Abstract
Background
We hypothesise that a high rate of tumour regrowth after the watch-and-wait (w&w) strategy may lead, despite salvage surgery, to a significant impairment of ultimate local control compared with immediate surgery.
Materials and methods
To test this hypothesis, we conducted meta-analyses of studies on the w&w strategy (both opportunistic and planned) with an ultimate local failure rate as an endpoint in three patient groups: (1) in all starting radio(chemo)therapy as potential w&w candidates, (2) in a subgroup starting w&w, and (3) in a subgroup with regrowth.
Results
We identified eight studies for evaluation of local failure in group 1 (N = 837) and 36 studies in group 2 (N = 1914) and in group 3 (N = 439). The meta-analysis revealed an ultimate local failure rate of 8.0% (95% CI 4.8%–12.1%) in group 1 and 5.4% (95% CI 3.9%–7.1) in group 2. These rates are similar to those reported in the literature following preoperative chemoradiation and surgery. However, in the most unfavourable group 3 (with regrowth), the rate of ultimate local failure was 24.1% (95% CI 17.9%–30.9%), with the most common causes being patients’ refusal of salvage total mesorectal excision (TME) (9.1%), recurrence after salvage TME (7.8%), distant metastases (4.1%), frailty (2.4%), and pelvic tumour unresectability (1.7%).
Conclusion
Nearly 25% of patients with regrowth (unfavourable subgroup) experienced ultimate local failure, primarily due to refusing salvage TME. The risk of ultimate local failure in patients initiating radio(chemo)therapy as potential w&w candidates, or in patients starting w&w, appears comparable to that reported after preoperative chemoradiation and surgery. However, this comparison may be biased, because w&w studies included more early tumours compared with surgical studies.
Keywords:
Background
Total mesorectal excision (TME) is the cornerstone of rectal cancer management, providing high local control. However, it can lead to adverse effects such as impaired anorectal function or stoma. Therefore, the watch-and-wait (w&w) strategy is increasingly used in patients achieving clinical complete response (cCR) or near-cCR after radio(chemo)therapy. However, the tumour regrowth rate is high, reaching 30% [Citation1,Citation2]. Regrowth is evidence that radio(chemo)resistant subclinical disease persists despite cCR. Thus, there are two groups of patients having cCR: with true complete response (a favourable group with sustained cCR during observation), and with radio(chemo)resistant subclinical disease (an unfavourable group with regrowth during observation). Despite the high rate of tumour regrowth, it is believed that ultimate local control is unaffected, since R0 salvage surgery is performed in most patients [Citation1,Citation3]. However, local failure has not been assessed in the meta-analysis, taking into account all causes of ultimate local failure in addition to the failure of salvage surgery. If all these causes are considered together, it is possible that the ultimate local failure is higher compared with immediate surgery.
Two w&w strategies exist: opportunistic (‘pick the winner’) or planned (intentional). In the opportunistic strategy, routine preoperative radio(chemo)therapy is given, and patients achieving cCR are observed without surgery. The planned strategy aims to increase a cCR rate via radiotherapy dose escalation or by adding induction or consolidation chemotherapy to the radio(chemo)therapy (i.e., total neoadjuvant therapy [TNT]). This increased rate of cCR may result in more aggressive regrowths in patients undergoing the planned strategy, leading to an increased ultimate local failure rate compared with those undergoing the opportunistic strategy.
For the above reasons, our first hypothesis is that regrowth leads to a meaningful increase of an ultimate local failure rate compared with immediate surgery. Our second hypothesis is that the ultimate local failure rate is higher in the planned strategy compared with the opportunistic strategy. To test these two hypotheses we performed meta-analyses with ultimate local failure rate as an endpoint. To test the first hypothesis, three meta-analyses were planned: (1) an intention-to-treat meta-analysis, evaluating outcomes in all patients starting radiotherapy as potential candidates for w&w (group 1), (2) a meta-analysis evaluating the ultimate local failure rate in the subgroup of patients with cCR or near-cCR starting opportunistic or planned w&w (group 2), and (3) a meta-analysis evaluating the ultimate local failure rate in the subgroup of patients with regrowth (group 3), along with an estimate of the reasons for this failure. Next, we compared the calculated rates of ultimate local failure with the corresponding values reported in the literature after preoperative radio(chemo) therapy and surgery. To test the second hypothesis, all the above three analyses were performed with stratification based on the type of the w&w strategy.
Material and methods
The definitions we adopted in this review were as follows:
W&w was defined as a strategy that involves observation without immediate surgery (neither local excision [LE] nor TME) in patients who achieved cCR or near-cCR.
Regrowth was defined as the (re)appearance of intraluminal tumour or pelvic nodal disease in patients starting w&w.
