Background
Meningiomas are the most common primary central nervous system tumors [Citation1] and they were classified as WHO Grade 1 (benign), 2 (atypical), and 3 (anaplastic) lesions on the basis of local invasiveness and cellular features of atypia [Citation2]. Currently, surgery is the mainstay of the treatment especially for lesions in favorable locations (e.g., convexity meningiomas). Different radiotherapy (RT) approaches (radiosurgery, stereotactic RT, conventional RT, and particle therapy) are often used adjuvantly for sub-totally resected tumors, recurrent tumors, or Grade 2/3 histologies and as an alternative to surgery in definitive setting when surgery could be associated with significant comorbidity.
Despite the 5-year recurrence-free survival rate after complete resection of meningioma being 95% for WHO Grade 1, recurrence of disease is not an anecdotal possibility for WHO Grade 2 and 3 even after adjuvant RT [Citation3,Citation4].
In the absence of clinical controlled trials, treatment decision for recurrent meningiomas is tailored by local experience and clinical practice. Further treatment options are currently limited and the morbidity and mortality among these patients are significantly high. In this scenario, reirradiation can be considered [Citation5–7], but the efficacy and toxicity, as well as the RT modality, treatment dose, and patient accrual are not well established.
Proton therapy (PT), thanks to the typical dose fall-off of the Bragg peak, could minimize the risk of side effects compared to conventional photon therapy, allowing for the treatment of recurrent tumors and ultimately reducing the possible detrimental effect of re-irradiation on the quality of life.
Based on this rationale, we report our institutional experience on re-irradiation with PT focusing on the clinical outcome, toxicity, and prognostic factors that condition survival. Data have been collected prospectively yet analyzed retrospectively.
The primary endpoint was progression-free survival (PFS). Secondary outcomes included overall survival (OS) and treatment-related toxicity.
Material and methods
Between January 2015 and June 2022, 32 consecutive patients with recurrent meningioma were re-irradiated with active scanning PT at our institution. All treated targets were defined as ‘in-field’ with respect to previous irradiation (95% of the volume of failure was within the 95% isodose line). Treatment planning was based on MRI with contrast enhancement medium administration. On the T1-weighted sequence, contrasted tumor enhancement was delineated as the gross tumor volume (GTV). While the clinical target volume (CTV) included GTV plus concerning areas for microscopic disease, such as surgical cavities for patients who underwent resections before re-irradiation. The planning tumor volume (PTV) added a 3-mm uniform expansion to the CTV to account for setup error and motion. All patients performed also a 68-Ga-DOTATOC-PET scan to identify the so-called Biological Tumor Volume (BTV). Treatment planning was generated with active beam scanning PT (Proteus Plus PT system – Ion Beam Application SA, Louvain‐la‐Neuve, Belgium) using 3–4 fields with single or multiple field optimization technique guided by the previously published data [Citation8]. Treatment plans were calculated and optimized in RayStation version 6.0.0.24 (RaySearch Laboratories AB, Stockholm, Sweden). The prescription dose ranged from 50.4 to 60 GyRBE (relative biologic effectiveness [RBE]), with a median dose of 54 GyRBE in 30 fractions. Biologically equivalent doses in 2 Gy fractions (EQD2) for normal tissue and tumor were estimated for each previous course (a/b = 2 for brain tissue and a/b = 4 for meningioma), as well as the biological effective dose (BED) [Citation9]. In order to estimate a cumulative total dose to the organs at risk, previous RT DICOM treatment plans were digitally fused with rigid registration to the PT plan; where the digital plan was not available dose statistics were obtained from the paper records. The dose constraints applied to critical normal structures were highly individualized. A partial recovery of (30%) of normal tissues at least was assumed from prior radiation therapy [Citation10]. Neither a uniform policy was used in making assumptions about normal tissue recovery from prior radiation, nor a minimum time interval from prior radiation was required. Desired dose constraints were then formulated, taking into account the maximum dose received by the organs at risk, the remaining radiotolerance, and the estimated recovery of the damage. Median age and KPS at re-irradiation were 61 years (range 34–89) and 90 (range 60–100), respectively. All patients recurred after the previous RT: 37.5% after Gamma-knife, 31.5% after Cyber-Knife, 25% after Linac-based RT, and 6% after PT. Twelve patients (37.5%) were identified with WHO Grade 1 disease, thirteen patients had Grade 2 disease (40%), and three had Grade 3 disease (10%). The four patients (12.5%) who had no histologic sampling were grouped with the Grade 1 patients for further analysis. As a consequence that patients have been treated over many years, the tumor was graded according to the WHO classification valid in use at the time of diagnosis. The median time from prior RT to reirradiation was 66 months (range 4–288 months) and the median GTV was 43 cc (range 1.2–225.5 cc). Twenty-one patients (65%) had additional surgery before re-irradiation and six patients showed a more aggressive histological sample. After surgery, re-irradiation was performed in an adjuvant setting or at the time of relapse in 11 and 10 patients, respectively.
