Abstract
Background
Cervical, liver and stomach cancers are the most common infection-associated malignancies and the leading cause of morbidity in non-Western regions. We compared the incidence and mortality of these cancers between non-Western immigrant and non-immigrant Nordic female populations. We also analysed the effect of age at immigration, duration of residence and education on cancer burden.
Material and Methods
Study population consisted of women residents in Denmark, Finland, Iceland and Norway in 1973–2020. Non-Western women contributed 3.1% of the total 260 million person-years at risk. All women were followed from their 20th birthday, or from the date of immigration if after, until the date of their first primary cancer diagnosis, death, emigration, or the end of the country-specific study period. All data were adjusted for 10-year age groups and calendar periods, and immigrant data was further broken down by region of birth, age at immigration and education level. Country-specific estimates were produced by multivariable Poisson regression and pooled in Finland with a random effects model.
Results
Altogether, there were 60 982 cases of cervical, liver and stomach cancer in the study population, causing 36 582 deaths. The immigrant women had significantly higher liver (rate ratio [RR] 1.78, 95% confidence interval (CI) 1.03–3.06) and stomach cancer incidence (RR 1.68, CI 1.29–2.18), and stomach cancer mortality (RR 1.49, CI 1.17–1.92) than non-immigrant women. In the immigrant population, high education was related to lower incidence and mortality of studied cancers. The rate ratio of cervical cancer decreased with duration of residence and increased with rising age at immigration.
Conclusion
Due to the increased incidence and mortality of infection-related cancers and changes in cancer patterns by age at immigration and duration of residence, attention should be paid to targeted health care services for immigrants. Special efforts should be given to women who have spent their youth in high-risk areas.
Introduction
Cancer incidence and mortality are increasing worldwide due to population growth and ageing, as well as changes in risk factors [Citation1]. There are, however, wide geographical variations in cancer burden, several of which are associated with socioeconomic developments, such as economic growth, medical and public health progress [Citation1, Citation2].
Cervical, stomach and liver cancers are the three most common infection-associated malignancies among women globally [Citation3]. In total, cervical cancer is the fourth most common malignancy and the fourth leading cause of cancer death, with around 600 000 new cases and 340 000 deaths worldwide [Citation4]. Stomach and liver cancers are followed by cervical cancer as seventh and ninth in incidence and fifth and sixth in mortality, totalling more than 650 000 annual cases and 500 000 annual deaths.
High-risk subtypes of the human papillomavirus (HPV) cause almost all cervical cancers [Citation5]. Other co-factors include sexually transmittable infections, high parity and smoking. Helicobacter pylori infection [Citation6] and chronic infections with hepatitis B or C viruses (HBV, HCV) are the main risk factors for stomach and liver cancers, respectively [Citation1]. Also, alcohol consumption, smoking and excess body weight have been shown to increase the risk, as well as food preservation by salting (stomach cancer) and type 2 diabetes (liver cancer) [Citation1].
Cervical cancer is preventable by HPV vaccine and screening [Citation1]. The key preventive measures for stomach and liver cancers are improvements in the preservation and storage of foods as well as HBV vaccination, well-performed infection control in health care and antiviral HCV agents. Unfortunately, these measures are non-equitably implemented across the world.
Migrant women are influenced by disease and risk patterns from both their country of origin and their new country [Citation7,Citation8]. Studies comparing migrant and non-immigrant female populations thus provide valuable insights into cancer aetiology, environmental risk factors and access to and effectiveness of health care services. In previous studies, migrants have shown differences in cancer risks between their home and host countries. Non-western migrants have also been shown to be more likely to develop cancers related to infectious diseases than populations in their host country. This seems logical since infections are the leading cause of morbidity in less developed regions and are often contracted at an early age [Citation1].
The Nordic countries represent regions with stable governance and economies, high education and well-functioning social and health care services. They also have nationwide population and cancer registries. Therefore, these countries provide excellent environments to study the transition in cancer patterns due to immigration. However, only Sweden, with the highest share of immigrants among the Nordic countries [Citation9], has consistently provided information on cancer developments in its migrant population since the early 2000s [Citation10–12]. From 2018 on, annual estimates on cancer incidence in different migrant populations have also been published in Norway [Citation13].