Ultimate local control was defined as sustained cCR or regrowth controlled by salvage TME or LE. Patients who had recurrence after salvage LE and subsequently underwent successful re-salvage TME were also considered locally controlled.
Salvage TME was defined as TME performed for regrowth with or without prior salvage LE.
In the intention-to-treat analysis, ultimate local failure was defined as pelvic recurrence after salvage TME or uncontrolled local disease caused by various other reasons, such as death during radio(chemo)therapy or patients’ refusal of TME.
The category of planned w&w with radiation dose escalation was assigned when the radiation dose was at least 60 Gy.
The category of planned w&w using TNT was assigned when induction or consolidation chemotherapy lasted for at least eight weeks.
In studies where more than one w&w strategy was used, the assigned category depended on the strategy used in the majority of patients.
For comparison, we chose patients who received chemoradiation before surgery in the recent randomised RAPIDO study by Bahadoer et al. [Citation4] and the PRODIGE23 study by Conroy et al. [Citation5] (standard-of-care arms) as comparators with w&w. Ultimate local failure was defined in the same way as for w&w.
Search strategy, study selection, and data extraction
The systematic review was conducted in accordance with the PRISMA guidelines [Citation6]. The PubMed database was searched from the first publication on w&w in 2002 until February 2023, using the search terms provided in Supplementary Appendix 1. Citation tracking of included articles was also performed. Only studies in English and Spanish were included. The inclusion criteria were based on the PICO model: Population: patients with adenocarcinoma of the rectum; Intervention: w&w; Control: immediate radical surgery after preoperative radio(chemo)therapy; Outcomes: ultimate local failure rate. Exclusion criteria included (1) studies in which the calculation of the ultimate local failure rate was impossible, such as those without data on recurrence after salvage TME, (2) studies in which w&w was used only in patients unfit for surgery, (3) studies in which planned LE was used for organ preservation in all patients with cCR, and (4) studies in which w&w or LE was used but results were not reported separately.
Articles were screened according to their titles and abstracts. Full texts of all relevant studies were obtained. If there were multiple publications on the same trial, data with the longest follow-up were used. The following data were extracted: patients’ and treatment characteristics, length of follow-up, and the number of patients in the following categories: (1) starting radio(chemo)therapy, (2) starting w&w, (3) with regrowth, (4) with ultimate local failure with reasons for this event, and (5) undergoing salvage TME along with the number of those with post-salvage recurrence. The data were extracted by two authors using a pre-designed form, and any disagreements were resolved by consensus. In addition, for the OPERA study [Citation7] and the MORPHEUS study [Citation8], missing relevant data were obtained from the corresponding authors. The modified Newcastle–Ottawa scale [Citation9] was used to evaluate the methodological quality of nonrandomized studies (Supplementary Table S1).
Statistical methods
The pooled weighted rates with 95% confidence intervals (CIs) were estimated using a random-effects meta-analytic approach allowing for between-study heterogeneity using MetaXL (www.epigear.com), an add-in for meta-analysis in Microsoft Excel. Between-study heterogeneity was tested using Cochrane’s Q (2) with a significance level of p < .10 and quantified using I2 statistics. To evaluate potential publication and related biases, a Doi plot and its associated Luis Furuya-Kanamori index [Citation10] were used. Sensitivity analysis was conducted by withdrawing one study at a time from the meta-analysis to evaluate its effect on the pooled rate. The meta-analysis was performed according to the MOOSE guidelines [Citation11] (Supplementary Table S2).
Results
The ultimate local failure in all patients starting radio(chemo)therapy as potential candidates for w&w (group 1)
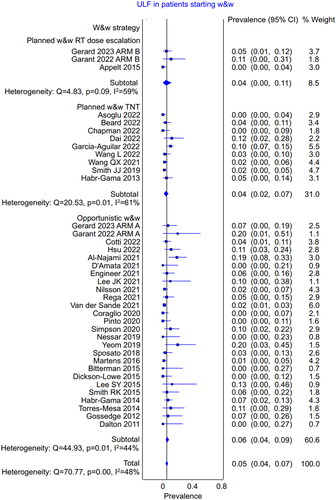
The systematic search (Supplementary Figure S1) identified eight trials (N = 837) that presented outcomes in all patients starting radio(chemo)therapy as potential candidates for w&w, allowing for intention-to-treat analysis () [Citation2,Citation7,Citation8,Citation12–17]. Seven studies were prospective, and one was retrospective. Three studies employed the planned strategy with radiation dose escalation, four used the planned strategy with TNT, and one used the opportunistic strategy. The meta-analysis of these studies showed that the pooled weighted rate of ultimate local failure was 8.0% (95% CI 4.8%–12.1%; heterogeneity, p = .001) (). There were no major differences in the ultimate local failure rate between the w&w strategies. In the planned strategy using radiation dose escalation (N = 143), the ultimate local failure rate was 6.9% (95% CI 2.6%–12.3%); in the planned strategy using TNT (N = 512), it was 7.3% (95% CI 2.3%–13.6%); and in the opportunistic strategy (N = 182), it was 10.8% (95% CI 1.2%–23.5%).
