Abstract
Background
Adjuvant radiotherapy (RT) after breast-conserving surgery for DCIS lowers the relative local recurrence risk by half. To identify a low-risk group with the minimal benefit of RT could avoid side effects and spare costs. In this study, the outcome was compared for different RT-strategies using data from the randomized SweDCIS trial.
Material and methods
Five strategies were compared in a Swedish setting: RT-to-none or all, RT to high-risk women defined by DCISionRT, modified Radiation Therapy Oncology Group (RTOG) 9804 criteria, and Swedish Guidelines. Ten-year recurrence risks and cost including adjuvant RT and local recurrence treatment cost were calculated.
Results
The mean age at recurrence was 64.4 years (36–90) and the mean cost for treating a recurrence was $21,104. In the SweDCIS cohort (n = 504), 59 women developed DCIS, and 31 invasive recurrence. Ten-year absolute local recurrence risk (invasive and DCIS) according to different strategies varied between 18.6% (12.5–23.6%) and 7.8% (5.0–12.6%) for RT-to-none or to-all, with an additional cost of $2614 US dollars per women and $24,201 per prevented recurrence for RT-to-all. The risk differences between other strategies were not statistically significant, but the larger proportion receiving RT, the fewer recurrences. DCISionRT spared 48% from RT with 8.1% less recurrences compared to RT-to-none, and a cost of $10,534 per prevented recurrence with additional cost depending on the price of the test. RTOG 9804 spared 39% from RT, with 9.7% less recurrences, $9525 per prevented recurrence and Swedish Guidelines spared 13% from RT, with 10.0% less recurrences, and $21,521 per prevented recurrence.
Conclusion
It seems reasonable to omit RT in pre-specified low-risk groups with minimal effect on recurrence risk. Costs per prevented recurrence varied more than two-fold but which strategy that could be considered most cost-effective needs to be further evaluated, including the DCISionRT-test price.
Keywords:
Background
Ductal breast carcinoma in situ (DCIS) constitutes about 10% of all diagnosed breast cancer in countries with mammography screening programs, and about 20% in screened populations [Citation1,Citation2]. Adjuvant radiotherapy (RT) after breast-conserving surgery (BCS) for DCIS lowers the relative local recurrence risk by approximately 50% after 10-years, but with no effect on survival [Citation3]. To identify a group with a low absolute risk and with minimal, or no, benefit of RT has been an aim for the last decades [Citation4,Citation5]. Different definitions of low risk, where RT might be safely omitted, have been included in different national guidelines [Citation6,Citation7]. Omitting RT could avoid negative side effects but also spare money and resources. However, data on the effect of using different low-risk definitions to decide who should be offered RT or not is lacking.
DCISionRT® is a biological signature for 10-years local recurrence risk in those treated with BCS with or without RT, based on seven biomarkers (PR, HER2, Ki67, FOXA1, p16/INK4A, SIAH2 and COX2) and four clinical factors (age, tumor size, surgical margins, and palpability). DCISionRT has shown to be prognostic and divides women with DCIS into Low Risk and Elevated Risk [Citation8] but also, it seems to be predictive for RT-benefit [Citation9,Citation10].
In this retrospective study, we define treatment costs for a recurrence after a primary DCIS and we compared different strategies for deciding on using RT after BCS for DCIS and the strategy impact on recurrence rate and costs, 10-years after primary surgery, using data from the randomized SweDCIS trial [Citation11].
Material and methods
We compared five different RT strategies for oncological outcome and costs after primary BCS for DCIS in a retrospective study of the SwedCIS randomized trial. The results are reported according to the REMARK guidelines [Citation12] (Supplementary Table 1). The different strategies were: (1) RT-to-none, (2) RT-to-all, (3) No RT to women with low-risk defined by DCISionRT, (4) No RT to women with low-risk DCIS, according to the modified Radiation Therapy Oncology Group 9804 study criteria (RTOG 9804) [Citation12], and (5) No RT to women with low-risk DCIS according to Swedish National Guidelines [Citation7].