We compared the incidence and mortality patterns of cervical, liver and stomach cancers between the first-generation non-Western immigrant and the non-immigrant female populations in Denmark, Finland, Iceland and Norway over several decades. Additionally, we analysed the effect of age at migration, duration of residence and education on the cancer burden within the immigrant populations.
Material and methods
We utilised individual data on immigrant and non-immigrant women from the Danish, Finnish, Icelandic and Norwegian national registries. In Denmark, Finland and Norway, the information on country of birth and residential history was retrieved from the population registry. In Iceland, these data were retrieved from the statistical office. In each country, the information on the first primary cancers of cervical, liver and stomach was from the cancer registry using ICD-10 codes, and the information on education for the immigrant women was from the statistical office. Information on causes of death was retrieved from statistical offices (Finland and Norway), the cancer registry (Iceland) and health data authority (Denmark). All these data were linked using a unique identification code, which exists for permanent residents in all four countries.
Our study population consisted of women registered as residents in 1986–2019 in Denmark, 1973–2017 in Finland, 1986–2020 in Iceland and 1990–2015 in Norway. The country-specific follow-up times varied depending on data availability, and the beginning and coverage of registration.
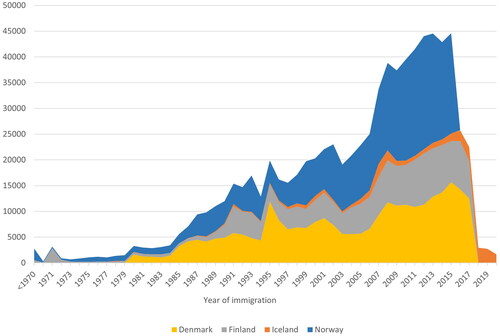
In each country, non-Western immigrants were defined as individual women born outside the Nordic countries, Western and Southern Europe, Northern America, Australia and New Zealand. The periodical accumulation of these women in each host country is presented in . Women who emigrated within one year after immigration, and women with a missing or clearly incorrect history of residence were excluded from the final study population (See Appendix A for further details).
Figure 1. Periodic accumulation of non-Western immigrant women in Denmark, Finland, Iceland and Norway. Since the populations in Denmark, Finland and Norway are close to each other, the absolute differences in the number of immigrant women also reflect the corresponding relative differences between these countries.
The non-Western study population was followed from their 20th birthday (or from the date of immigration if it happened after the 20th birthday) until death, emigration, age 95 years or the end of the year of the country-specific study period, whichever occurred first. For the cancer incidence analysis, the follow-up ended on the date of the first diagnosis of the cancer in question, or the censoring date (emigration, death, age 95 years or country-specific study end), whichever came first. For the cancer mortality analysis, the follow-up ended on the date of death due to the cancer in question, or the censoring date (emigration, death, age 95 years, or country-specific study end), whichever came first. The date of immigration was the start date, and the date of emigration was the end date of the first period of residence in the Nordic country. The non-immigrant study population was followed-up similarly to the immigrant population in Denmark, Iceland and Norway. In Finland, we used aggregate census data on non-immigrants. These data were modified to closely resemble the data from other Nordic countries.
Aggregate data on the number of incident cancers, cancer deaths and person-years at risk were formed by 10-year age groups and 10-year calendar periods separately for non-Western immigrant and non-immigrant women in each Nordic country. For immigrant women, the data were also adjusted for the region of birth (Central and South Asia, East Asia and the Pacific, Latin America and the Caribbean, the Middle East and North Africa, Russia and Eastern Europe, Sub-Saharan Africa), age at immigration (0–19, 20–29, 30–39, 40+ years), duration of residence (1–9, 10–19, 20+ years) and education level (primary 0–9 years or missing, secondary 9–12 years, tertiary 12+ years). The Nordic Statistical Offices include both primary 0–9 years and missing education into the same group, and thus it was not possible to distinguish between those.