Figure 1. The pooled weighted rate of the ultimate local failure in the intention-to-treat analysis of all the patients starting radio(chemo)therapy (potential candidates for w&w).
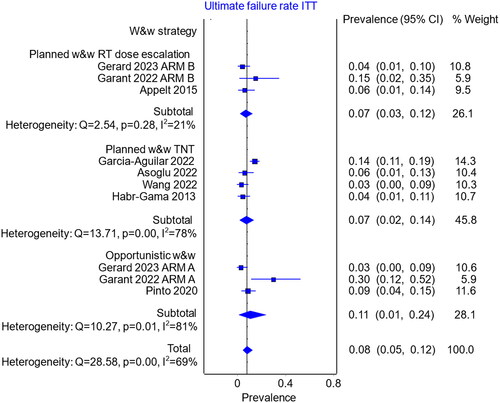
Table 1. Ultimate local failure among all the patients starting radio(chemo)therapy as potential candidates for w&w.
The ultimate locoregional failure in patients starting w&w (group 2)
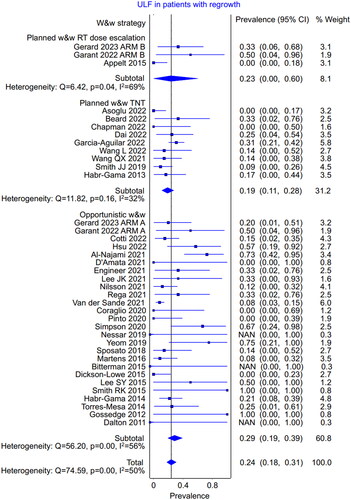
Thirty-six relevant references (N = 1914) were identified for the evaluation of outcomes in patients starting w&w () [Citation2,Citation7,Citation8,Citation12–45]. Ten studies were prospective, and 26 were retrospective. In three studies, the planned strategy with radiation dose escalation was employed, in nine studies, the planned strategy with TNT, and in the remaining 24 trials, the opportunistic strategy. The rate of ultimate local failure among patients starting w&w was 5.4% (95% CI 3.9%–7.1%; heterogeneity, p = .001). There were no major differences in the ultimate local failure rate between the w&w strategies (). In the planned strategy using radiation dose escalation (N = 121), the ultimate local failure rate was 4.4% (95% CI 0.0%–11.5%); in the planned strategy using TNT (N = 650), it was 4.3% (95% CI 1.8%–7.3%); and in the opportunistic strategy (N = 1143), it was 6.2% (95% CI 4.1%–8.5%).
Table 2. Ultimate local failure among the patients starting w&w strategy.
The ultimate locoregional failure in patients with regrowth (group 3), and the reasons for this failure
The rate of ultimate local failure among the subgroup with regrowth (36 references, N = 439) was 24.1% (95% CI 17.9%–30.9%; heterogeneity, p < .001). There were no major differences in the ultimate local failure rate between the w&w strategies (). In the planned strategy using radiation dose escalation (N = 22), the ultimate local failure rate was 23.3% (95% CI 0.0%–60.4%); in the planned strategy using TNT (N = 161), it was 18.9% (95% CI 11.1%–27.8%); and in the opportunistic strategy (N = 256), it was 28.7% (95% CI 19.2%–38.9%).
Among patients who experienced regrowth, the most common causes of ultimate local failure were patients’ refusal of salvage TME (9.1%, 95% CI 5.3%–13.7%), recurrence after salvage TME (7.8%, 95% CI 5.2%–11.0%), distant metastases (4.1%, 95% CI 2.4%–6.1%), frailty (2.4%, 95% CI 1.2%–4.0%), unresectable pelvic tumours (1.7%, 95% CI 0.7%–3.2%), and other reasons (2.3%, 95% CI 1.1%–3.9%) (Supplementary Figures S5–S10). There were no significant differences between the w&w strategies for each of the above-mentioned causes of ultimate local failure (Supplementary Figures S5–S10).
There might be two reasons for the patients’ refusal of salvage TME: (1) patients participating in w&w already refuse TME at diagnosis or (2) patients decide to refuse TME later at the time of regrowth. To explore this further, we conducted an additional post-hoc meta-analysis, which found a lower rate of patients refusing surgery among those with persistent tumours at response evaluation, at 1.9% (95% CI 0.6%–3.9%, Supplementary Figure S11), compared with 9.1% (95% CI 5.3%–13.7%) among those with regrowth.