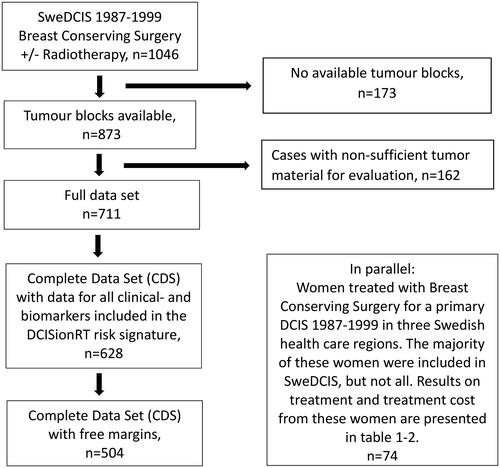
The oncological outcome was calculated from data in a validation study of DCISionRT in the SweDCIS cohort [Citation10]. In SweDCIS, women with primary DCIS were randomized to RT or not after BCS between 1987 and 1999, in Sweden [Citation11]. Formalin embedded paraffin tumor blocks were collected in Sweden and new slides were sent to PreludeDx, Laguna Hills, CA, USA, for staining and scoring of the biomarkers and nuclear grade (NG), by board-certified pathologists blinded to outcome [Citation8]. Of 1046 randomized women, 504 women with all clinical and biomarker data and negative margins were included in the study cohort. DCISionRT decision scores (DS, range 0–10 points) were calculated and women were divided into Low Risk, DS ≤3 and Elevated Risk, DS >3 (). All statistical analyses were conducted in Sweden independent from PreludeDx. The 10-year absolute and relative recurrence risks were calculated for total and invasive breast cancer events. Invasive events were defined as either local, regional, or metastatic. Total events were defined as either a new DCIS or an invasive ipsilateral event. In the current comparison of the different RT strategies, we only used total events.
Figure 1. Flow chart describing available tumor material from the randomized SweDCIS trial, for evaluation of 10-year absolute total local recurrence risk according to five different radiotherapy strategies.
In the RTOG 9804 [Citation13], good-risk patients were identified as those with mammographically detected, non-palpable, low- or intermediate-grade DCIS, measuring ≤2.5 cm and margins >3 mm. In this study, we had to modify this to screen-detected, non-palpable, NG 1–2 lesions, size ≤2.5 cm, and with negative margins. According to Swedish National Guidelines [Citation7], women with DCIS NG 1–2, <1.5 cm with negative margins and ‘preferably post-menopausal’ can be spared RT after informed consent. We defined post-menopausal as 52 years or older.
We defined numbers needed to treat (NNT) as how many women must be given adjuvant RT to prevent one recurrence, and an example of harm was defined according to reference Citation13 as breast pain due to RT at least once a week the previous six months, 10–17 years post-radiotherapy. Harm was estimated to occur in one of 12 having RT [Citation14].
In 74 women from Västra Götaland, Uppsala, and Västerås health care regions in Sweden with a local ipsilateral recurrence as the first event after primary BCS for DCIS between 1988 and 1999, treatment of the recurrence was documented through medical records (). The majority of these women were from the SweDCIS cohort but some additional women from the same region and time period were included. Treatment included breast and axillary surgery, additional re-operations, reconstruction either direct or delayed (with an implant or autologous tissue), radiotherapy, and chemotherapy. Costs were based on diagnostic-related grouping (DRG) in Sweden, 2020 (SEK) and then converted to US dollars ($1 = 9.16 SEK, per 31st of May 2021). The initial cost for adjuvant RT was also based on DRG. The cost for the DCISionRT test was set to $1010 in a study by Kim et al. [Citation15] but as no fixed price is available in Sweden, we decided to exclude test costs in our analyses. Mean recurrence costs for four different treatment- and recurrence options were calculated, i.e., BCS with or without adjuvant RT, with either a new DCIS or an invasive recurrence.