Multivariable Poisson regression was used to model the relative differences in incidence and mortality as rate ratios (RR) with 95% confidence intervals in the above-listed categories and subgroups. First, we compared the incidence and mortality rates of cervical, liver and stomach cancers between non-Western immigrant women and the non-immigrant female population. Second, we analysed the impact of age at immigration, duration of residence and education on the incidence and mortality of these three cancers within the immigrant female population. All analyses were performed using the R program version 4.0.2.
All models were adjusted for the 10-year age group and 10-year calendar period. These rather wide categories were chosen to avoid strata with no cases or deaths among immigrants. Models related to the non-Western immigrant population only, and assessing education, duration of residence and age at immigration, were additionally adjusted for geographical region (Central and South Asia, Middle East and North Africa, Russia and Eastern Europe and Sub-Saharan Africa).
Data were processed and analysed with a federated approach, where the Finnish Cancer Registry was responsible for coordination, instructions, and the development of R scripts. First, all data variables and formats were harmonised in each Nordic country following the instructions. Thereafter, the contents of these harmonised individual data were checked for consistency and analysed using similar R scripts in each country. Finally, Denmark, Iceland and Norway sent their respective modelling results to Finland, where a random effects meta-analysis model was used to pool the country-specific estimates [Citation14]. The weights were calculated using generic inverse variance method.
The country-specific estimates were pooled because the proportion and number of non-Western immigrant women and, as a result, the number of cancers (especially in the categories of length of stay and age of immigration) were low in each Nordic country.
The STROBE cohort reporting guidelines were used in the study [Citation15].
Results
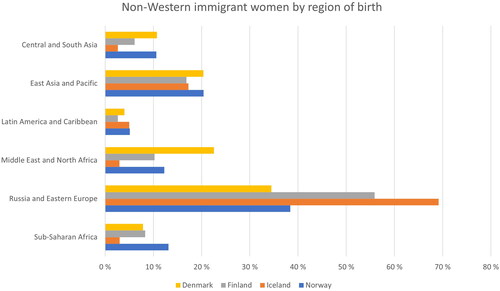
In total, there were 766 033 non-Western immigrant women in the study population, and they contributed 3.1% of the total of 260 million person-years at risk. In all countries, the largest immigrant group consisted of women born in Russia or Eastern Europe (). The second and third biggest immigrant populations were from East Asia and the Pacific, and from the Middle East and North Africa.
In the study population, there were altogether 60 982 cervical, liver and stomach cancers, and 36 582 deaths due to these cancers (). In non-Western women, cervical cancer was the most common cancer, followed by stomach and liver cancers, whereas stomach cancer had the highest number of deaths.
Table 1. The number of cancer cases and deaths, and the corresponding person-years (pyrs) among the non-immigrant and non-Western immigrant study population.
The number of cancer cases and deaths and the corresponding person-years (pyrs) among the non-immigrant and non-Western immigrant study populations.
The adjusted rate ratios showed both higher incidence (rate ratio (RR) 1.68, 95% confidence interval (CI) 1.29–2.18) and higher mortality (RR 1.49, CI 1.17–1.92) in stomach cancer in non-Western immigrant women compared to the non-immigrant female population (). Immigrant women also had a higher risk of liver cancer than non-immigrant women (RR 1.78, CI 1.03–3.06). This was most pronounced in Norway but could not be seen in Finland. Further analyses by region of birth showed elevated risks for stomach cancer in women originating from the Middle East and North Africa, Russia and Eastern Europe, East Asia and the Pacific and Sub-Saharan Africa (Appendix B). The risk of liver cancer was highest in women from East Asia and the Pacific, Sub-Saharan Africa and Latin America.
Figure 3. Adjusted cancer incidence and mortality rate ratios (RR) among non-Western immigrant women compared to non-immigrant women. (Adjusted by attained age, calendar year and region of birth.)