Sensitivity analysis and publication bias assessment
Sensitivity analyses showed no significant impact on the outcomes when individual studies were removed (Supplementary Tables S3–S5). Publication and related bias assessments suggest that the pooled effect may have been overestimated in patients starting w&w and those with regrowth (Supplementary Figures S2–S4).
Discussion
The results of our systematic review and meta-analysis showed an unexpectedly high rate of ultimate local failure in patients with regrowth, affecting nearly a quarter of them, predominantly attributable to their refusal of salvage TME. This finding contradicts the belief that regrowth poses no meaningful oncological risk [Citation1,Citation3].
The ultimate local failure in all patients starting radio(chemo)therapy as potential candidates for w&w (group 1)
The incidence of ultimate local failure in the intention-to-treat analysis of all patients who underwent radio(chemo)therapy as potential candidates for w&w was 8.0% (95% CI 4.8%–12.1%) (, ). This rate is comparable to the corresponding rates of 8.7% (95% CI 6.1%–11.3%, 39 of 449) observed in surgically treated patients after chemoradiation in the RAPIDO trial [Citation4,Citation46] and 12.1% (95% CI 7.9%–16.3%, 28 of 230) in the PRODIGE23 trial [Citation5]. However, this comparison is biased with respect to the tumour size and stage, because the tumours in these trials were more advanced. For example, most studies included in our meta-analysis did not enrol patients with cT4 tumours (), whereas in the RAPIDO and PRODIGE23 trials they constituted 31% and 16% of the patients, respectively. Consequently, our analysis may substantially underestimate the additional risk of ultimate locoregional failure in patients who underwent radio(chemo)therapy as potential candidates for w&w compared with those undergoing immediate surgery.
The ultimate locoregional failure in patients starting w&w (group 2)
The incidence of ultimate locoregional failure was 5.4% (95% CI 3.9%–7.1%) in patients starting w&w (, ). This rate was comparable to the locoregional failure rate of 5.6% (95% CI 3.4%–7.8%, 24 of 426) observed in patients who underwent R0 or R1 resection after chemoradiation in the RAPIDO trial [Citation4,Citation46] and 6.1% (95% CI 2.9%–9.3%, 13 of 212) in the PRODIGE23 trial [Citation5]. Of note, patients achieving cCR represent a selected subgroup with favourable cancer biology. For example, in the OnCoRe study by Renehan et al. [Citation47], the overall survival at 3 years was 96% in patients undergoing w&w compared with 87% in patients undergoing immediate surgery for persistent tumour, p = .024. Thus, in our analysis the selection bias is present. The additional risk of ultimate locoregional failure in patients starting w&w compared with those undergoing immediate surgery may therefore be underestimated.
The ultimate locoregional failure in patients with regrowth (group 3)
Our meta-analysis found a locoregional recurrence rate of 7.8% (95% CI 5.2%–11.0%) after salvage TME (Supplementary Figure S6). This rate is similar to the rates of locoregional recurrence in patients who underwent R0 or R1 resection after chemoradiation in the RAPIDO trial (5.6%, 95% CI 3.4%–7.8%) [Citation4,Citation46] and the PRODIGE23 trial (6.1%, 95% CI 2.9%–9.3%) [Citation5]. Therefore, our meta-analysis suggests that patients undergoing salvage TME for regrowth do not have a meaningfully higher risk of ultimate locoregional failure compared with those undergoing immediate TME.
However, when all causes of ultimate local failure were considered (such as recurrence after salvage TME, patients’ refusal of salvage TME, distant metastases, frailty, or unresectable pelvic tumour), the rate of ultimate locoregional failure in the patients with regrowth was 24.1% (95% CI 17.9%–30.9%). This appears to be in contradiction with the results showing 5.4% of ultimate locoregional failure among patients starting w&w. However, this contradiction can be explained by the different denominators used in the calculations—the former only included cases with regrowth, while the latter consists of two quite different subgroups: (1) the larger subgroup with sustained cCR having excellent prognosis (winners), and the smaller subgroup with regrowth hawing much worse prognosis (losers). This issue is further illustrated by a pooled analysis of the surgical series showing a 5-year risk of local recurrence of 2.8% in the pathological complete response (pCR) group and 9.7% in the no pCR group, p < .0001) [Citation48].
Due to such a high rate of ultimate local failure in patients with regrowth, we suggest measures to reduce the risk of regrowth (see ). These measures also aim to decrease the risk of distant metastases, which may result from seeding from an uncontrolled primary tumour [Citation53,Citation54].
