Abstract
Background
We explored the relationship between benefit finding (BF)/posttraumatic growth (PTG) at baseline and health-related quality of life (HRQOL) at baseline and follow-up in long-term cancer survivors (LTCS; ≥5-year post-diagnosis).
Materials and methods
HRQOL was assessed in LTCS in 2009–2011 (5- to 16-year post-diagnosis, baseline) and re-assessed in 2018/2019 (14- to 24-year post-diagnosis, follow-up). BF and PTG were measured at baseline; mean scores were dichotomized into ‘none-to-low’ (<3) and ‘moderate-to-high’ (> =3). Linear regression models and linear mixed regression models were employed to assess the association of BF/PTG with HRQOL.
Results
Of the 6057 baseline participants, 4373 were alive in 2019, of whom 2704 completed the follow-up questionnaire. Cross-sectionally, LTCS with none-to-low BF reported better HRQOL at baseline and at follow-up than LTCS with higher BF. Longitudinally, no difference was found between none-to-low and moderate-to-high BF on the HRQOL change from baseline to follow-up. HRQOL differences between the PTG groups were not statistically significant cross-sectionally and longitudinally, except those participants with moderate-to-high PTG reported higher role functioning and global health status/QOL.
Conclusions
Cross-sectionally, BF was significantly negatively related to subscales of HRQOL, while PTG was positively correlated to role functioning and global health status/QOL. The results add further evidence that BF and PTG are two different positive psychological concepts.
Background
Longer survival with high quality of life is one of the aims of oncology. Survival rates for many types of cancer have increased [Citation1] due to improvements in early detection and treatment advances. Despite these advances, cancer and its treatments can still negatively affect health-related quality of life (HRQOL) and these detriments can persist indeterminately [Citation2]. Likewise, survivors can also attribute positive growth to their cancer experiences [Citation3]. The positive growth which cancer survivors experience during their trajectory is an important aspect of mental well-being for their life after cancer. According to stress, coping, and adaptation theories, positive growth is thought to be positively associated with HRQOL [Citation4,Citation5]. However, more empirical research on the relationship between positive growth and HRQOL in cancer survivors is needed, especially in long-term cancer survivors (LTCS; 5 or more years post-diagnosis).
Theoretical and empirical studies label positive growth from cancer as benefit finding (BF) and posttraumatic growth (PTG) [Citation6,Citation7]. Although the two concepts are related and often used synonymously in publications [Citation8], slight differences exist [Citation9]. BF is a form of cognitive adaptation to adversity via positive evaluation of the circumstances survivors encountered [Citation10], whereas PTG refers to the positive psychological changes experienced after struggling with a traumatic event [Citation11]. BF encompasses broader and less specific aspects and is not necessarily triggered by a traumatic event, in contrast to PTG [Citation12]. Another difference between BF and PTG is the initiation process. BF can be experienced immediately after cancer diagnosis, while the traumatic event of cancer needs to be processed before PTG is supposed to be initiated [Citation13].
Research into the association of BF with HRQOL in cancer survivors is limited. Studies used heterogeneous samples (e.g., age groups, cancer types) and instruments, and their results are inconsistent [Citation14]. Even less research has been done on BF and HRQOL in LTCS. In contrast, there has been considerable research addressing the relationship between PTG and HRQOL in cancer survivors. A recent systematic review of the relationship between PTG and overall HRQOL in cancer survivors concluded that there is a statistically significant positive relationship [Citation8]. However, this relationship is small and no inferences could be made to the direction of discovered associations because the included studies (a) had small sample sizes and (b) were largely of cross-sectional design [Citation8]. Evidence from prospective studies of good quality on PTG and HRQOL that specifically include LTCS is lacking.
It is important to study the relationship between positive growth and HRQOL in LTCS in order to promote better HRQOL in cancer survivors in the long run. This population-based, prospective cohort study that consisted of LTCS of breast, colorectal, and prostate cancer was conducted to address the following aims: (1) investigate the cross-sectional association between baseline BF/PTG with baseline HRQOL, and the association between baseline BF/PTG and HRQOL at follow-up, and (2) explore whether baseline BF/PTG was associated with differences in HRQOL between baseline and follow-up. As we previously found that the age-adjusted prevalence of BF and PTG differed according to cancer type (breast > colorectal > prostate) and sex (female > male) [Citation15], we also posed the third aim: (3) examine whether the relationship between BF/PTG and HRQOL differ by cancer type and by sex.