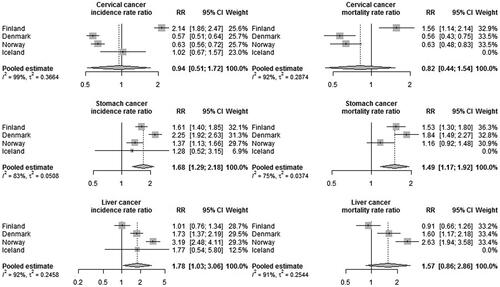
Cervical cancer incidence and mortality were significantly lower among the non-Western immigrants in Denmark and Norway compared to the non-immigrant population whereas in Finland the opposite was observed (). The same phenomena were also seen in the results by region of birth (Appendix B).
To study further the country-specific variations of cervical cancer, we performed sensitivity analyses with the Finnish data using pooled estimate from the Nordic countries as a reference. (Nordcan database 2.0.) In these analyses, the Finnish incidence rate ratio compared to the pooled estimate from Nordcan was reduced to 1.04 (0.91–1.14), and the corresponding mortality rate ratio to 0.83 (0.61–1.14), suggesting that the low national incidence and mortality figures among non-immigrant women in Finland drove the larger rate ratios we observed in the main analysis.
All in all, incidence and mortality figures followed each other in all three cancer sites, showing e.g., that low incidence results in low mortality. This may reveal that treatment facilities are similarly available for both non-immigrant and immigrant populations in the Nordic countries.
shows the number of cancer cases and deaths as well as the adjusted rate ratio in cancer incidence and mortality in non-Western women by duration of residence, age at immigration and education level. Compared to the primary education level, secondary or tertiary education decreased both the incidence and mortality of all studied cancers. Additionally, the rate of cervical cancer decreased with duration of residence and increased with rising age at immigration.
Table 2. Number of cancer cases and deaths and adjusted cancer incidence and mortality rate ratios (RR) among non-Western immigrant women by duration of residence, age at immigration and education level.
Number of cancer cases and deaths and adjusted cancer incidence and mortality rate ratios (RR) among non-Western immigrant women by duration of residence, age at immigration and education level.
Discussion
Our study is a collaborative study on the incidence and mortality patterns of cervical, liver and stomach cancers in non-Western immigrant women. We provide for the first time pooled information over several decades on the incidence and mortality of these cancers in a first-generation migrant female population from Denmark, Finland, Iceland and Norway. Our results show that non-Western immigrant women in the Nordic countries have significantly higher liver and stomach cancer incidence and mortality than non-immigrant women. High education is related to lower incidence and mortality rates for all three studied cancers. The risk of cervical cancer decreases with the duration of residence and increases with increasing age at immigration.
Strengths and limitations
The strength of our study lies in the individual, long-term registry data and uniform analyses which were performed using the same R-scripts in every participating country. Our study thus provides comparable long-term insights on post-migration changes in cancer incidence and mortality from Denmark, Finland, Iceland and Norway. Furthermore, these are the first results on cervical, liver and stomach cancer incidence and mortality in non-Western immigrant women from Finland and Iceland.
Since immigrant women are still relatively few and young in Denmark, Iceland, Finland and Norway (), we observed only a small number of cancers and cancer deaths, and thus the region-specific results may be sporadic. Also, information on potential contributing factors, such as the reason for migration, marital status and origin of spouses, was not available. Additionally, we did not account for return migration and thus excluded women at their first emigration. There might also have been small differences in reporting and registering data practices in each Nordic country [Citation16]. These, together with minor imperfections in the registration of the residing history and causes of deaths, are, however, unlikely to affect our results.
Comparison to European and other Nordic studies
European studies have consistently reported lower overall cancer incidence and mortality in non-Western immigrants compared to non-immigrant population [Citation8,Citation17]. Nonetheless, there has been considerable diversity between the sites [Citation8,Citation18]. Results from the Nordic countries reveal a higher burden of liver and stomach cancers in non-Western immigrants, especially in men and women from Asia (liver) and Eastern Europe (stomach) [Citation12,Citation19,Citation20], and a lower incidence and mortality of cervical cancer, compared to non-immigrant citizens [Citation11,Citation21,Citation22].