Toxicity was assessed according to Common Terminology Criteria for Adverse Events version 5.0 (U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES). Follow-up consisted in serial magnetic resonance imaging (MRI) and clinical examination; the first MRI was performed after 3 months from the end of PT and then at 3–6 monthly intervals for a total of 5 years depending on the tumor grade.
Patient baseline clinical and treatment characteristics are summarized in .
Table 1. Patient baseline clinical and treatment characteristics.
The protocol was reviewed and approved by the local ethics committee and informed consent was obtained by each patient.
Statistical analysis
Median, interquartile range (IQR) and/or range were obtained for quantitative measurements, such as months of follow-up and time between 2 RT treatments. The descriptive analysis of patient’s samples also involved the distribution frequency (absolute and percentage).
Associations between qualitative variables were analyzed with the Fisher exact test (significant p value ≤ .05).
The cutoff values for EQD2 tumor dose and BED cumulative of the brain for the risk of RN were established with higher sensitivity and specificity. Receiver-operating characteristic (ROC) curve analyses were performed for EQD2 tumor dose and BED cumulative brain.
PFS and OS were estimated from the start of PT using the Kaplan–Meier method. The log-rank test was performed to compare the entire survival experience between groups (significant p value ≤ .05).
The statistical analysis was conducted using Sas System 9.4 software (SAS Institute Inc., Cary, NC).
Results
Local tumor control and survival
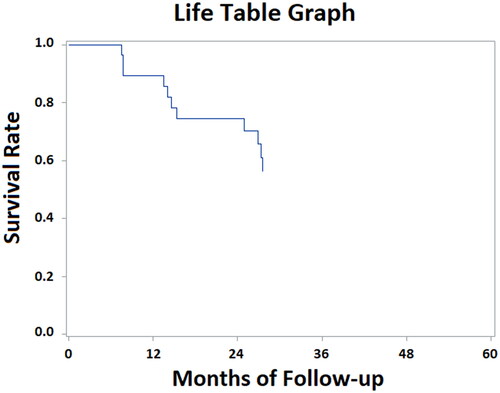
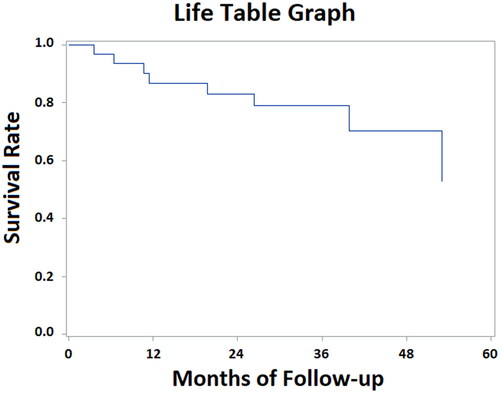
At the median follow-up of 27 months (range 1–72 months), 1-year and 2-year PFS were 89.4% and 74.5%, respectively (). Median PFS was not reached in the WHO Grade 1 meningioma (> 72 months) whilst PFS for WHO Grade 2 and 3 meningioma was 27.5 and 14.1 months, respectively. Histology appears to be relatively significant in terms of PFS (p: 0.06): 1-year and 2-year PFS were 100% for Grade 1, 100%, and 76% for Grade 2, 66%, and 50% for Grade 3, respectively. Other several factors analyzed (tumor location, gender, age, time between radiation courses, KPS, PT dose, surgery before re-irradiation, tumor volume, and total EQD2 tumor dose) did not point out any statistically significant correlation on PFS.
1-Year and 2-year OS were 86.6% and 83.0%, respectively (). Median OS was 47.5 months for WHO Grade 2 meningioma; however, it was not reached in the WHO Grade 1 and Grade 3 groups. Regarding histology, no significant difference in terms of OS was found (p: 0.6). 2-year OS was 87% for Grade 1, 86% for Grade 2, 66% for Grade 3, respectively. Similarly, the same factors analyzed for PFS confirmed no significant impact on OS.