This study was approved by the ethics committee at Umeå University, Sweden, Fek 8705052-2 and Dnr 05–65 M with amendment 2020-02132.
Statistical analyses
Absolute 10-year total recurrence rates were calculated by Kaplan–Meier analyses, and rates and differences with 95% confidence intervals (CI) were calculated using Cox proportional models by means of flexible parametric survival model (stpm2) [Citation16] and standardized failure function (stand-surv) [Citation17]. A competing risk model was tested for DCISionRT with a minor effect on estimated risks [Citation10]. Hazard ratios (HR) for association of RT, clinicopathologic factors, and year of diagnosis were determined by multivariable Cox proportional hazards analysis. In the validation cohort, HRs for the association of RT were determined within categorical DS groups (DS ≤3, DS >3) or according to clinicopathology factors defining low-risk or high-risk by RTOG 9804 and Swedish Guidelines. Clinicopathologic and treatment factors were summarized by counts and percentages. T-test or Fisher’s exact testing was used to assess differences between subsets. Statistical analyses were performed with Stata/MP 16.1.
Results
Treatment and cost of recurrences
Of the 74 selected women with recurrence studied for the cost calculations, 33 recurrences were new DCIS, and 41 invasive cancers. Fifty-four of those 74 primarily had BCS without adjuvant RT and 20 had BCS with adjuvant RT (). The mean age at primary diagnosis was 58.4 years (range 30–84 years) and the mean time to recurrence was 6.0 years (1–22 years). Time to recurrence was statistically significantly longer for those with invasive recurrences than for those with a new DCIS: 7.2 vs. 4.6 years (p = .03), but similar after BCS with RT and BCS without RT, 5.7 vs. 6.3 years (p = .79), respectively. In all, 68.9% (51/74) had a salvage mastectomy: 70.0% (14/20) after BCS with RT and 68.5% (37/54) after BCS without RT (p = 1.0). Of those having a mastectomy at the time of recurrence, reconstruction was performed in 35.7% (5/14) and 29.7% (11/37) (p = .74) of those with BCS with RT and BCS without RT, respectively (). The reconstruction rate was higher, but not statistically significantly higher, after a mastectomy due to a new DCIS compared to an invasive recurrence, 40.9% (9/22) versus 24.1% (7/29) (p = .24).
Table 1. Treatment of invasive or in situ recurrences in 74 women after initial breast conserving surgery for primary DCIS, with or without adjuvant radiotherapy.
The costs per treatment modality based on data from the 74 women are presented in . The mean cost for treating a recurrence was $21,104 and the median cost was $16,904 (range $0–78,850). One woman, 82 years old at the time of recurrence, did not receive any treatment due to congestive heart disease and one woman aged 83 only received antioestrogen treatment. The mean cost was used for the calculation of cost per strategy and the costs were not statistically significantly different for women who had had adjuvant RT or not, or for the different types of recurrence. The mean recurrence costs were: BCS with RT and DCIS recurrence $17,719 ($6707–47,986), BCS with RT and invasive recurrence $22,506 ($0–66,709), BCS without RT and DCIS recurrence $20,625 ($6707–78,850), and BCS without RT and invasive recurrence $21,870 ($4900–66,709), respectively.
Table 2. Costs for different treatments according to diagnostic related grouping in Sweden 2020 and converted to US dollars.
RT-strategies
In the nationwide SweDCIS study cohort (n = 504), 90 women had developed a recurrence after 10-years, of which 59 were a new DCIS and 31 invasive recurrences. There were no metastatic first events. The calculated 10-year absolute total breast event risks according to the different strategies and risk groups within the strategies are presented in . The difference in local recurrence risk was of borderline statistical significance between Strategies 1 (RT-to-none) and 2 (RT-to-all), otherwise, the confidence intervals were overlapping between strategies and risk groups. Based on these data, the number of recurrences and the total cost for adjuvant RT, and treatment of recurrences, and per strategy are presented in . The confidence intervals are not presented but were wide and overlapping. Comparing strategies the larger proportion receiving adjuvant RT, the fewer recurrences.