Table 3. Measures potentially leading to a reduction of regrowth risk in the watch-and-wait (w&w) strategy.
Notably, the most common reason for ultimate local failure was patients’ refusal of salvage TME, reported in 9.1% (95% CI 5.3%–13.7%) of those with regrowth (Supplementary Figure S5), whereas the rate of patients refusing surgery among those with persistent tumours at response evaluation was only 1.9% (95% CI 0.6%–3.9%) (Supplementary Figure S11). Similarly, the intention-to-treat analysis showed 1.1% (95% CI 0.9%−3.0%, 5 of 449) of patients refusing surgery in the standard-of-care arm of the RAPIDO trial [Citation4]. This suggests that the desire for organ preservation, initially arising from achieving cCR, may have been deeply rooted in these patients’ consciousness, making it difficult to accept salvage TME, especially abdominoperineal resection. Thus, the high rate of patients refusing a salvage TME may be regarded as a drawback of the w&w strategy. This information can be communicated to patients as a warning. Frailty was the reason for ultimate local failure only in 2.4% of patients with regrowth. Nevertheless, it cannot be excluded that some patients who refused salvage TME were driven by a subjective sense of frailty. On the other hand, in the OPERA trial 21% (4 of 19, ) of patients with regrowth did not agree for salvage TME, despite only fully operable patients being eligible for entry into this study.
Does planned strategy jeopardise ultimate locoregional control compared with the opportunistic strategy?
We did not observe major differences in the ultimate failure rates between the opportunistic strategy and the planned strategy (using either radiation dose escalation or TNT) (). Thus, our hypothesis that ultimate local failure is higher in the planned strategy compared with the opportunistic strategy is not supported. It is worth noting that a higher number of patients achieving cCR in the planned strategy, together with a similar ultimate local failure rate, results in a clinically significant increase in the number of patients with long-term organ preservation compared with the opportunistic strategy [Citation1,Citation2,Citation7,Citation8].
Limitations
The calculated rates of ultimate local failure are uncertain for various reasons. First, they could be underestimated because the follow-up periods were shorter than 3 years in 38% (3 of 8) of the studies included in our intention-to-treat analysis () and in 44% (16 of 36) of the studies included in our analysis of patients starting w&w (). In addition, these follow-up periods were measured from radiotherapy, which means that they would be approximately one year shorter if measured from salvage TME. Second, the ultimate local failure rates could be overestimated because some patients with recurrence after salvage TME could be cured after re-salvage surgery; such attempts or outcomes were usually not reported. However, achieving a cure from recurrence following TME is rare; a median overall survival of only about 1.5 years from the onset of local recurrence has been reported [Citation46]. Finally, the ultimate local failure rates could also be overestimated because some patients who initially refused salvage TME might have changed their minds later.
The aforementioned sources of bias, variability in patient population and treatment modalities (reflected by statistically significant tests for heterogeneity), as well as low quality of evidence (most included studies were small retrospective or cohort studies), and a possible systematic bias from the use of language restrictions, make our estimations and conclusions uncertain. Thus, the evidence from our meta-analysis is weak and does not allow for ruling out a small additional risk of an increased ultimate locoregional failure rate associated with w&w.
A call for standardisation of reporting outcomes
While performing our meta-analysis, we noticed that the reporting of results varied among publications, making it difficult to compare outcomes. Therefore, we suggest standardising the reporting of outcomes. First, it would be helpful in assessing treatment efficacy to report outcomes not only in patients who started w&w but also in all patients who started radio(chemo)therapy as potential candidates for w&w. Second, outcomes after w&w are reported in two ways: (1) in the total group of patients who initiate w&w with either cCR or near-CR [Citation2,Citation7,Citation14,Citation18,Citation40]. In this approach, patients with near-CR who do not eventually achieve cCR and subsequently require salvage surgery are categorised as having ‘regrowth’ (although they actually present with the persistent disease rather than tumour reappearance), and (2) only in patients with cCR and those with near-CR who eventually achieve cCR [Citation19–27,Citation30–37,Citation41–45]. This approach excludes patients with near-cCR who do not eventually reach cCR and require salvage surgery due to persistent or progressive near-CR from the analyses. This leads to presenting artificially lower regrowth risk in the second way compared with the first. We believe that reporting outcomes in all patients starting w&w (way 1) is desirable, because it includes all patients at risk of deferred surgery.
Conclusion
Nearly a quarter of patients with regrowth (an unfavourable subgroup) experience ultimate local failure, primarily due to their refusal of salvage TME. The risk of ultimate local failure in patients starting radio(chemo)therapy as potential candidates for w&w, or those starting w&w, seems to be comparable to that reported after preoperative chemoradiation and surgery. This comparison may be biased because w&w studies included more early tumours compared with surgical studies.
