Introduction
Soft tissue sarcomas (STSs) are a group of rare malignant tumours with heterogeneous histological subtypes. Tumours occur most commonly in the extremities and the retroperitoneum [Citation1,Citation2]. Standard treatment for localised adult-type STS is wide surgical resection and radiotherapy in high-grade tumours at least when wide marginals are not achieved [Citation3].
The role of adjuvant chemotherapy is still controversial. Doxorubicin with ifosfamide is the most used combination [Citation4]. A systematic meta-analysis of 18 adjuvant trials found that compared to local treatment adjuvant combination chemotherapy had an OR of 0.56 (95% CI, 0.36-0.85; p = .01) in overall survival (OS) and an OR of 0.69 (95% CI, 0.56-0.86; p = .0008) in the overall recurrence rate. The absolute risk reduction in death was 11%, from 41% to 30% [Citation5].
Scandinavian countries often offer adjuvant chemotherapy to high-risk patients, but European Society for Medical Oncology is more conservative in recommendations [Citation3]. Common criteria for chemotherapy are high-grade sarcoma with vascular invasion or tumour size over 8 centimetres, infiltrative growth pattern and microscopic tumour necrosis [Citation6]. However, doxorubicin is known for its dose-dependent irreversible cardiotoxicity, which in turn affects prognosis [Citation7–9]. In an early retrospective study has been reported a 3% incidence of cardiotoxicity at a cumulative dose of 400 mg/m2, 7% at 550 mg/m2 and 18% at 700 mg/m2 [Citation10]. Another retrospective analysis of three trials indicated that 26% of patients would experience doxorubicin-related heart failure (HF) at a cumulative dose of 550 mg/m2 [Citation11]. Other risk factors that are associated with doxorubicin-induced cardiotoxicity include female gender, age > 65 years or < 18 years, pre-existing cardiovascular risk factors, doxorubicin administered as an intravenous bolus and radiation therapy involving the heart [Citation12,Citation13]. Currently accepted safe cumulative dose of doxorubicin is < 550 mg/m2 [Citation14].
There are scarce previous publications of the life span of sarcoma patients after adjuvant treatments. In a Danish national cohort of 1,187 STS patients the incidence of cardiac death was 2.7 (95% CI: 1.6–4.5) per 1,000 person years. Doxorubicin-based chemotherapy associated strongly to overall mortality (HR =4.92, 95% CI: 3.67–6.59). [Citation15] Thus, this retrospective study was aimed to analyse the clinical data of patients treated in a large tertiary centre with (neo)adjuvant doxorubicin-based chemotherapy alone or with combined radiotherapy to assess the incidence of late complications. Survival and recurrence during follow-up were also analysed.
Material and methods
The clinical data of adult STS patients were collected retrospectively from original medical records. Patients were initially collected according to diagnosis code (ICD-10 codes C48 and C49). There were primarily 1156 patients listed and in the final analysis altogether 67 patients were included. Eligibility criteria were pathological diagnosis of STS, age ≥ 18 years, resectable disease and neoadjuvant or adjuvant therapy including doxorubicin-based chemotherapy with or without radiation therapy. Exclusion criteria were age < 18 years, inoperable disease, diagnosis of Ewing’s sarcoma or osteosarcoma and missing clinical data. Nine patients with completely operated metastatic disease or local recurrence were included in the analyses on long-term complications. Patients were treated at the Tampere University Hospital from the 1st of January 2008 to the 31st of December 2017. Patients were followed according to the normal clinical practice, which included chest x-ray, laboratory values and visits every three months for the first year, every four months for the second year, and every six months in years 3–5. The imaging of the primary tumour location was done during every other visit. Echocardiography was performed when clinically indicated. Other risk factors for heart complications were recorded. The data was collected in spring 2021. Patient characteristics, treatment-related late-onset adverse events, OS, disease-specific survival (DSS), disease-free survival (DFS) and local- and distant recurrences were evaluated.
The primary outcome measure was the incidence of doxorubicin-induced cardiotoxicity, defined as the onset of clinical HF. Secondary outcome measures were other treatment-related late-onset adverse events, such as secondary malignities and renal failure, and the time elapsed between the end of (neo)adjuvant treatment and the development of complications. Kidney failure was recorded when GFR was <57mL/min. For evaluation of recurrence and survival, patients were divided into chemotherapy and a chemo- and radiation (later: combination) therapy groups. DFS was calculated as the time between the end of the treatment and the occurrence of distant metastasis or a local recurrence. Death for other reason than cancer was not considered as an event for DFS and patients were censored when death occurred. Survival analysis was performed using the Kaplan-Meier method. The groups were compared with Kruskal–Wallis test and Chi-Square test. Cox regression was used to report the hazard ratios (HR) for death in treatment groups. All data were analysed using IBM SPSS Statistics software version 27.