Table 3. The calculated 10-year absolute total breast event risks (invasive and in situ) for patients treated with breast conserving surgery with negative margins and with adjuvant radiotherapy given according to different strategies in the SweDCIS randomized study.
Table 4. Proportion of women having adjuvant radiotherapy, number of recurrences at 10-years, and mean total cost per RT-strategy per woman undergoing breast conserving surgery for DCIS.
Ten-year absolute local recurrence risk (invasive and DCIS) was 18.6% (12.5–23.6%) for RT-to-none and 7.8% (5.0–12.6%) for RT to all, with an additional cost of $2614 US dollars per women for RT-to-all and a cost per prevented recurrence of $24,201. Strategy 3 (DCISionRT) spared 48% from RT with 8.1% less recurrences after 10-years compared to RT-to-none, at a cost of $10,534 per prevented recurrence (additional cost of the test is not included). Strategy 4 (RTOG 9804) spared 39% from RT, with 9.7% less recurrences, at a cost of $9525 per prevented recurrence and Strategy 5 (Swedish Guidelines) spared 13% from RT, with 10.0% less recurrences, at a cost of $21,521 per prevented recurrence. The corresponding numbers for invasive recurrences (with even larger confidence intervals) were 8.4% invasive recurrences after 10-years for RT-to none and 3.7% for RT-to-all with a cost of $70,649 per prevented invasive recurrence. Strategy 3 (DCISionRT) had a 5.3% absolute risk with a cost of $27,548 per prevented invasive recurrence, Strategy 4 (RTOG 9804) had a 3.5% absolute risk with a cost of $18,857 per prevented invasive recurrence, and finally Strategy 5 (Swedish Guidelines) with an absolute risk of 3.6% with a cost of $44,833 per prevented invasive recurrence.
Looking at NNT and harm by RT for the different strategies, for Strategy 2 (RT-to-all) the NNT was 9.3 and RT caused breast pain at least once a week in 8.3% of women. For Strategy 3 (DCISionRT) NNT was 6.4 and caused pain in 4.3% of women. The corresponding numbers for Strategy 4 (RTOG 9804) was: NNT 6.3 and causing pain in 5.1%, and for Strategy 5 (Swedish Guidelines) NNT 8.7 and causing pain in 7.2% of women ().
Table 5. Numbers needed to treat (NNT) and harm for the different radiotherapy strategies in relation to cost and recurrence risk at 10-years compared to RT to none, in the SweDCIS cohort with women undergoing breast conserving surgery for primary DCIS.
Discussion
The main finding from this retrospective study of a randomized trial was that using DCISionRT, RTOG 9804 criteria, or Swedish national guidelines criteria could spare 48%, 39%, and 13%, of women radiotherapy respectively after breast-conserving surgery for DCIS with a low impact on 10-year recurrence risk. The excess 10-year recurrence rates were 2.7%, 1.1%, and 0.8% for the different strategies, compared to giving radiotherapy to all. Cost comparisons between strategies are presented but must be looked upon as approximative. Also, the test cost for DCISionRT was not included. However, the cost of preventing a recurrence varied more than two-fold.
A strength of the study is that the calculations are based on data from 504 women randomized to RT or not after BCS for a primary DCIS in the SweDCIS study. RT was associated with an absolute reduction of local recurrences of 16% at 10-years [Citation18]. At 20-years, the reduction was 12% of which only 2% were prevented invasive recurrences [Citation11]. In our current study, only women with free margins were included to make the cohort more contemporary and representative of today, making the 10-year local recurrences rates somewhat lower than in the entire SweDCIS population, 7.8% vs. 10.3% and 18.6% vs. 21.6%, with and without RT, respectively. We applied different criteria for giving RT on the cohort and even if the initial study cohort was not designed for this and the numbers were small in the different subgroups, the results show that giving RT to all is a considerable overtreatment.