At the last follow-up, the rate of local recurrence was 34% (11 patients) and 6 meningioma-related deaths were registered (18.5%). Median time of recurrence was 13.4 months (range 6.1–26.3 months). Seven patients (64%) had in-field failures, one patient (9%) had marginal failure and three patients (27%) had both in and out-of-field failures. One patient (9%) underwent salvage surgery, one patient was treated with a third course of RT and salvage surgery thereafter (9%), one patient received chemotherapy (9%), and one patient had radio-metabolic treatment (9%); the remaining seven patients (64%) received only best supportive care.
Treatment toxicity
All patients completed the treatment without breaks. The treatment was well tolerated with mild symptoms; no registered acute Grade 3 or higher toxicities were reported except a case of Grade 3 erythema, two cases of Grade 3 seizure and a case of Grade 3 hydrocephalus that needed ventriculoperitoneal drain after three months from the end of PT. Similarly, late toxicities were limited to Grade 2 or less, except two cases of Grade 3 seizure (). At the median follow-up of 27 months 20 patients (62%) had stable symptoms, seven patients (21%) had improved symptoms while five patients (17%) had a worsening deficit.
Table 2. Acute and late treatment-related toxicity.
Five patients (14%) developed radionecrosis (RN – diagnosed at imaging) with a median time of 3.4 months from the end of PT (range 3–8.8 months) and with a median time from prior RT to reirradiation of 29.5 months (range 12.5–45.6 months). RN was defined as any areas of MRI contrast-enhancement (T1 sequence post contrast medium administration) involving the brain tissue and outside the target volume, with low perfusion, and no pathological metabolic uptake at 68-Ga-DOTATOC-PET image. Two patients developed Grade 1 RN, one patient with Grade 2 and two patients had Grade 3 RN. Grade 2 RN was positively treated with dexamethasone, however in Grade 3 RN and due to the poor response to dexamethasone, treatment was with bevacizumab (median 6 cycles) with a stability/decrease of RN and improvement of symptoms. All patients with RN were WHO Grade 2 (40%) or WHO Grade 3 meningiomas (60%) and were re-irradiated with a median dose of 60 GyRBE (range 54–60 GyRBE). The median cumulative EQD2 tumor prescription dose and EQD2 brain max dose for patients with RN was 120 GyRBE (IQR = 9 GyRBE) and 122 GyRBE (IQR = 4 GyRBE), respectively. In the whole population, the median cumulative EQD2 tumor prescription dose was 98 GyRBE (IQR = 18 GyRBE) and the ROC curve (area under curve 0.878) identified the highest sensitivity (100%) and specificity (70%) values at cutoff 104 GyRBE for the risk of RN. For cumulative max BED brain (median = 206 and IQR = 49) the cutoff identified by the ROC curve (area under curve 0.570), in predicting the risk of RN, is 235 (sensitivity = 60% and specificity = 81%). No cumulative EQD2 brain dose showed a significant correlation with the occurrence of RN. No cumulative EQD2 tumor dose and EQD2 brain dose were statistically correlated with local control. Moreover, in our analysis, we did not see any statistical correlation between time from radiation treatments and RN appearance.
Discussion
In the last decades, recurrences after RT in patients with meningioma are an evolving and challenging situation, but so far, their management is often driven by a local experience without the support of clinical randomized trials with strong evidence. In several cases, the indication of re-irradiation is excluded for a number of reasons: initial RT has often fully exhausted the radiation tolerance of surrounding normal tissue, an extension of meningioma, and poor performance status of the patient; in this contest clinical reports of re-irradiation are limited and consequently, the evidence is moderate.
The physical dose distribution of protons (typical dose fall-off of the Bragg peak) makes the PT an attractive therapeutic option. In the past years, it is well established from pre-clinical and plan comparison studies that PT not only can deliver higher dose conformity but also reduces the mean and minimum dose to the surrounding tissue ultimately providing a better sparing of the organs at risk near the target [Citation11–13].
Previous published studies have shown a satisfying clinical outcome after re-irradiation for Grade 1 meningioma, but high-grade meningioma seem to have a poor prognosis, with a worse PFS comparing with benign lesions [Citation5,Citation14–19].
Wojcieszynski et al. in their analysis of 19 re-irradiated patients with either fractionated-SRT (median dose = 50.4 Gy) or SRS (median dose = 15 Gy) reported a median 1-year PFS of 57 months (92%) for Gade 1 vs. 8 months and 17% for Grade 2/3, respectively. Lin et al. reviewed 43 patients treated with SRS or EBRT showing a global 1-year, 2-year PFS of 73%, 63% and 1-year, 2-year OS of 93%, 80%, respectively. Their results confirmed the relatively poor results in terms of PFS for aggressive meningiomas (median for Grade 2/3 and Grade 1, respectively, 26 months vs. 41 months; 2-year, 50% vs. 92%). Furthermore, Kim et al. proved in 33 patients who repeated SRS with a median dose of 14 Gy, a benefit of re-irradiation for benign lesions and worse outcome for Grade 2 and 3 (median PFS Grade 1 vs. Grade 2 and 3 60 months vs. 12 months, respectively).
