.438).
Figure 5. Progression-free survival (PFS) in patients with high-volume metastatic hormone-sensitive prostate cancer (mHSPC). (A) PFS for each intervention compared with androgen deprivation therapy (ADT). (B) PFS for each intervention compared with docetaxel (DOC). (C) Ranking plot based on the probabilities of interventions. (D) Surface under the cumulative ranking (SUCRA) score for each intervention. (E) League table of PFS.
Discussion
The best standard treatment for mHSPC is systemic therapies, including ARIs (ABI, APA or ENZ) or DOC plus ABI or DOC plus DAR on the basis of ADT. Many NMAs have been conducted to compare the above first-line treatment options [Citation27–36]. The novel androgen-receptor inhibitor REZ has been reported to significantly prolong OS and PFS in patients with high-volume mHSPC, compared with ADT + bicalutamide [Citation15]. To our knowledge, the present NMA is the first to compare the efficacy of REZ with other first-line therapies. The ranking analysis showed that REZ had the greatest improvement in OS compared with common doublet therapy (ABI, ENZ, APA or DOC combined with ADT) in patients with high-volume mHSPC, and it was significantly superior to ADT alone. The effect of REZ in reducing the risk of progression was similar to that of other doublet treatments. REZ was not significantly less effective than triplet therapies in reducing the risk of death. Based on the ranking analysis, REZ + ADT is the most effective doublet therapy for improvement of OS in patients with high-volume mHSPC, only second to DAR + DOC + ADT and ABI + DOC + ADT triplet therapy.
We found no significant difference in OS and PFS between REZ, ABI, ENZ, APA, or DOC in patients with high-volume mHSPC, except that REZ and ENZ significantly improved PFS over DOC, and these doublet therapies significantly improved OS and PFS compared with ADT alone. In some previous NMAs, there was no significant difference in OS between ENZ + ADT and ADT alone in patients with high-volume mHSPC [Citation33,Citation35,Citation36]. It was previously believed that ENZ only had a favorable effect in the low-volume mHSPC patients and had no survival benefit in the high-volume group. The previous NMAs did not include ARCHES that updated the OS data of the high-volume subgroup, which resulted in an underestimation of the efficacy of ENZ in high-volume mHSPC. We included the updated ARCHES result which reported the final follow-up data, which reported that patients in the high-volume subgroup who received ENZ + ADT had a 43% significantly lower risk of death compared with patients who received ADT alone [Citation18]. The results for APA in most NMAs were consistent with our results; only Mori et al. reported that APA + ADT did not have significant survival benefits compared with ADT alone [Citation33]. It is worth noting that Mori et al. used the frequentist method for NMA, and the follow-up data of TITAN in their analysis is less mature [Citation10]. The final survival analysis of TITAN reported that APA, compared with placebo, significantly prolonged OS (HR = 0.70, 95% CI: 0.56–0.88) in patients with high-volume disease [Citation19]. The efficacy of other doublet therapies compared with ADT alone in all NMAs was consistent with our results.
Compared with DOC, ARIs-based first-line doublet therapies was not significantly associated with longer OS in high-volume mHSPC patients. We found that, compared with DOC, there were marginal differences in PFS (p = .05) for ABI, and a significant difference in PFS for REZ and ENZ in high-volume patients. Meri et al. reported that, compared with DOC, ENZ significantly improved PFS in high-volume patients, but ABI and APA did not [Citation33]. Yanagisawa et al. found that, compared with DOC, ABI significantly reduced the risk of progression in mHSPC patients with high-volume [Citation37]. Unfortunately, no PFS data have been reported for a head-to-head comparison of ARSI-based doublet therapy with DOC in high-volume patients, so our results need to be validated in high-quality randomized trials.
Our NMA revealed that REZ ranked first in terms of the likelihood of OS benefit in high-volume patients, and ABI ranked second among the current doublet regimens. At present, NMA for mature follow-up has reached a unified conclusion that ABI ranked first among first-line therapies for improvement of OS in high-volume mHSPC patients [Citation28,Citation31,Citation33,Citation35,Citation38]. Our findings were similar to those studies, and surprisingly, REZ ranks higher than ABI and may be a more effective treatment. In contrast, previous NMAs have reported that ENZ has a greater likelihood of being the preferred therapy for improving PFS in high-volume disease [Citation33], which is consistent with our results. In our study, REZ was second only to ENZ in providing the maximal PFS in patients with high-volume disease.