Supplemental Material
Download PDF (2.4 MB)Acknowledgements
The authors thank Prof. Te Vuong from the Jewish General Hospital in Montreal and Prof. Jean Pierre Gerard from Centre Antoine Lacassagne in Nice for providing unpublished data from the MORPHEUS and the OPERA trials.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
- van der Valk MJM, Hilling DE, Bastiaannet E, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the international watch & wait database (IWWD): an international multicentre registry study. Lancet. 2018;391(10139):2537–2545. doi: 10.1016/S0140-6736(18)31078-X.
- Garcia-Aguilar J, Patil S, Gollub MJ, et al. Organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy. J Clin Oncol. 2022;40(23):2546–2556. doi: 10.1200/JCO.22.00032.
- Kong JC, Guerra GR, Warrier SK, et al. Outcome and salvage surgery following “watch and wait” for rectal cancer after neoadjuvant therapy: a systematic review. Dis Colon Rectum. 2017;60(3):335–345. doi: 10.1097/DCR.0000000000000754.
- Bahadoer RR, Dijkstra EA, van Etten B, et al. RAPIDO collaborative investigators. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):29–42. doi: 10.1016/S1470-2045(20)30555-6.
- Conroy T, Bosset JF, Etienne PL, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(5):702–715. doi: 10.1016/S1470-2045(21)00079-6.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71.
- Gerard JP, Barbet N, Schiappa R, et al. Neoadjuvant chemoradiotherapy with radiation dose escalation with contact x-ray brachytherapy boost or external beam radiotherapy boost for organ preservation in early cT2-cT3 rectal adenocarcinoma (OPERA): a phase 3, randomised controlled trial. Lancet Gastroenterol Hepatol. 2023;8(4):356–367. doi: 10.1016/S2468-1253(22)00392-2.
- Garant A, Vasilevsky C-A, Boutros M, et al. MORPHEUS phase II-III study: a pre-planned interim safety analysis and preliminary results. Cancers (Basel). 2022;14:3665. doi: 10.3390/cancers14153665.
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute, 2011. [cited 2023 March 25]. Available from: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Furuya-Kanamori L, Barendregt JJ, Doi SAR. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int J Evid Based Healthc. 2018;16(4):195–203. doi: 10.1097/XEB.0000000000000141.
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008.
- Habr-Gama A, Sabbaga J, Gama-Rodrigues J, et al. Watch and wait approach following extended neoadjuvant chemoradiation for distal rectal cancer: are we getting closer to anal cancer management? Dis Colon Rectum. 2013;56(10):1109–1117. doi: 10.1097/DCR.0b013e3182a25c4e.
- Appelt AL, Pløen J, Harling H, et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015;16(8):919–927. doi: 10.1016/S1470-2045(15)00120-5.
- Wang L, Zhang XY, Zhao YM, et al. Intentional watch & wait or organ preservation surgery following neoadjuvant chemoradiotherapy plus consolidation CAPEOX for MRI-defined low-risk rectal cancer: findings from a prospective phase 2 trial (PKUCH-R01 trial, NCT02860234). Ann Surg. 2023;277(4):647–654. doi: 10.1097/SLA.0000000000005507.
- Asoglu O, Bulut A, Aliyev V, et al. Chemoradiation and consolidation chemotherapy for rectal cancer provides a high rate of organ preservation with a very good long-term oncological outcome: a single-center cohort series. World J Surg Oncol. 2022;20(1):358. doi: 10.1186/s12957-022-02816-7.
- Asoglu O, Tokmak H, Bakir B, et al. The impact of total neo-adjuvant treatment on nonoperative management in patients with locally advanced rectal cancer: the evaluation of 66 cases. Eur J Surg Oncol. 2020;46(3):402–409. doi: 10.1016/j.ejso.2019.07.012.
- Pinto JC, Pereira AD, Pimenta A, et al. Low rectal cancer treatment strategies: a cohort study assessing watch and wait. J Cancer Res Clin Oncol. 2020;146(10):2631–2638. doi: 10.1007/s00432-020-03248-0.
- van der Sande ME, Figueiredo N, Beets GL. Management and outcome of local regrowths in a watch-and-wait prospective cohort for complete responses in rectal cancer. Ann Surg. 2021;274(6):e1056–e1062. doi: 10.1097/SLA.0000000000003738.
- Habr-Gama A, Gama-Rodrigues J, São Julião GP, et al. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys. 2014;88(4):822–828. doi: 10.1016/j.ijrobp.2013.12.012.
- Martens MH, Maas M, Heijnen LA, et al. Long-term outcome of an organ preservation program after neoadjuvant treatment for rectal cancer. J Natl Cancer Inst. 2016;108:djw171. doi: 10.1093/jnci/djw171.