Chronic HBV infections are rare in the Nordic countries [Citation23], and most of the HBV-infected persons are immigrants from high-risk areas [Citation12]. Liver cancer typically develops two or more decades after viral infection, and the risk is specifically high for those infected at birth [Citation1]. Therefore, the higher incidence and mortality levels in immigrants compared to non-immigrants are likely to depend on infections contracted before immigration. Our results showed a non-significant increase in the incidence and mortality of liver cancer due to the rising age of immigration and thus contribute to this conclusion. The high risk of liver cancer in immigrants was most evident in Norway, as has been reported earlier [Citation20]. This may reflect a larger proportion of immigrant women from high-risk areas (EastAsia, Pacific and Sub-Saharan Africa) compared to that in Denmark and Finland (see ). To note, however, the incidence of liver cancer in the non-immigrant Norwegian female population over the period 1990–2016 was lower than the corresponding incidence in Denmark and Finland (Age standardised rate (World) 1.2 vs. 1.8/2.0) [Citation24].
Incidence and mortality rates of stomach cancer have been steadily declining over the last decade in most populations [Citation4]. These trends have been attributed to a decreased prevalence of Helicobacter pylori and improvements in the preservation and storage of foods [Citation1]. However, high incidence and mortality figures are still seen in Asia and Eastern Europe, and through immigrants from these areas, also in the Nordic countries. Studies on smoking, salt and meat consumption have demonstrated increased risks of developing stomach cancer among H. pylori-infected individuals, while studies relating the intake of fruits, vegetables and vitamins have demonstrated opposite developments [Citation25]. Small indications of a decrease in the risk of stomach cancer due to changes in living standards and lifestyle in the new host country were also seen in this study (see ).
Many studies have shown that cervical cancer risk among non-Western immigrants is lower than in their countries of origin [Citation22]. This may reflect a lower prevalence of human papillomavirus in the new host country as well as changes in lifestyle, including sexual behaviour [Citation1,Citation26]. An efficient cervical cancer screening program may also play a role since people immigrating at an older age have consistently had a higher risk, and the risk has decreased after living in the new country for several years. Our overall results support all of these. The initial results from Finland showed both a higher incidence and a higher mortality rate of cervical cancer in non-Western immigrants compared to the non-immigrant population. The sensitivity analyses demonstrated, however, that the low national incidence and mortality figures in Finland mainly induced this outcome. The larger share of immigrants from Russia or Eastern Europe, 56% in Finland compared to 34% in Denmark and 38% in Norway (data not shown), could also play a role since the incidence and mortality of cervical cancer were high in women immigrating from these areas (see Appendix B).
We analysed the impact of educational level on the incidence and mortality of cervical, liver and stomach cancers among migrants. Education was chosen to represent socioeconomic position, since it was comparable and available in all four countries. Our results revealed that high education decreased both the incidence and mortality of all studied cancers. This is in line with previous Nordic studies among non-immigrant and immigrant populations [Citation27–31].
Conclusion
Our collaborative study in four Nordic countries shows decreased risk of cervical cancer and increased incidence and mortality of liver and stomach cancers in the first-generation non-Western immigrant female population compared to non-immigrant women. Within the immigrant population, we show clear (cervical cancer) and directional (liver and stomach cancer) changes in cancer patterns by age at immigration and duration of residence. Additionally, we show a decrease in both cancer incidence and cancer mortality by increasing the level of education. All this reveals, that integration into society and targeted healthcare services are important in the prevention and treatment of cancers within the immigrant female population. The latter is also emphasised by studies on second-generation immigrants, which have shown the first two decades of life to be those setting the patterns for cancer development in subsequent lives [Citation32,Citation33]. Preventive measures for infection-related cancers should therefore be targeted especially at women who have spent their youth in high-risk areas.
Author contributions
TS: Conceptualisation, Funding acquisition, Supervision, Validation, Writing-Original draft preparation, Reviewing and Editing (LEAD). ML: Conceptualisation, Data curation, Funding acquisition, Project administration, Visualisation, Writing-Original draft preparation, Reviewing and Editing. MN: Validation, Writing-Reviewing and Editing. SN: Validation, Writing- Reviewing and Editing. AV: Writing- Reviewing and Editing. AL: Conceptualisation, Data curation, Formal analysis, Methodology, Visualisation, Writing- Reviewing and Editing. EH: Data curation. ST: Formal analysis, Methodology, Writing- Reviewing and Editing. SC: Data curation, Formal analysis, Writing-Reviewing and Editing. HS: Data curation, Formal analysis, Validation, Writing-Reviewing and Editing. GU: Supervision, Writing-Reviewing and Editing. SH: Conceptualisation, Methodology, Supervision, Validation, Writing-Reviewing and Editing.