Compared with REZ + ADT, triplet therapy did not significantly prolong OS and PFS in high-volume patients, except for a significant PFS benefit from DAR + DOC + ADC. Similarly, the addition of DOC to ARIs (ABI or ENZ) did not bring significant OS benefits compared with ABI, ENZ, or APA, or PFS benefits compared with ABI or ENZ. Nevertheless, compared with APA, triplet therapy had a significant PFS benefit, and DAR + DOC + ADT had a significant OS benefit. DAR + DOC + ADT significantly improved PFS in high-volume patients over all doublet therapies (REZ, ENZ, ABI, APA, and DOC). Yanagisawa et al. reported that, compared with ABI, triplet therapy had no significant benefit for OS in high-volume patients, whereas triplet therapy significantly improved PFS [Citation37]. In ranking analysis for the maximal OS benefit, DAR + DOC + ADT ranked first, followed by ABI + DOC + ADT, REZ + ADT, ABI + ADT, and ENZ + ADT ranked fifth. The data for ENZ + DOC + ADT were derived from a subgroup analysis of ENZAMET [Citation11]. The follow-up time of ENZAMET was so short that the effect on OS was highly unreliable, which was acknowledged by the authors. Regarding the preferred therapies for prolonging PFS, DAR + DOC + ADT, ABI + DOC + ADT and ENZ + DOC + ADT ranked first, second and third, respectively, and higher than all the doublet regimens. The number of cases with clinical progression during ENZ treatment in ENZAMET was sufficient so that PFS, an earlier outcome endpoint, was statistically reliable [Citation11].
Some limitations of our NMA were inevitable and may have affected interpretation of the results. First, most of the survival data for high-volume mHSPC patients were derived from subgroup analysis of the RCTs. Second, the proportion of non-Asian patients in the CHART study was small, although it has been confirmed that the safety and efficacy of other second-generation ARSIs are similar in Asian and non-Asian people. Third, the data for high-volume patients in CHART and ENZAMET have not reached the final follow-up maturity, and there was no median follow-up time.
Conclusions
We did not find significant differences in OS and PFS between REZ + ADT and available doublet or triplet therapies in patients with high-volume mHSPC, except for superiority to DOC + ADT and inferiority to DAR + DOC + ADT in terms of PFS benefit. However, Bayesian ranking analysis showed that REZ + ADT was the most effective doublet treatment to improve OS for high-volume mHSPC, second only to DAR + DOC + ADT and ABI + DOC + ADT. Triplet therapies had a high likelihood of being the preferred approach for prolonging PFS, followed by ENZ + ADT and REZ + ADT.
Authors’ contributions
G.Z.: Conceptualization, Data curation, Formal analysis, Investigation, Validation, Supervision; Z.Z.: Conceptualization, Investigation, Data Curation, Methodology, Writing—Original Draft, Project administration; S.L.: Investigation, Data Curation, Writing—Original Draft; J.M., T.L. and F.L.: Writing—Original Draft, Writing—Review and Editing, Supervision.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data is available upon request from corresponding author.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708.
- Zelefsky MJ, Eastham JA, Cronin AM, et al. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. J Clin Oncol. 2010;28(9):1508–1513. doi: 10.1200/JCO.2009.22.2265.
- Ichikawa T, Suzuki H, Ueda T, et al. Hormone treatment for prostate cancer: current issues and future directions. Cancer Chemother Pharmacol. 2005;56(Suppl 1):58–63. doi: 10.1007/s00280-005-0100-x.
- Xie W, Regan MM, Buyse M, et al. Metastasis-free survival is a strong surrogate of overall survival in localized prostate cancer. J Clin Oncol. 2017;35(27):3097–3104. doi: 10.1200/JCO.2017.73.9987.
- Tangen CM, Hussain MHA, Higano CS, et al. Improved overall survival trends of men with newly diagnosed M1 prostate cancer: a SWOG phase III trial experience (S8494, S8894 and S9346). J Urol. 2012;188(4):1164–1169. doi: 10.1016/j.juro.2012.06.046.
- De Bruycker A, Lambert B, Claeys T, et al. Prevalence and prognosis of low-volume, oligorecurrent, hormone-sensitive prostate cancer amenable to lesion ablative therapy. BJU Int. 2017;120(6):815–821. doi: 10.1111/bju.13938.
- Kinsey EN, Zhang T, Armstrong AJ. Metastatic hormone-sensitive prostate cancer: a review of the current treatment landscape. Cancer J. 2020;26(1):64–75. doi: 10.1097/PPO.0000000000000418.
- Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. Prostate. 2022;82(13):1237–1247. doi: 10.1200/JCO.19.00799.
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):13–24. doi: 10.1056/NEJMoa1903307.
- Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121–131. doi: 10.1056/NEJMoa1903835.
- Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352–360. doi: 10.1056/NEJMoa1704174.
- Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–746. doi: 10.1056/NEJMoa1503747.
- Smith MR, Hussain M, Saad F, et al. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med. 2022;386(12):1132–1142. doi: 10.1056/NEJMoa2119115.
- Gu W, Han W, Luo H, et al. Rezvilutamide versus bicalutamide in combination with androgen-deprivation therapy in patients with high-volume, metastatic, hormone-sensitive prostate cancer (CHART): a randomised, open-label, phase 3 trial. Lancet Oncol. 2022;23(10):1249–1260. doi: 10.1016/S1470-2045(22)00507-1.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269, W64. doi: 10.7326/0003-4819-151-4-200908180-00135.
- Higgins JP, Altman DG, Gøtzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928.
- Armstrong AJ, Azad AA, Iguchi T, et al. Improved survival with enzalutamide in patients with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2022;40(15):1616–1622. doi: 10.1200/JCO.22.00193.
- Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in patients with metastatic castration-sensitive prostate cancer: final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol. 2021;39(20):2294–2303. doi: 10.1200/JCO.20.03488.
- Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686–700. doi: 10.1016/S1470-2045(19)30082-8.
- Hoyle AP, Ali A, James ND, et al. Abiraterone in “high-” and “low-risk” metastatic hormone-sensitive prostate cancer. Eur Urol. 2019;76(6):719–728. doi: 10.1016/j.eururo.2019.08.006.
- Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-Term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36(11):1080–1087. doi: 10.1200/JCO.2017.75.3657.
- Gravis G, Boher JM, Joly F, et al. Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 trial. Eur Urol. 2016;70(2):256–262. doi: 10.1016/j.eururo.2015.11.005.
- Clarke NW, Ali A, Ingleby FC, et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results from the STAMPEDE trial. Ann Oncol. 2020;31(3):442. doi: 10.1093/annonc/mdz396.
- Fizazi K, Foulon S, Carles J, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet. 2022;399(10336):1695–1707. doi: 10.1016/S0140-6736(22)00367-1.
- Hussain M, Tombal B, Saad F, et al. Darolutamide plus androgen-deprivation therapy and docetaxel in metastatic hormone-sensitive prostate cancer by disease volume and risk subgroups in the phase III ARASENS trial. J Clin Oncol. 2023;41(20):3595–3607. doi: 10.1200/JCO.23.00041.
- Wallis CJD, Klaassen Z, Bhindi B, et al. Comparison of abiraterone acetate and docetaxel with androgen deprivation therapy in high-risk and metastatic hormone-naive prostate cancer: a systematic review and network meta-analysis. Eur Urol. 2018;73(6):834–844. doi: 10.1016/j.eururo.2017.10.002.
- Chen J, Ni Y, Sun G, et al. Comparison of current systemic combination therapies for metastatic hormone-sensitive prostate cancer and selection of candidates for optimal treatment: a systematic review and Bayesian network meta-analysis. Front Oncol. 2020;10:519388. doi: 10.3389/fonc.2020.519388.
- Marchioni M, Di Nicola M, Primiceri G, et al. New antiandrogen compounds compared to docetaxel for metastatic hormone sensitive prostate cancer: results from a network meta-analysis. J Urol. 2020;203(4):751–759. doi: 10.1097/JU.0000000000000636.
- Sathianathen NJ, Koschel S, Thangasamy IA, et al. Indirect comparisons of efficacy between combination approaches in metastatic hormone-sensitive prostate cancer: a systematic review and network meta-analysis. Eur Urol. 2020;77(3):365–372. doi: 10.1016/j.eururo.2019.09.004.
- Wang Y, Gui H, Wang J, et al. Comparative efficacy of combined radiotherapy, systemic therapy, and androgen deprivation therapy for metastatic hormone-sensitive prostate cancer: a network meta-analysis and systematic review. Front Oncol. 2020;10:567616. doi: 10.3389/fonc.2020.567616.
- Ferro M, Lucarelli G, Crocetto F, et al. First-line systemic therapy for metastatic castration-sensitive prostate cancer: an updated systematic review with novel findings. Crit Rev Oncol Hematol. 2021;157:103198. doi: 10.1016/j.critrevonc.2020.103198.
- Mori K, Mostafaei H, Sari Motlagh R, et al. Systemic therapies for metastatic hormone-sensitive prostate cancer: network meta-analysis. BJU Int. 2022;129(4):423–433. doi: 10.1111/bju.15507.
- Roy S, Sayyid R, Saad F, et al. Addition of docetaxel to androgen receptor axis-targeted therapy and androgen deprivation therapy in metastatic hormone-sensitive prostate cancer: a network meta-analysis. Eur Urol Oncol. 2022;5(5):494–502. doi: 10.1016/j.euo.2022.06.003.
- Wenzel M, Wurnschimmel C, Nocera L, et al. Overall survival after systemic treatment in high-volume versus low-volume metastatic hormone-sensitive prostate cancer: systematic review and network meta-analysis. Eur Urol Focus. 2022;8(2):399–408. doi: 10.1016/j.euf.2021.04.003.
- Menges D, Yebyo HG, Sivec-Muniz S, et al. Treatments for metastatic hormone-sensitive prostate cancer: systematic review, network meta-analysis, and benefit-harm assessment. Eur Urol Oncol. 2022;5(6):605–616. doi: 10.1016/j.euo.2022.04.007.
- Yanagisawa T, Rajwa P, Thibault C, et al. Androgen receptor signaling inhibitors in addition to docetaxel with androgen deprivation therapy for metastatic hormone-sensitive prostate cancer: a systematic review and meta-analysis. Eur Urol. 2022;82(6):584–598. doi: 10.1016/j.eururo.2022.08.002.
- Mandel P, Hoeh B, Wenzel M, et al. Triplet or doublet therapy in metastatic hormone-sensitive prostate cancer patients: a systematic review and network meta-analysis. Eur Urol Focus. 2023. doi: 10.1016/j.euf.2022.08.007.