Material and methods
Study participants
The study population came from the CAESAR study (CAncEr Survivorship – A multi-Regional population-based study – an observational cohort study) [Citation16] conducted by the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) in close collaboration with six population-based cancer registries in Germany (including the federal states of Bremen, Hamburg, Münster/North Rhine-Westphalia, Rhineland-Palatinate, Saarland, and Schleswig-Holstein). In brief, the study included breast, prostate, and colorectal cancer survivors diagnosed during 1994–2004 as registered in one of the six participating cancer registries and with an age at diagnosis of 20–75 years. Eligible participants were contacted in 2008–2011 (baseline) and recontacted in 2018–2019 (follow-up) [Citation17]. The ethics committee of the University of Heidelberg and the ethics committees responsible for the involved cancer registries approved both the baseline and the follow-up study protocol. All participants gave written informed consent for each assessment. As illustrated in the study flowchart (Supplementary Figure 1), of the 14,526 cancer survivors eligible for the CAESAR core study, 6057 completed the long questionnaire in the baseline study. According to records in the cancer registries, 4373 were still alive in 2019, of whom 2704 completed the follow-up questionnaire. Differences between participants who dropped out and those with follow-up data had been assessed previously [Citation17]: those who participated in the follow-up study were younger at cancer diagnosis, less frequently diagnosed with stage IV cancer, had shorter time from diagnosis, were more frequently to be breast cancer survivors, and less frequently to be colorectal cancer survivors.
Outcomes and measurements
Sociodemographic and clinical data
Cancer registry data at recruitment included sex, year of birth, year of diagnosis, cancer diagnosis, and stage. Education was collected at baseline by survivors’ self-report. Number of comorbidities, recurrence, metastasis, and new cancer since index diagnosis were collected both in the baseline and follow-up questionnaires by self-report. Age at survey for both the baseline and the follow-up study was calculated as the year of survey completion minus the year of birth. Time since diagnosis at baseline and follow-up survey was defined as the year of survey minus the year of diagnosis.
Benefit finding
BF was evaluated by the German short form of the Benefit Finding Scale (BFS) with 10 items [Citation18]. For each item, agreement to a pre-defined statement was rated on 5-point Likert scale ranging from 1 (not at all) to 5 (extremely). BF was measured at baseline.
Posttraumatic growth
PTG was measured by three subscales (appreciation of life/spiritual change/new possibilities) of the German Posttraumatic Growth Inventory (PTGI). The other two scales of the original questionnaire (personal strengths/relationship to others) were not included to reduce overall questionnaire length [Citation13]. The 10 items were measured on 6-point Likert scale (0 = I did not experience this change as a result of my cancer, 5 = I experienced this change to a very great degree as a result of my cancer). PTG was measured at baseline.
Intensity of BF and PTG
According to previously reported prevalence rates [Citation15], the intensity levels of BF and PTG were each determined using a cutoff of 3 (indicated as ‘moderate’ according to a previous report [Citation19]). As such, the mean scores for the overall scales were dichotomized into ‘none-to-low’ (<3) and ‘moderate-to-high’ (> = 3).
Health-related quality of life
HRQOL was measured by the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–Core 30 (EORTC QLQ-C30) Version 3.0. This internationally validated questionnaire with 30 items includes a global health status/quality of life (QOL) and five functioning scales, and nine aspects on symptoms and financial difficulties. Answers range from 1 (not at all) to 4 (very much), except for the global health status/QOL scale which ranges from 1 (very poor) to 7 (excellent). HRQOL was measured at baseline and follow-up. We performed linear transformations of the raw sum scores to a scale of 0–100 according to the EORTC scoring manual [Citation20]. Higher scores on the functioning and global health status/QOL scales imply better functioning and global health status/QOL, whereas higher scores on the symptom and financial difficulties scales imply greater symptom severity and financial problems.