This retrospective study is based on clinical data. For the use of medical records, permission was sought from the Pirkanmaa Hospital District, which is competent as their registrar, in accordance with its licencing practices. This study did not affect the patient’s treatment. Thus, a statement of the ethics committee or the informed consent of the subjects was not required in order to conduct the study.
Results
A total of 67 patients (age range 24.4-78.9 years) met the eligibility criteria. The median follow-up time was 4.16 years. The general characteristics of the patients are summarised in . Patients generally received 6 cycles of doxorubicin as a one-hour intravenous infusion (70 mg/m2) combined with 2000 mg/m2 ifosfamide which was given in days 1-3 in 21-day cycles. Five elderly patients (≥70 years) received doxorubicin with a reduced dose (50–60 mg/m2).
Table 1. Patient characteristics of the two treatment groups.
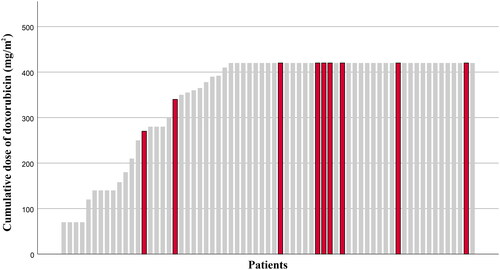
Among all the chemotherapy patients, 9 patients (13.4%) developed HF (). The median cumulative dose of doxorubicin was the planned 420 mg/m2. The median time to the onset of HF was 14.0 months. Among patients who received doxorubicin at a minimum dose of 400 mg/m2 17.1% developed HF, while in patients who received doxorubicin less than 400 mg/m2 the rate was 7.7%. Seven of the nine HF patients were men and two were women, four (44.4%) were ≥ 65 years old and three (33.3%) were smokers. Two (22.2%) had previous heart conditions (coronary artery disease and atrial fibrillation) and five (55.6%) had hypertension, while in patients without HF hypertension was diagnosed in 36.2%. Two patients had postoperative radiation therapy to chest area. First patient received radiotherapy to the heart at a mean dose of 3.5 Gy. The second patient’s radiotherapy was conducted in another hospital and therefore more detailed information is not available.
Figure 1. Cumulative dose of doxorubicin for patients. Patients who developed heart failure are highlighted with red colour.
One patient who had received adjuvant chemotherapy with doxorubicin (dose 420 mg/m2) and ifosfamide and preoperative radiation therapy with a dose of 50 Gy developed acute myeloid leukaemia one year after the end of the treatment. Clinically significant permanent skin pigmentation was recorded in medical records in two combination therapy patients. These patients received radiation therapy with doses of 50–60 Gy. Six patients discontinued chemotherapy and three patients received reduced doses due to adverse events, the most common of which were neutropenic infections and nausea. Six patients (9.0%) developed kidney failure. The median time elapsed between the end of treatment and the onset of kidney failure was 6.0 months.
In the chemotherapy group, 48.8% of the patients developed distant metastases. The respective rate in the combination therapy group was 33.3%, with no statistically significant difference (p = 0.258). Local recurrence rates in chemotherapy and in the combination therapy group were 40.0% and 12.1%, respectively, with a statistically significant dependence (p = 0.014) and explained by differences in the percentage of retroperitoneal tumours which are more difficult to treat with radiation therapy. Median DFS was 5.0 months in the chemotherapy and 17.0 months in the combination therapy group (p = 0.087).
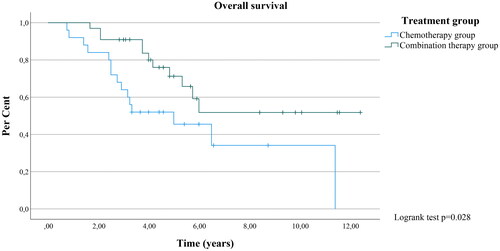
In the combination therapy group, 66.7% of the patients survived the entire follow-up time and the median OS could not be calculated. The respective OS rate in the chemotherapy group was 40.0% with a median OS of 60.0 months (p = 0.028, 95% CI: 22.787-97.213) (). Compared to combination therapy the HR for death in the chemotherapy group was 2.446 (p = 0.025, 95% CI: 1.120-5.342). The DSS rates in the chemotherapy and combination therapy groups were 45.5% and 73.3%, respectively (p = 0.088, not significant). HR for death was 2.664 (p = 0.032, 95% CI: 1.086-6.536). The median DSS in the chemotherapy group was 40.0 months (95% CI: 13.619-66.381).