One could argue that patients today do better in general but in a study by Chua et al. non-low-risk DCIS patients from 2007 to 2014, had a 5-year local recurrence rate of 7.3% after RT [Citation19], which is in line with the results for the different high-risk groups receiving RT in this study. Also, the better the patients do, the stronger the concern of overtreatment. In two low-risk populations using the RTOG 9804 and ECOG criteria, the recurrence rate at 10-years without RT was still about 10% [Citation20,Citation21]. Altogether, applying low-risk criteria for RT treatment omission seems to be a reasonable and cost-effective practice [Citation22,Citation23], even if the relative benefit of RT still is about 50%. On an individual level, however, it must be a joint decision between physician and patient to omit RT.
The treatment cost calculations in this study were built on 74 women and the treatment they received for their recurrence according to medical records. Costs were added for surgery and oncological treatment and the costs were very similar regardless of if they had RT or not at primary surgery, and regardless of if the recurrence was a new DCIS or invasive. We did not have data on RT-boost, a specific type of chemotherapy, or exact type of axillary staging, making the cost calculations approximative. About the same proportion of women had a salvage mastectomy at the time of recurrence even if the option of a new BCS with adjuvant RT would have been possible. To find the reasons behind these treatment decisions was not within the scope of this study but maybe the fear of having yet another recurrence drives the patient’s decision toward mastectomy. It is difficult to compare costs as treatment policies and prices might vary between countries. As an example of this, in a Swedish study from 1997 the treatment cost for a recurrence after BCS for early invasive breast cancer was about $3000 (adjusted to $4000 for 2020) [Citation24], the 10-year cost per local recurrence was $19,596 in another study from the USA, based on SEER and Medical Claims linked data from 2008 [Citation25], and in a poster presentation at the San Antonio Breast Cancer Symposium 2022, the full cost after early breast cancer recurrence, also based on SEER and Medical Claims data, was $96,465 [Citation26]. We did not do a cost-effectiveness analysis according to the CHEERS [Citation27] criteria as we missed some crucial information.
The comparison of the effect of different strategies and the corresponding cost per woman and cost per prevented recurrence can be seen mainly as an eye-opener regarding overtreatment. The estimated cost per prevented recurrence by RT was in the range of $9500 to $24,000 for any recurrence and $20,000 to $70,000 for invasive recurrences, and the cost was lower for Strategies 3, 4, and 5 compared to giving RT to all. In the study by Liljegren et al. [Citation24], the estimated cost to prevent a local recurrence was about $44,000 (adjusted to $57,000, 2020) including indirect costs as travel expenses and lost production. The authors also calculated the cost for every gained quality adjusted life-year (QALY) for RT after BCS for early breast cancer. The cost was estimated to $210,526 ($26,315–$513,158) (adjusted to $274,800, 2020) [Citation24]. The cost per prevented recurrence and gained QALY was estimated to be reduced by 38%–45% by giving RT only to high and intermediate-risk patients [Citation24].
The least costly strategy, just looking at money, was to omit RT for all. To balance the harm and the benefit in relation to the cost is, however, something that must be done. Based on the data from this study, we cannot draw any far going conclusion on what is the preferred strategy. Also, we only used one side effect as a representative for harm. We did not include data on possible side effect caused to the lungs or heart. Notwithstanding, RT to all seems to be a costly overtreatment with an NNT of 9.3. By using the Swedish National Guidelines 87% received RT. For DCISionRT and RTOG 9804, 52% and 61% were irradiated with 1.9% and 0.3% over risk at 10-years. In Sweden, a switch to RTOG 9804 criteria for omitting RT could be proposed while awaiting more DCISionRT data and cost.
In a Markov modeled study, the cost-effectiveness of DCISionRT was examined in an American setting. Using DCISionRT was not cost-effective compared to RT-to-none [Citation28]. In another cost-effectiveness analysis by Kim et al. [Citation14], RT decisions were simulated based on traditional clinicopathological factors or DCISionRT. The test cost was set to $1010 and an acceptable threshold of $100,000 per QALY was assumed. DCISionRT was cost-effective, costing $177.20 more per treated woman, compared to treatment based on clinicopathological factors. This is of course depending on the test price.