Statistical analysis
Descriptive statistics were conducted on sociodemographic and clinical variables in the baseline and follow-up sample ().
Table 1. Baseline characteristics of samples at baseline study and follow-up study.
Linear regression models were used to assess the relationship of BF/PTG with HRQOL at baseline and HRQOL at follow-up. The covariate ‘active cancer disease’ included stage IV at diagnosis or self-report of subsequent recurrence/metastasis/new cancer after initial diagnosis. To achieve aim 1, least-square means of HRQOL scores at baseline and at follow-up were computed and compared between the none-to-low and moderate-to-high BF/PTG groups adjusted for age at survey, time since diagnosis, comorbidity, active cancer disease, sex, cancer type, UICC stage at diagnosis, and education. To achieve aim 2, linear mixed regression models (LMM) were employed to compare the baseline and follow-up HRQOL scores of LTCS with moderate-to-high versus none-to-low BF or PTG. An unstructured covariance matrix structure and maximum likelihood estimation were used for LMM. The study round was defined as baseline or follow-up. The study time interval was defined as the number of years between baseline study and follow-up study, and it was included in the models as a continuous predictor. The models were adjusted for time-variant covariates (age at diagnosis, time since diagnosis, comorbidity, and active cancer disease both at baseline and follow-up), and time-invariant baseline factors (sex, cancer type, UICC stage at diagnosis, education at baseline). The collinearity among the variables in the regression models was assessed by the variance inflation factor (VIF), with a VIF value ≥5 indicating the presence of collinearity [Citation21]. The model effect ‘BF/PTG groups’ described the HRQOL difference at baseline between the none-to-low BF/PTG group and the moderate-to-high BF/PTG group (control group). The model effect ‘study time interval’ revealed the HRQOL change in the years between baseline study and follow-up study. The interaction effect of ‘BF/PTG group’ and ‘study time interval’ compared the difference between the relationship of BF/PTG and HRQOL at baseline versus the relationship of BF/PTG at baseline and HRQOL at follow-up. The significance testing of the results is based on F tests ( and ). To achieve aim 3, the linear regression models as described before were additionally tested in four subgroups of LTCS, namely breast cancer, colorectal cancer – females, colorectal cancer – males, and prostate cancer (Supplementary Figure 2 for BF, Supplementary Figure 3 for PTG).
Table 2. Tests of effects on longitudinal relationship between benefit finding and health-related quality of life (functional scales and global health status/QOL of EORTC QLQ-C30).
Table 3. Tests of effects on longitudinal relationship between posttraumatic growth and health-related quality of life (functional scales and global health status/QOL of EORTC QLQ-C30).
As there were nearly 20% missings in the variable UICC stage at diagnosis, a separate category was created for these cases to reduce possible bias due to an exclusion of these participants in analyses that require complete cases. Sensitivity analysis was carried out for the BF prevalence and PTG prevalence by excluding participants who had been lost to follow-up. The HRQOL differences between the none-to-low and moderate-to-high BF/PTG groups at baseline were also compared in three subsamples: the sample who participated in both rounds (n = 2704), the sample who only participated in the baseline study (n = 3353), and the whole sample (n = 6057) to detect potential survival bias.
SAS statistical software (Version 9.4) was used to perform all analyses. For all analyses, a p value <0.05 (two-sided) was considered statistically significant. The p values refer to individual tests rather than a global test for differences and were not adjusted for multiple testing.
Results
Descriptive statistics
Of the baseline sample (N = 6057), 52.1% were female, 55.2% were over 69 years old at survey, 52.2% had less than 10 years of education, 43.8% were breast LTCS, 36.1% were prostate LTCS, and 20.1% were colorectal LTCS. Among the baseline sample, 61.3% had initially early-stage cancer diagnosis, 48.5% had more than one comorbidity, and 83.1% had no active cancer disease at the survey (). In the follow-up sample (N = 2704, 62.6% of the 4321 survivors who had survived and were eligible to participate in the follow-up), 53.2% were female, 43.9% were over 69 years old at baseline survey, 46.6% had less than 10 years of education, 65.0% had early-stage disease, 44.4% had more than 1 comorbidity, and 86.4% had no active cancer disease at survey. The prevalence of moderate-to-high BF was 63% and the prevalence of moderate-to-high PTG was 19%, both for the entire baseline sample (n = 6057) and the baseline subsample who completed the follow-up survey (n = 2704). Sensitivity analysis revealed that the prevalence for BF/PTG at baseline was comparable between the subsamples according to participation at follow-up. BFS and PTGI were highly correlated (odds ratio = 24.6, co-prevalence of BF and PTG are presented in Supplementary Table 1).