Discussion
In this study, we found that the cumulative incidence of doxorubicin-induced HF was 13.4% with a median cumulative doxorubicin dose of 420 mg/m2. The incidence was higher with greater doses of doxorubicin (17.1% vs 7.7%). Although no direct cardiac deaths were recorded during the study period, doxorubicin-induced cardiotoxicity can be fatal despite of an otherwise successful treatment of the malignancy. The cardiotoxicity-associated mortality is a matter to be weighed against the recurrence and survival advantages that are previously reported [Citation5].
Radiation therapy to chest area may have contributed the development of HF. There is no previous data of sarcoma patients, but in a randomised controlled trial of cardiotoxicity among Asian women with breast cancer receiving adjuvant therapies authors found that HR for major heart events was 1.92 (1.65–2.23) for patients who received adjuvant radiation therapy with anthracycline-based chemotherapy, while anthracycline-based chemotherapy alone had an HR of 1.48 (1.25–1.75) (p < 0.0001) [Citation16].
The definition of cardiotoxicity varies between studies. In this retrospective study, we hadn’t any systematic evaluation of heart function. All recorded cases were clinically significant and symptomatic HFs confirmed by echocardiography. When screening procedures are used, chemotherapy-related cardiomyopathy is a disease spectrum ranging from a measurable biomarker rise before detectable changes to asymptomatic decline in systolic function and finally HF with its clinical signs and symptoms [Citation17–19]. If an asymptomatic decline in left ventricular ejection fraction (LVEF) was included in the definition of cardiotoxicity, the cardiac event rate would be higher and there would be more events with lower doses of anthracycline [Citation11]. To patients with advanced disease and limited survival, clinical HF is the most significant definition of cardiotoxicity. However, also the subclinical cardiac damage and asymptomatic changes in LVEF are significant to patients with curatively treated local STS [Citation20].
Limiting peak plasma levels by administrating anthracycline as a longer infusion rather than a bolus is known to reduce cardiotoxicity in adults [Citation21,Citation22]. In a meta-analysis of five randomised controlled trials and a total of 557 cancer patients receiving anthracyclines, the authors found that there was a statistically significantly lower rate of clinical HF with infusion duration of six hours or longer compared to a shorter infusion duration (RR 0.27, 95% CI: 0.09-0.81, p = 0.02) [Citation23]. Thus the infusion duration of 1 h may have contributed to cardiotoxicity in patients and prolonging infusion duration might be a feasible aspect to consider.
Randomised trials have shown a significant correlation between doxorubicin dose and response rate with doses of 60 to 70 mg/m2 versus lower doses. In the meta-analysis the doxorubicin dosage ranged from 50 mg/m2 to 90 mg/m2 [Citation5,Citation24] and no proven difference in efficacy between doses of 60 to 70 mg/m2 was found. After this analysis, we lowered the dose of doxorubicin in adjuvant treatments to 60 mg/m2.
Usually, the first peak of secondary cancers is seen from 1 to 3 years after primary cancer and the second peak is observed from 7 to 10 years being mostly caused by radiotherapy [Citation20]. Thus our median follow-up time of 4.16 years may be short to detect all late cases.
Our study as a single-centre experience had a limited sample size which affects the credibility of the results. Nevertheless, the results that we obtained suggest that adjuvant therapy should be individually designed considering the patient’s risk factors and prognosis. As the dose of doxorubicin can be variable, lower, but still efficient doses (60 mg/m2) could prevent many clinical heart failures. The earlier detection of heart complications also enables earlier treatment to prevent these cases to escalate to clinically significant HF. The number of late complications is an important quality measurement in cancer treatment and every centre should be aware of its own results. Further evaluation of possible preventive strategies for cardiotoxicity is still needed.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
At present, the Finnish legislation on secondary uses of healthcare data and patient records does not permit sharing this type of data. Therefore, the datasets generated and used for this study cannot be made available. Documentation on data collection process and dataset are available from the corresponding author on reasonable request.
References
- Stiller CA, Trama A, Serraino D, The RARECARE Working Group., et al. Descriptive epidemiology of sarcomas in Europe: report from the RARECARE project. Eur J Cancer. 2013;49(3):684–695. doi: 10.1016/j.ejca.2012.09.011.
- Abaricia S, Van Tine BA. Management of localized extremity and retroperitoneal soft tissue sarcoma. Curr Probl Cancer. 2019;43(4):273–282. doi: 10.1016/j.currproblcancer.2019.06.002.