Ethics approval
All phases of this study were conducted in accordance with relevant guidelines and regulations in each country. All administrative permissions to access the raw data used in the study were applied and granted in each country according to the European Union’s General Data Protection Regulation (article 30). In Denmark, the project was listed at the record of processing activities for research projects in the Central Denmark Region (J. No: 1-16-02-210-20). In Finland, the data were used in accordance with the Act on the National Institute of Health and Welfare (668/2008) and based on authorisations (VRK VRK/3059/2018–2 and THL/1081/6.02.00/2018) granted under the Act on Secondary Use of Health and Social Data (552/2019). In Iceland, the study was approved by The National Bioethics Committee of Iceland (VSN-20-204, VSN-20-204-V1, VSN-20-204-V2, VSN-20-204-V3), and in Norway, it was approved by the Regional Committee for Medical and Health Research Ethics South East A (reference code 17772). The analyses were performed using country-specific anonymised data.
Acknowledgements
We thank Bo Søborg for his work in data formulation for Denmark.
Disclosure statement
No potential conflict of interest was reported by the authors.
Availability of data and materials
Country-specific summary data are available from the corresponding author upon reasonable request. Due to data protection regulations, the register data are not openly shared.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660.
- Omran AR. The epidemiologic transition. A theory of the epidemiology of population change. Milbank Mem Fund Q. 1971;49(4):509–538.
- Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118(12):3030–3044. doi: 10.1002/ijc.21731.
- Globocan Cancer Observatory [Internet]. Cancer Today. Lyon: IARC; [cited 2023 Jan 9]. Available from: https://gco.iarc.fr/.
- Cohen PA, Jhingran A, Oaknin A, et al. Cervical cancer. Lancet. 2019;393(10167):169–182. doi: 10.1016/S0140-6736(18)32470-X.
- Lee Y-C, Chiang T-H, Chou C-K, et al. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology. 2016;150(5):1113–1124.e5. doi: 10.1053/j.gastro.2016.01.028.
- Kolonel LN, Altshuler D, Henderson BE. The multiethnic cohort study: exploring genes, lifestyle, and cancer risk. Nat Rev. 2004;4:1–9.
- Arnold M, Razum O, Coebergh J-W. Cancer risk diversity in non-Western migrants to Europe: an overview of the literature. Eur J Cancer. 2010;46(14):2647–2659. doi: 10.1016/j.ejca.2010.07.050.
- Statistics Sweden [Internet]. Population Statistics. Summary of Population Statistics 1960–2021; [cited 2023 Jan 9]. Available from: https://www.scb.se/en/finding-statistics/statistics-by-subject-area/population/population-composition/population-statistics/pong/tables-and-graphs/population-statistics–-summary/summary-of-population-statistics/.
- Hemminki K, Li X, Czene K. Cancer risk in first-generation immigrants to Sweden. Int J Cancer. 2002;99(2):218–228. doi: 10.1002/ijc.10322.
- Beiki O, Allebeck P, Nordqvist T, et al. Cervical, endometrial and ovarian cancers among immigrants in Sweden: importance of age at migration and duration of residence. Eur J Cancer. 2009;45(1):107–118. doi: 10.1016/j.ejca.2008.08.017.
- Hemminki K, Mousavi SM, Brandt A, et al. Liver and gallbladder cancer in immigrants to Sweden. Eur J Cancer. 2010;46(5):926–931. doi: 10.1016/j.ejca.2009.12.031.
- Cancer Registry of Norway [Internet]. Cancer in Norway 2018. [cited 2023 Aug 15]. Available from: https://www.kreftregisteret.no/globalassets/cancer-in-norway/2018/cin2018_and_special_issue.pdf.