Relationships between BF and HRQOL
HRQOL differences between BF groups at baseline and at follow-up
LTCS with none-to-low BF reported higher scores in the functioning scales at baseline (). Statistically significant differences between none-to-low and moderate-to-high BF were noted for physical functioning, role functioning, emotional functioning, and social functioning at baseline. The differences in follow-up HRQOL scores showed a similar pattern but were not statistically significant. In both BF groups, there was a decrease in HRQOL scores from baseline to follow-up.
Figure 1. Distribution of HRQOL scores in none-to-low and moderate-to-high BF group (upper line) and the differences of HRQOL between none-to-low and moderate-to-high BF (lower line). Note. HRQOL: health-related quality of life; BF: benefit finding. PF: physical functioning; EF: emotional functioning; CF: cognitive functioning; RF: role functioning; SF: social functioning; QL: the global health status/quality of life. LowerCL/upperCL: lower level/upper level of 95% confidence interval on the HRQOL difference between none-to-low group and moderate-to-high BF group. Baseline HRQOL was adjusted for cancer type, sex, age at survey, education, time since diagnosis, UICC stage, comorbidities, and active cancer disease. Follow-up HRQOL was adjusted for baseline HRQOL, other baseline variables (cancer type, sex, education, UICC stage), and follow-up variables (age at survey, time since diagnosis, comorbidities, and active cancer disease).
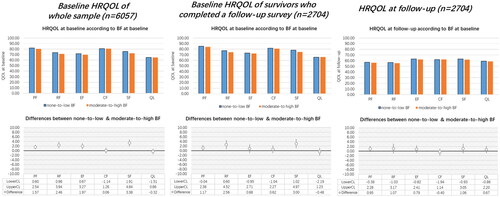
Differences in the relationship between BF and HRQOL at baseline versus follow-up
When including study time interval as an independent predictor in the LMM models, the interaction between BF groups and study time interval was not statistically significant ().
Relationships between BF and HRQOL by cancer type and sex
When stratifying the sample by cancer type and sex, the results were similar to the overall sample: There was a decrease in HRQOL scores from baseline to follow-up for both BF groups, and LTCS with none-to-low BF tended to report higher scores in functioning scales (Supplementary Figure 2).
Relationship between PTG and HRQOL
HRQOL differences between PTG groups at baseline and at follow-up
LTCS with none-to-low PTG reported lower HRQOL scores compared to those with moderate-to-high PTG, at baseline and at follow-up (). However, statistically significant differences between none-to-low and moderate-to-high PTG were only noted for role functioning and global health status/QOL at baseline. The differences in follow-up HRQOL were not statistically significant. A decrease in HRQOL scores from baseline (n = 6057) to follow-up (n = 2704) for both PTG groups was observed.
Figure 2. Distribution of HRQOL scores in none-to-low and moderate-to-high PTG group (upper line) and the differences of HRQOL between none-to-low and moderate-to-high PTG (lower line). Note. HRQOL: health-related quality of life; PTG: posttraumatic growth. PF: physical functioning; EF: emotional functioning; CF: cognitive functioning; RF: role functioning; SF: social functioning; QL: the global health status/quality of life. LowerCL/upperCL: lower level/upper level of 95% confidence interval on the HRQOL difference between none-to-low group and moderate-to-high PTG group. Baseline HRQOL was adjusted for cancer type, sex, age at survey, education, time since diagnosis, UICC stage, comorbidities, and active cancer disease. Follow-up HRQOL was adjusted for baseline HRQOL, other baseline variables (cancer type, sex, education, UICC stage), and follow-up variables (age at survey, time since diagnosis, comorbidities, and active cancer disease).