The psychological effects of a recurrence, QoL, or QALY were not included in our calculations. In SweDCIS the adjuvant RT was routinely given for five weeks. The proportion of women with pain was 21% in the Swedish population undergoing such a regime [Citation14]. In an Australian study, 80% of women receiving RT reported a difference in breast pain between sides (34% moderate to large) after at least 12 months [Citation29]. Today, hypo-fractionation is becoming more common, and it seems like the preventive effect is as good as for five weeks RT, but long-term side effects may still be underestimated, e.g., regarding cardio-pulmonary effects. However, in a cost-effectiveness analysis in the UK, a five-day hypo-fractioning had both the least cost and greatest expected QALYs compared to 15-d RT [Citation30]. The psychological effect of a recurrence might also be different between in situ and invasive recurrences as a new DCIS does not have any effect on survival while the risk of breast cancer death is reported to be 18-fold higher after an invasive recurrence [Citation31,Citation32]. There is also an increased risk of secondary cancers including angiosarcomas after breast radiation. The risk is small but nevertheless, it may affect the individual therapy decision [Citation33–35].
To conclude, it seems reasonable to omit RT in pre-specified low-risk groups with minimal effect on recurrence rates at 10-years. The total cost per woman (adjuvant radiotherapy and cost for treating the recurrences) varies almost two-fold between RT-strategies. The cost per prevented recurrence varies even more, especially regarding invasive local recurrences. Risk perception is however individual and the decision to give, or omit RT, must be discussed with every woman based on available scientific data.
Supplemental Material
Download MS Word (22.3 KB)Disclosure statement
FW: Institutional funding by PreludeDx for the conduct of previous studies. AK: Institutional and travel grants by EndoMag Ltd, Honoraria by Pfizer, Consultation for Resitu AB, Associate Editor for the EJSO, Elsevier. ROB: Institutional research grants from Bristol-Myers Squibb (BMS), Endomagnetics Ltd (Endomag) and SkyLineDx, speaker honorarium from Roche, Pfizer and Pierre-Fabre, and has served on advisory boards for Amgen, BD/BARD, Bristol-Myers Squibb (BMS), Merck Sharp & Dohme (MSD), Novartis, Roche and Sanofi Genzyme, and is a shareholder in SATMEG Ventures AB. EH: Exact Sciences (patents pending, royalty) and Prelude Dx (patents pending, royalty). FV: is a consultant for ImpediMed, a research advisor for PreludeDx, and an employee of GenesisCare and Michigan Health care Professionals. GBM: is an employee of The Royal Melbourne Hospital, which received research grant funding from PreludeDx, PK: Astrazeneca (advisory role), Exact Sciences (patents pending, royalty), and Prelude Dx (patents pending, royalty). No other disclosures were reported.
Data availability statement
All data can be provided by the corresponding author.
Additional information
Funding
References
- Wadsten C, Heyman H, Holmqvist M, et al. A validation of DCIS registration in a population-based breast cancer quality register and a study of treatment and prognosis for DCIS during 20 years. Acta Oncol. 2016;55(11):1338–1343. doi: 10.1080/0284186X.2016.1211317.
- Karlsson P. Postoperative radiotherapy after DCIS: useful for whom? Breast. 2017;34(Suppl 1):S43–S46. doi: 10.1016/j.breast.2017.06.026.
- Correa C, McGale P, Taylor C, et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010(41):162–177.
- Barrio AV, Van Zee KJ. Controversies in the treatment of ductal carcinoma in situ. Annu Rev Med. 2017;68:197–211. doi: 10.1146/annurev-med-050715-104920.
- Rakovitch E, Nofech-Mozes S, Hanna W, et al. A population-based validation study of the DCIS score predicting recurrence risk in individuals treated by breast-conserving surgery alone. Breast Cancer Res Treat. 2015;152(2):389–398. doi: 10.1007/s10549-015-3464-6.