- Gronchi A, Miah AB, Dei Tos AP, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(11):1348–1365. doi: 10.1016/j.annonc.2021.07.006.
- Casali PG. Adjuvant chemotherapy for soft tissue sarcoma. Am Soc Clin Oncol Educ Book. 2015;35:e629-33–e633. doi: 10.14694/EdBook_AM.2015.35.e629.
- Pervaiz N, Colterjohn N, Farrokhyar F, et al. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113(3):573–581. doi: 10.1002/cncr.23592.
- Gustafson P, Åkerman M, Alvegård TA, et al. Prognostic information in soft tissue sarcoma using tumour size, vascular invasion and microscopic tumour necrosis—the SIN-system. Eur J Cancer. 2003;39(11):1568–1576. doi: 10.1016/s0959-8049(03)00369-1.
- Shamai S, Rozenbaum Z, Merimsky O, et al. Cardio-toxicity among patients with sarcoma: a cardio-oncology registry. BMC Cancer. 2020;20(1):609. doi: 10.1186/s12885-020-07104-9.
- Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines. Eur Heart J. 2016;37(36):2768–2801. doi: 10.1093/eurheartj/ehw211.
- Tian Z, Yang Y, Yang Y, et al. High cumulative doxorubicin dose for advanced soft tissue sarcoma. BMC Cancer. 2020;20(1):1139. doi: 10.1186/s12885-020-07663-x.
- von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-lnduced congestive heart failure. Ann Intern Med. 1979;91(5):710–717. doi: 10.7326/0003-4819-91-5-710.
- Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin. Cancer. 2003;97(11):2869–2879. doi: 10.1002/cncr.11407.
- Kim YA, Cho H, Lee N, et al. Doxorubicin-induced heart failure in cancer patients: a cohort study based on the korean national health insurance database. Cancer Med. 2018;7(12):6084–6092. doi: 10.1002/cam4.1886.
- Kongbundansuk S, Hundley WG. Noninvasive imaging of cardiovascular injury related to the treatment of cancer. JACC Cardiovasc Imaging. 2014;7(8):824–838. doi: 10.1016/j.jcmg.2014.06.007.
- Meinardi MT, Gietema JA, van Veldhuisen DJ, et al. Long-term chemotherapy-related cardiovascular morbidity. Cancer Treat Rev. 2000;26(6):429–447. doi: 10.1053/ctrv.2000.0175.
- Shantakumar S, Olsen M, Vo T, et al. Cardiac dysfunction among soft tissue sarcoma patients in Denmark. Clin Epidemiol. 2016;8:53–59. doi: 10.2147/CLEP.S100779.
- Lee CH, Zhang JF, Yuan KSP, et al. Risk of cardiotoxicity induced by adjuvant anthracycline-based chemotherapy and radiotherapy in young and old asian women with breast cancer. Strahlenther Onkol. 2019;195(7):629–639. doi: 10.1007/s00066-019-01428-7.
- McGowan JW, Chung R, Maulik A, et al. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther. 2017;31(1):63–75. doi: 10.1007/s10557-016-6711-0.
- Sawicki KT, Sala V, Prever L, et al. Preventing and treating anthracycline cardiotoxicity: new insights. Annu Rev Pharmacol Toxicol. 2021;61(1):309–332. doi: 10.1146/annurev-pharmtox-030620-104842.
- Curigliano G, Lenihan D, Fradley M, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31(2):171–190. doi: 10.1016/j.annonc.2019.10.023.
- Jung HK, Park S, Kim NW, et al. Development of second primary cancer in korean breast cancer survivors. Ann Surg Treat Res. 2017;93(6):287–292. doi: 10.4174/astr.2017.93.6.287.
- Payne DL, Nohria A. Prevention of chemotherapy induced cardiomyopathy. Curr Heart Fail Rep. 2017;14(5):398–403. doi: 10.1007/s11897-017-0353-9.
- Armenian S, Bhatia S. Predicting and preventing Anthracycline-Related cardiotoxicity. Am Soc Clin Oncol Educ Book. 2018;38(38):3–12. doi: 10.1200/EDBK_100015.
- van Dalen EC, Pal HJH, Kremer LCM. Different dosage schedules for reducing cardiotoxicity in people with cancer receiving anthracycline chemotherapy. Cochrane Database Syst Rev. 2016;2020(6):CD005008.
- Antman K, Suit H, Amato D, et al. Preliminary results of a randomized trial of adjuvant doxorubicin for sarcomas: lack of apparent difference between treatment groups. J Clin Oncol. 1984;2(6):601–608. doi: 10.1200/JCO.1984.2.6.601.