- Brockwell SE, Gordon IR. A comparison of statistical methods for meta-analysis. Stat Med. 2001;20(6):825–840. doi: 10.1002/sim.650.
- von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies.J Clin Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008.
- Andersson TM, Myklebust TÅ, Rutherford MJ, et al. Five ways to improve international comparisons of cancer survival: lessons learned from ICBP SURVMARK-2. Br J Cancer. 2022;126(8):1224–1228. doi: 10.1038/s41416-022-01701-0.
- Visser O, van Leeuwen FE. Cancer risk in first generation migrants in Noth-Holland/Flevoland, The Netherlands, 1995-2004. Eur J Cancer. 2007;43(5):901–908. doi: 10.1016/j.ejca.2006.12.010.
- Stirbu I, Kunst AE, Vlems FA, et al. Cancer mortality rates among first and second generation migrants in The Netherlands: convergence towards the rates of the native Dutch population. Int J Cancer. 2006;119(11):2665–2672. doi: 10.1002/ijc.22200.
- Hjerkind KV, Qureshi SA, Moller B, et al. Ethnic differences in the incidence of cancer in Norway. Int J Cancer. 2017;140(8):1770–1780. doi: 10.1002/ijc.30598.
- Hjerkind K, Larsen IK, Aaserud S, et al. Cancer incidence in non-immigrants and immigrants in Norway. Acta Oncol. 2020;59(11):1275–1283. doi: 10.1080/0284186X.2020.1817549.
- Azerkan F, Zendehdel K, Tillgren P, et al. Risk of cervical cancer among immigrants by age at immigration and follow-up time in Sweden, from 1968 to 2004. Int J Cancer. 2008;123(11):2664–2670. doi: 10.1002/ijc.23843.
- Mousavi SM, Sundquist K, Hemminki K. Morbidity and mortality in gynecological cancers among first- and second-generation immigrants in Sweden. Int J Cancer. 2012;131(2):497–504. doi: 10.1002/ijc.26395.
- World Health Organization (WHO) [Internet]. Global hepatitis report 2017. Geneva: WHO. [cited 2023 Aug 15]. Available from: https://www.who.int/publications/i/item/9789241565455.
- Nordcan database 2.0 [Internet]. Cancer statistics for the Nordic countries. [cited 2023 Aug 15]. Available from: https://nordcan.iarc.fr/en/dataviz/tables.
- Venneman K, Huybrechts I, Gunter MJ, et al. The epidemiology of Helicobacter pylori infection in Europe and the impact of lifestyle on its natural evolution toward stomach cancer after infection: a systematic review. Helicobacter. 2018;23(3):e12483. doi: 10.1111/hel.12483.
- Crosbie EJ, Einstein MH, Franceschi S, et al. Human papillomavirus and cervical cancer. Lancet. 2013;382(9895):889–899. doi: 10.1016/S0140-6736(13)60022-7.
- Jensen KE, Gerd Hannibal C, Nielsen A, et al. Social inequality and incidence and survival from cancer of the female genital organs in a population-based study in Denmark, 1994-2003. Eur J Cancer. 2008;44(14):2003–2017. doi: 10.1016/j.ejca.2008.06.014.
- Baastrup R, Sorensen M, Hansen J, et al. Social inequality and incidence and survival from cancers of the oesophagus, stomach, and pancreas in a population-based study in Denmark, 1994-2003. Eur J Cancer. 2008;44(14):1962–1977. doi: 10.1016/j.ejca.2008.06.013.
- Dalton SO, Schüz J, Engholm G, et al. Social inequality in incidence and survival from cancer in a population-based study in Denmark, 1994-2003: summary of findings. Eur J Cancer. 2008;44(14):2074–2085. doi: 10.1016/j.ejca.2008.06.018.
- Lamminmäki M, Leivonen A, Sarkeala T, et al. Health inequalities among Russian-born immigrant women in Finland: longitudinal analysis on cervical cancer incidence and participation in screening. J Migr Health. 2022;6:100117. doi: 10.1016/j.jmh.2022.100117.