- Bitterman DS, Resende Salgado L, Moore HG, et al. Predictors of complete response and disease recurrence following chemoradiation for rectal cancer. Front Oncol. 2015;5:286. doi: 10.3389/fonc.2015.00286.
- Dalton RS, Velineni R, Osborne ME, et al. A single-Centre experience of chemoradiotherapy for rectal cancer: is there potential for nonoperative management? Colorectal Dis. 2012;14(5):567–571. doi: 10.1111/j.1463-1318.2011.02752.x.
- Torres-Mesa PA, Oliveros R, Mesa J, et al. Desenlaces del manejo no quirúrgico posterior a neoadyuvancia del cáncer localmente avanzado de recto. Rev Colomb Cancerol. 2014;18(3):109–119. doi: 10.1016/j.rccan.2014.05.003.
- Gossedge G, Montazeri A, Nandhra A, et al. Complete clinical response to chemoradiotherapy for rectal cancer. Is it safe to ‘watch and wait’? Colorectal Dis. 2012;14(Suppl 2):20–21.
- Dickson-Lowe RA, Hanek P, Kalaskar S, et al. Non-operative management of low rectal cancer with complete response to standard neoadjuvant chemoradiotherapy. Gut. 2015;64(Suppl 1):A554–A555. doi: 10.1136/gutjnl-2015-309861.1217.
- Coraglio MF, Eleta MA, Kujaruk MR, et al. Analysis of long-term oncological results of clinical versus pathological responses after neoadjuvant treatment in locally advanced rectal cancer. World J Surg Oncol. 2020;18(1):313. doi: 10.1186/s12957-020-02094-1.
- Lee JK, Cho JR, Song KS, et al. Oncologic comparison between nonradical management and total mesorectal excision in good responders after chemoradiotherapy in patients with mid-to-low rectal cancer. Ann Surg Treat Res. 2021;101(2):93–101. doi: 10.4174/astr.2021.101.2.93.
- Yeom SS, Lee SY, Kim CH, et al. Non-operative treatment outcome for rectal cancer patient with clinical complete response after neoadjuvant chemoradiotherapy. Asian J Surg. 2019;42(8):823–831. doi: 10.1016/j.asjsur.2018.12.007.
- Al-Najami I, Jones HJ, Dickson EA, et al. Rectal cancer: watch-and-wait and continuing the rectal-preserving strategy with local excision for incomplete response or limited regrowth. Surg Oncol. 2021;37:101574. doi: 10.1016/j.suronc.2021.101574.
- Wang QX, Zhang R, Xiao WW, et al. The watch-and-wait strategy versus surgical resection for rectal cancer patients with a clinical complete response after neoadjuvant chemoradiotherapy. Radiat Oncol. 2021;16(1):16. doi: 10.1186/s13014-021-01746-0.
- Nilsson PJ, Ahlberg M, Kordnejad S, et al. Organ preservation following short-course radiotherapy for rectal cancer. BJS Open. 2021;5:zrab093. doi: 10.1093/bjsopen/zrab093.
- Cotti GC, Pandini RV, Braghiroli OFM, et al. Outcomes of patients with local regrowth after nonoperative management of rectal cancer after neoadjuvant chemoradiotherapy. Dis Colon Rectum. 2022;65(3):333–339. doi: 10.1097/DCR.0000000000002197.
- Lee SY, Chang HK, Young JK, et al. Oncologic outcomes according to the treatment strategy in radiologic complete responders after neoadjuvant chemoradiation for rectal cancer. Oncology. 2015;89(6):311–318. doi: 10.1159/000439279.
- Hsu YJ, Chern YJ, Lai IL, et al. Usefulness of close surveillance for rectal cancer patients after neoadjuvant chemoradiotherapy. Open Med. 2022;17(1):1438–1448. doi: 10.1515/med-2022-0555.
- Neşşar G, Demirbağ AE, Mısırlıoğlu HC, et al. “Watch and wait" approach in rectal cancer patients following complete clinical response to neoadjuvant chemoradiotherapy does not compromise oncologic outcomes. Turk J Gastroenterol. 2019;30(11):951–956. doi: 10.5152/tjg.2019.18984.
- Sposato LA, Lam Y, Karapetis C, et al. Observation of “complete clinical response” in rectal cancer after neoadjuvant chemoradiation: the flinders experience. Asia Pac J Clin Oncol. 2018;14(6):439–445. doi: 10.1111/ajco.12993.
- D'Amata G, Manzi F, Florio G, et al. The “watch and wait” approach following chemoradiotherapy for rectal cancer: a case series and review of literature. Ann Ital Chir. 2021;10:S0003469X2103534X.