Consistent with the above-mentioned data, our results reported a similar or better outcome in terms of PFS (1-year and 2-year PFS: 89.4% and 74.5%, respectively) and OS (1-year and 2-year OS: 86.6% and 83%, respectively). Also in our population, histology appeared to be an important prognostic factor for PFS as well as OS (median PFS not reached, 27.5 months and 14.1 months for Grade 1–3, respectively; median OS not reached and 47.5 months for Grade 1–3, respectively), but generally with better results compared with the historical data.
To our knowledge, this study represents the largest monocentric experience of re-irradiation with PT for recurrence meningioma, with at least a similar outcome compared with the other published researches [Citation7,Citation19].
El Shafie et al. analyzed 42 patients re-irradiated with ions (only 19% treated with PT) with 1-year and 2-year PFS of 71% and 56,5% and a median PFS of 34,3 months. 1-year OS after re-irradiation was 89,6% and 2-year OS 71.4% with a median OS of 61 months. The authors confirmed the negative effect of histology on PFS (median PFS of 25,7 months for Grade 2 and 3, not reached for Grade 1) and OS (median OS not reached for Grade 1, 45.5 months for Grade 2 and 3). In addition, Imber et al. reported in their analysis of 16 patients irradiated with PT, 1-year and 2-year PFS of 80% and 43% and 1-year and 2-year OS of 94% and 73%, respectively. Also, their results were negative for aggressive meningiomas (Grade 2 and 3).
Till now, radiation oncology community is unwilling to strongly support re-irradiation for recurrent meningiomas because of concern of late radiation toxicity, in particular RN. Actually, these studies showed that re-irradiation is an acceptable treatment modality with a low-moderate risk of Grade 2 or more RN, around 15–21% [Citation5,Citation7,Citation14–17]. In some cases, this risk may increase above a cumulative EQD2 brain dose of 120 Gy [Citation5].
The hesitation to propose re-irradiation is further accentuated with the possibility to use PT due to the historical concern about physical characteristics of proton (range uncertainties, RBE variable). However, several trials showed that PT was associated with significantly higher rates of MRI sequences T1c + T2 changes compared with photon therapy for brain tumors but the rates of symptomatic RN following PT was as uncommon as the conventional photon-based group [Citation20,Citation21]. In our series, we reported a rate of RN of 14% (8.5% for RN Grade 2 or more), with a median cumulative EQD2 brain dose of 122 GyRBE (range 111.7–123.2), in line with trials previously published, despite of the significantly large irradiated volume (median GTV 43 cc, range 1.2−225.5 cc).
Due to a lack of available data, the optimal choice of RT modality for reirradiation of recurrent meningiomas remains poorly defined. Dose sparing with protons or photons in meningioma re-irradiation is highly case-specific and the optimal treatment modality needs to be assessed on an individual basis. Based on the results of the clinical studies discussed above, SRS seems to be a safe and effective treatment for small Grade 1 meningioma. For large recurrences of Grade 2 and 3 meningioma, where probably larger margins are recommended, conventional fractionation (EBRT, fractionated-SRT, and IMRT) and in particular PT should be the preferred options.
There were several limitations to our study including its retrospective nature, the relatively small size of patients, the heterogenous population and different prior radiation treatments and the lack of quality-of-life analysis. Furthermore, the median follow-up of 27 months may still be short to assess late events of radiation toxicity and the durability of responses observed.
Conclusion
Re-irradiation with PT of meningiomas progressing after previous RT appears to be feasible with promising clinical outcomes and an acceptable toxicity profile, even for aggressive and large-volume meningiomas. Longer follow-up and prospective trials are necessary to assess the definitive efficacy of particle therapy in the contest of re-irradiation for recurrent meningioma.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author [DS]. The data are not publicly available due to ethical restriction.
Additional information
Funding
References
- Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other Central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017;19(5):v1–v88. doi: 10.1093/neuonc/nox158.
- Louis DN, Perry A, Reifenberger G, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1.
- Rogers L, Barani I, Chamberlain M, et al. Meningiomas: knowledge base, treatment outcomes, and uncertainties: a RANO review. J Neurosurg. 2015;122(1):4–23. doi: 10.3171/2014.7.JNS131644.