- NHS England. Clinical guidelines for the management of breast cancer. Available from: https://www.england.nhs.uk/mids-east/wp-content/uploads/sites/7/2018/02/guidelines-for-the-management-of-breast-cancer-v1.pdf.
- Regionala Cancercentrum I Samverkan. Kunskapsbanken. Available from: https://kunskapsbanken.cancercentrum.se/diagnoser/brostcancer/vardprogram/.
- Bremer T, Whitworth PW, Patel R, et al. A biological signature for breast ductal carcinoma In situ to predict radiotherapy benefit and assess recurrence risk. Clin Cancer Res. 2018;24(23):5895–5901. doi: 10.1158/1078-0432.CCR-18-0842.
- Weinmann S, Leo M, Francisco M, et al. Validation of a ductal carcinoma in situ biomarker profile for risk of recurrence after breast-conserving surgery with and without radiation therapy. Clin Cancer Res. 2020;26(15):4054–4063. doi: 10.1158/1078-0432.CCR-19-1152.
- Wärnberg F, Karlsson P, Holmberg E, et al. Prognostic risk assessment and prediction of radiotherapy benefit for women with ductal carcinoma in situ (DCIS) of the breast, in a randomized clinical trial (SweDCIS). Cancers. 2021;13(23):6103. doi: 10.3390/cancers13236103.
- Wärnberg F, Garmo H, Emdin S, et al. Effect of radiotherapy after breast-conserving surgery for ductal carcinoma in situ: 20 years follow-up in the randomized SweDCIS trial. J Clin Oncol. 2014;32(32):3613–3618. doi: 10.1200/JCO.2014.56.2595.
- McShane LM, Altman DG, Sauerbrei W, et al. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005;93(4):387–391. doi: 10.1038/sj.bjc.6602678.
- McCormick B, Winter K, Hudis C, et al. RTOG 9804: a prospective randomized trial for good-risk ductal carcinoma in situ comparing radiotherapy with observation. J Clin Oncol. 2015;33(7):709–715. doi: 10.1200/JCO.2014.57.9029.
- Lundstedt D, Gustafsson M, Malmström P, et al. Symptoms 10–17 years after breast cancer radiotherapy data from the randomised SWEBCG91-RT trial. Radiother Oncol. 2010;97(2):281–287. doi: 10.1016/j.radonc.2010.09.018.
- Kim H, Vargo JA, Smith KJ, et al. Cost-Effectiveness analysis of biological signature DCISionRT use for DCIS treatment. Clin Breast Cancer. 2021;21(3):e271–e278. doi: 10.1016/j.clbc.2020.10.007.
- Royston P, Lambert PC. Flexible parametric survival analysis in stata: beyond the cox model. Texas: StataPress. 2011.
- Crowther MJ, Lambert PC. Parametric multi-state survival models: flexible modelling allowing transition-specific distributions with application to estimating clinically useful measures of effect differences. Stat Med. 2017;36(29):4719–4742. doi: 10.1002/sim.7448.
- Holmberg L, Garmo H, Granstrand B, et al. Absolute risk reductions for local recurrence after postoperative radiotherapy after sector resection for ductal carcinoma in situ of the breast. J Clin Oncol. 2008;26(8):1247–1252. doi: 10.1200/JCO.2007.12.7969.
- Chua BH, Link EK, Kunkler IH, et al. Radiation doses and fractionation schedules in non-low-risk ductal carcinoma in situ in the breast (BIG 3-07/TROG 07.01): a randomised, factorial, multicentre, open-label, phase 3 study. Lancet. 2022;400(10350):431–440. doi: 10.1016/S0140-6736(22)01246-6.
- McCormick B, Winter KA, Woodward W, et al. Randomized phase III trial evaluating radiation following surgical excision for Good-Risk ductal carcinoma in situ: long-term report from NRG oncology/RTOG 9804. J Clin Oncol. 2021;39(32):3574–3582. doi: 10.1200/JCO.21.01083.