- Beard BW, Rettig RL, Ryoo JJ, et al. Watch-and-Wait compared to operation for patients with complete response to neoadjuvant therapy for rectal cancer. J Am Coll Surg. 2020;231(6):681–692. doi: 10.1016/j.jamcollsurg.2020.08.775.
- Dai D, Liu G, Liu H, et al. Clinical feasibility of the therapeutic strategies total neoadjuvant therapy and “watch and wait” in the treatment of rectal cancer patients with recurrence after clinical complete response. Front Surg. 2022;9:1006624. doi: 10.3389/fsurg.2022.1006624.
- Engineer R, Saklani A, D'souza A, et al. Watch and wait approach after neoadjuvant chemoradiotherapy in rectal cancer: initial experience in the Indian subcontinent. Indian J Surg Oncol. 2021;12(4):664–670. doi: 10.1007/s13193-021-01421-6.
- Simpson G, Hopley P, Wilson J, et al. Long-term outcomes of real world 'watch and wait’ data for rectal cancer after neoadjuvant chemoradiotherapy. Colorectal Dis. 2020;22(11):1568–1576. doi: 10.1111/codi.15177.
- Rega D, Granata V, Romano C, et al. Watch and wait approach for rectal cancer following neoadjuvant treatment: the experience of a high volume cancer center. Diagnostics. 2021;11:1507. doi: 10.3390/diagnostics11081507.
- Chapman BC, Lai S, Friedrich T, et al. Rectal cancer: clinical and molecular predictors of a complete response to total neoadjuvant therapy. Dis Colon Rectum. 2023;66(4):521–530. doi: 10.1097/DCR.0000000000002245.
- Smith JJ, Strombom P, Chow OS, et al. Assessment of a watch-and-Wait strategy for rectal cancer in patients with a complete response after neoadjuvant therapy. JAMA Oncol. 2019;5(4):e185896. doi: 10.1001/jamaoncol.2018.5896.
- Smith RK, Fry RD, Mahmoud NN, et al. Surveillance after neoadjuvant therapy in advanced rectal cancer with complete clinical response can have comparable outcomes to total mesorectal excision. Int J Colorectal Dis. 2015;30(6):769–774. doi: 10.1007/s00384-015-2165-2.
- Dijkstra EA, Nilsson PJ, Hospers GAP, et al. Locoregional failure during and after short-course radiotherapy followed by chemotherapy and surgery compared to long-course chemoradiotherapy and surgery – A five-year follow-up of the RAPIDO trial. Ann Surg. 2023;4:e288. doi: 10.1097/SLA.0000000000005799.
- Renehan AG, Malcomson L, Emsley R, et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol. 2016;17(2):174–183. doi: 10.1016/S1470-2045(15)00467-2.
- Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11(9):835–844. doi: 10.1016/S1470-2045(10)70172-8.
- Jankowski M, Pietrzak L, Rupiński M, et al. Watch-and-wait strategy in rectal cancer: is there a tumour size limit? Results from two pooled prospective studies. Radiother Oncol. 2021;160:229–235. doi: 10.1016/j.radonc.2021.05.014.
- Bulens PP, Smets L, Debucquoy A, et al. Nonoperative versus operative approach according to the response to neoadjuvant chemoradiotherapy for rectal cancer: a prospective cohort study. Clin Transl Radiat Oncol. 2022;36:113–120. doi: 10.1016/j.ctro.2022.07.009.
- Garant A, Magnan S, Devic S, et al. Image guided adaptive endorectal brachytherapy in the nonoperative management of patients with rectal cancer. Int J Radiat Oncol Biol Phys. 2019;105(5):1005–1011. doi: 10.1016/j.ijrobp.2019.08.042.
- Martens MH, van Heeswijk MM, van den Broek JJ, et al. Prospective, multicenter validation study of magnetic resonance volumetry for response assessment after preoperative chemoradiation in rectal cancer: can the results in the literature be reproduced? Int J Radiat Oncol Biol Phys. 2015;93(5):1005–1014. doi: 10.1016/j.ijrobp.2015.09.008.
- Fernandez LM, São Julião GP, Renehan AG, et al. The risk of distant metastases in patients with clinical complete response managed by watch and wait after neoadjuvant therapy for rectal cancer: the influence of local regrowth in the international watch and wait database. Dis Colon Rectum. 2023;66(1):41–49. doi: 10.1097/DCR.0000000000002494.
- Socha J, Kępka L, Michalski W, et al. The risk of distant metastases in rectal cancer managed by a watch-and-wait strategy – A systematic review and meta-analysis. Radiother Oncol. 2020;144:1–6. doi: 10.1016/j.radonc.2019.10.009.