- Hug EB, Devries A, Thornton AF, et al. Management of atypical and malignant meningiomas: role of high-dose, 3D-conformal radiation therapy. J Neurooncol. 2000;48(2):151–160. doi: 10.1023/a:1006434124794.
- Lin AJ, Hui C, Dahiya S, et al. Radiologic response and disease control of recurrent intracranial meningiomas treated with reirradiation. Int J Radiat Oncol Biol Phys. 2018;102(1):194–203. doi: 10.1016/j.ijrobp.2018.05.011.
- Moinay K, Do Hee L, Hyun Jung K. RN analysis of the results of recurrent intracranial meningiomas treated with re-radiosurgery. Clin Neurol Neurosurg . 2017;153:93–101.
- Imber BS, Neal B, Casey DL, et al. Clinical outcomes of recurrent intracranial meningiomas treated with proton beam reirradiation. Int J Part Ther. 2019;5(4):11–22. doi: 10.14338/IJPT-18-00045.1.
- Tommasino F, Widesott L, Fracchiolla F, et al. Clinical implementation in proton therapy of multi-field optimization by a hybrid method combining conventional PTV with robust optimization. Phys Med Biol. 2020;65(4):045002. doi: 10.1088/1361-6560/ab63b9.
- van Leeuwen CM, Oei AL, Crezee J, et al. The alfa and beta of tumours: a review of parameters of the linear-quadratic model, derived from clinical radiotherapy studies. Radiat Oncol. 2018;13(1):96. doi: 10.1186/s13014-018-1040-z.
- Nieder C, Milas L, Ang KK. Tissue tolerance to reirradiation. Semin Radiat Oncol. 2000;10(3):200–209. doi: 10.1053/srao.2000.6593.
- Simone CB, Ly D, Dan TD, et al. Comparison of intensity-modulated radiotherapy, adaptive radiotherapy, proton radiotherapy, and adaptive proton radiotherapy for treatment of locally advanced head and neck cancer. Radiother Oncol. 2011;101(3):376–382. doi: 10.1016/j.radonc.2011.05.028.
- De Ruysscher D, Mark Lodge M, Jones B, et al. Charged particles in radiotherapy: a 5-year update of a systematic review. Radiother Oncol. 2012;103(1):5–7. doi: 10.1016/j.radonc.2012.01.003.
- Poel R, Lobmaier AS, Andratschke N, et al. Dosimetric comparison of protons vs photons in re-irradiation of intracranial meningioma. Br J Radiol. 2019;92(1100):20190113. doi: 10.1259/bjr.20190113.
- Laperriere NJ, Bahl G, Menard C, et al. Reirradiation for recurrent meningiomas: outcomes and complications. Int J Radiat Oncol Biol Phys. 2008;72(1):S220. doi: 10.1016/j.ijrobp.2008.06.679.
- Wojcieszynski AP, Ohri N, Andrews DW, et al. Reirradiation of recurrent meningioma. J Clin Neurosci. 2012;19(9):1261–1264. doi: 10.1016/j.jocn.2012.01.023.
- Lanning RM, Chohan MO, Ryan C, et al. Outcomes after salvage reirradiation for recurrent meningioma. Int J Radiat Oncol Biol Phys. 2015;93(3):E86–E87. doi: 10.1016/j.ijrobp.2015.07.767.
- Kim M, Lee DH, Kim RNHJ, et al. Analysis of the results of recurrent intracranial meningiomas treated with re-radiosurgery. Clin Neurol Neurosurg. 2017;153:93–101. doi: 10.1016/j.clineuro.2016.12.014.
- Pritchard AG, Nguyen TK, Bauman GS. Lessons learned from reirradiation of recurrent skull base meningioma: a case report and review of the literature. Adv Radiat Oncol. 2017;2(1):1–5. doi: 10.1016/j.adro.2017.01.002.
- El Shafie RA, Czech M, Kessel KA, et al. Evaluation of particle radiotherapy for the re-irradiation of recurrent intracranial meningioma. Radiat Oncol. 2018;13(1):86. doi: 10.1186/s13014-018-1026-x.
- Song J, Aljabab S, Abduljabbar L, et al. Radiation-induced brain injury in patients with meningioma treated with proton or photon therapy. J Neurooncol. 2021;153(1):169–180. doi: 10.1007/s11060-021-03758-y.
- Harrabi SB, von Nettelbladt B, Gudden C, et al. Radiation induced contrast enhancement after proton beam therapy in patients with low grade glioma - how safe are protons? Radiother Oncol. 2022;167:211–218. doi: 10.1016/j.radonc.2021.12.035.