- Rakovitch E, Gray R, Baehner FL, et al. Refined estimates of local recurrence risks by DCIS score adjusting for clinicopathological features: a combined analysis of ECOG-ACRIN E5194 and ontario DCIS cohort studies. Breast Cancer Res Treat. 2018;169(2):359–369. doi: 10.1007/s10549-018-4693-2.
- Gupta A, Jhawar SR, Sayan M, et al. Cost-Effectiveness of adjuvant treatment for ductal carcinoma in situ. J Clin Oncol. 2021;39(21):2386–2396. doi: 10.1200/JCO.21.00831.
- Ward MC, Vicini F, Al-Hilli Z, et al. Cost-Effectiveness analysis of no adjuvant therapy versus partial breast irradiation alone versus combined treatment for treatment of low-risk DCIS: a microsimulation. J Clin Oncol Oncol Pract. 2021;17(8):e1055–e1074. doi: 10.1200/OP.20.00992.
- Liljegren G, Karlsson G, Bergh J, et al. The cost-effectiveness of routine postoperative radiotherapy after sector resection and axillary dissection for breast cancer stage I. Results from a randomized trial. Ann Oncol. 1997;8(8):757–763. doi: 10.1023/a:1008230000822.
- Stokes ME, Thompson D, Montoya EL, et al. Ten-year survival and cost following breast cancer recurrence: estimates from SEER-medicare data. Value Health. 2008;11(2):213–220. doi: 10.1111/j.1524-4733.2007.00226.x.
- Vitko AS, Martin PA, Zhang S, et al. Costs of breast cancer recurrence after initial treatment for high risk early breast cancer using SEER-Medicare. 44th Annual San Antonio Breast Cancer Symposium; 2022 Dec 6 − 10, San Antonio TX, USA; 2022
- Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Eur J Health Econ. 2013;14(3):367–372. doi: 10.1007/s10198-013-0471-6.
- Raldow AC, Sher D, Chen AB, et al. Cost effectiveness of DCISionRT for guiding treatment of ductal carcinoma in situ. JNCI Cancer Spectr. 2020;4(2):pkaa004.
- Tian Y, Schofield PE, Gough K, et al. Profile and predictors of long-term morbidity in breast cancer survivors. Ann Surg Oncol. 2013;20(11):3453–3460. doi: 10.1245/s10434-013-3004-8.
- Glynn D, Bliss J, Brunt AM, et al. Cost-effectiveness of 5 fraction and partial breast radiotherapy for early breast cancer in the UK: model-based multi-trial analysis. Breast Cancer Res Treat. 2023;197(2):405–416. doi: 10.1007/s10549-022-06802-1.
- Sopik V, Iqbal J, Sun P, et al. Impact of a prior diagnosis of DCIS on survival from invasive breast cancer. Breast Cancer Res Treat. 2016;158(2):385–393. doi: 10.1007/s10549-016-3894-9.
- Sagara Y, Freedman RA, Vaz-Luis I, et al. Patient prognostic score and associations with survival improvement offered by radiotherapy after breast-conserving surgery for ductal carcinoma in situ: a population-based longitudinal cohort study. J Clin Oncol. 2016;34(11):1190–1196. doi: 10.1200/JCO.2015.65.1869.
- Burt LM, Ying J, Poppe MM, et al. Risk of secondary malignancies after radiation therapy for breast cancer: comprehensive results. Breast. 2017;35:122–129. doi: 10.1016/j.breast.2017.07.004.
- Friedrich A-KU, Reisenbichler ES, Heller DR, et al. Characteristics and long-term risk of breast angiosarcoma. Ann Surg Oncol. 2021;28(9):5112–5118. doi: 10.1245/s10434-021-09689-2.
- Karlsson P, Holmberg E, Samuelsson A, et al. Soft tissue sarcoma after treatment for breast cancer–a Swedish population-based study. Eur J Cancer. 1998;34(13):2068–2075. doi: 10.1016/s0959-8049(98)00319-0.