- Sarkar S, Dauer MJ, In H. Socioeconomic disparities in gastric cancer and identification of a single SES variable for predicting the risk. J Gastrointest Cancer. 2022;53(1):170–178. doi: 10.1007/s12029-020-00564-z.
- Hemminki K, Li X. Cancer risk in second generation immigrants to Sweden. Int J Cancer. 2002;99(2):229–237. doi: 10.1002/ijc.10323.
- Hemminki K, Försti A, Khyatti M, et al. Cancer in immigrants as a pointer to the causes of cancer. Eur J Public Health. 2014;24(suppl 1):64–71. doi: 10.1093/eurpub/cku102.
Appendix A
(Material and Methods)
Data details by country
Denmark 1986–2019
Individual level data on all female residents
All women born 1933–1996
Finland 1973–2017
Individual level data on female immigrants and aggregate data on general female population
Iceland 1986–2020
Individual level data on all female residents
Norway 1990–2015
Individual level data on all female residents
Regions of birth (non-Western immigrants) and country groupings
Central and South Asia
Afghanistan, Armenia, Azerbaijan, Bangladesh, Bhutan, Ceylon, Georgia, India, Kazakhstan, Kyrgyz Republic, Maldives, Nepal, Pakistan, Sri Lanka, Tajikistan, Turkmenistan, Uzbekistan
East Asia and Pacific
American Samoa, Brunei, Burma, Cambodia, China, Fiji, Hong Kong, Indonesia, Japan, Korea Dem. People’s Rep., Lao PDR, Macao, Malaysia, Mongolia, Myanmar, Papua New Guinea, Philippines Republic of Korea, Samoa, Singapore, Solomon Islands, South Vietnam, Taiwan, Thailand, Vietnam
Latin America and Caribbean
Antigua and Barbuda, Antilles, Argentina, Aruba, Bahamas, Barbados, Belize, Bolivia, Brazil, Chile, Colombia, Costa Rica, Cuba, Curacao, Dominican Republic, Ecuador, El Salvador, Grenada, Guatemala, Guyana, Haiti, Honduras, Jamaica, Mexico, Nicaragua, Panama, Paraguay, Peru, Puerto Rico, St. Kitts and Nevis, St. Lucia, St. Martin, Suriname, Trinidad and Tobago, Turks and Caicos Islands, Uruguay, Venezuela, Middle East and North Africa
Middle East and North Africa
Algeria, Bahrain, Egypt, Iran, Iraq, Israel, Jordan, Kuwait, Lebanon, Libya, Morocco, Oman, Palestine, Qatar, Saudi Arabia, South Yemen, Syria, Tunisia, Turkey, United Arab Emirates, Yemen
Russia and Eastern Europe
Albania, Belarus, Bosnia and Herzegovina, Bulgaria, Croatia, Czech Republic, Czechoslovakia, Estonia, Hungary, Kosovo, Latvia, Lithuania, Moldova, Montenegro, North Macedonia, Poland, Romania, Russia, Serbia, Slovak Republic, Slovenia, Soviet Union, Ukraine, Yugoslavia
Sub-Saharan Africa
Angola, Benin, Botswana, Burkina Faso, Burundi, Cabo Verde, Cameroon, Central African Republic Chad , Comoros, Congo rep., Cote d‘Ivoire, Djibouti, Equatorial Guinea, Eritrea, Eswatini , Ethiopia, Gabon, Gambia, Ghana, Guinea, Guinea-Bissau, Kenya, Liberia, Madagascar, Malawi, Mali, Mauritania, Mauritius, Mozambique, Namibia, Niger, Nigeria, Rhodesia, Rwanda, Senegal, Seychelles, Sierra Leone, Somalia, South Africa, South Sudan, South West Africa, Sudan, Tanzania, Togo, Uganda, Zaire, Zambia, Zimbabwe
Appendix B
Adjusted cancer incidence and mortality rate ratios (RR) among non-Western immigrant women compared to native women by region of birth.
Figure B.1. Adjusted cervical cancer incidence and mortality rate ratios (RR) among non-Western immigrant women compared to native women. (Adjusted by attained age and calendar year.)