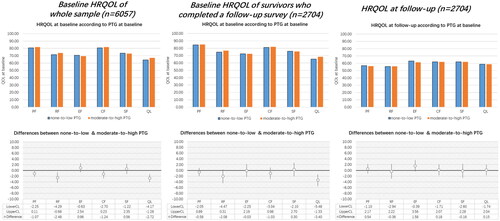
Differences in the relationships between PTG and HRQOL at baseline versus follow-up
When study time interval was included as an independent predictor, the LMM models showed no significance on the interaction between PTG groups and study time interval in any subscale of HRQOL ().
Relationships between PTG and HRQOL by cancer type and sex
The relationships between PTG and HRQOL for breast cancer survivors were the same as in the overall sample, but not for colorectal cancer survivors and prostate cancer survivors (Supplementary Figure 3).
Discussion
This study assessed the longitudinal relationship between baseline BF/PTG and changes in HRQOL from baseline to follow-up in a population-based cohort of LTCS. In most HRQOL scales, including global health status, the results revealed significant difference between none-to-low and moderate-to-high BF, in which LTCS with none-to-low BF tended to report better HRQOL. We found no statistically significant difference between the relationship of BF and HRQOL at baseline versus the relationship of BF at baseline and HRQOL at follow-up. For PTG, the pattern was contrary to that of BF, with the moderate-to-high PTG group reporting higher HRQOL than the none-to-low PTG group. However, the differences did not reach statistical significance, except for role functioning and global health status/QOL. Interactions between PTG and study time interval were not statistically significant in any subscale. Overall, the relationship between BF/PTG and HRQOL by cancer type and sex showed the same trend as for the overall group.
In this study, none of the included HRQOL scales revealed a positive relationship with BF, which is inconsistent with previous research that suggests BF was associated with specific QOL subscales, e.g., better social/family, functional, and colorectal cancer-specific well-being [Citation22], and increased physical health [Citation23]. The sample in our study was older than in other studies and living with cancer for more than 5 years. A possible explanation for the different findings could be that the relationship weakened with a longer time since diagnosis. The most stressful memories of having cancer and the consequences of cancer treatment may have declined with time. Another possible explanation could be that survivors who did not view cancer as a stressful event were less likely to experience BF. These survivors may have better health or have better adaption to cancer and therefore were more likely to report better HRQOL. The relationship between BF and HRQOL might also be mediated through factors such as social support [Citation24] or depression and anxiety [Citation25], which were not controlled for in the current study.
In concordance with a previous study [Citation13], PTG in our study had a positive relationship with global health status/QOL and no statistically significant relationship with functioning scales at baseline (5- to 16-year post-diagnosis). However, baseline PTG was not associated with HRQOL at follow-up (14- to 24-year post-diagnosis) in our study. This is inconsistent with another study involving LTCS of various cancer types that found a positive association, even 13.7 ± 6.0 years after diagnosis [Citation26]. The discrepancy in the results might be explained by the younger age of the samples in those studies and the cross-sectional assessment of the outcomes. In our study, the time interval between the measurements of PTG and HRQOL (on average 8.5 years) was much longer than in previous studies [Citation27,Citation28]. Our results add new insight into the longitudinal relationship between PTG and HRQOL in LTCS.
In our previous analysis, we observed some overlaps between BFS and PTGI [Citation15], as well as differences between BF and PTG. An explanation for this difference could be that BF contains more extensive and less specific changes than PTG [Citation15]. In the present analysis, we found that BF and PTG were associated with different aspects of HRQOL. This suggests that BF and PTG reflect heterogeneous aspects in the experience of survivors after cancer diagnosis, which is in line with another study [Citation13]. Taken together, our study supports the view that BF and PTG are two associated but slightly different concepts in the framework of positive psychology.
Strengths and limitations
The strengths of our study are the large, longitudinal cohort study of LTCS encompassing three common cancer types, and HRQOL measured with the validated EORTC QLQ-C30.
However, the following limitations should also be noted. One caveat is the possibility of healthy survivor bias [Citation17]. Another caveat is a limited generalizability with only the most common cancers for cancer type and sex-specific analyses (lung cancer survivors were not included, for which the proportion of long-term survivors is very low). Another factor to be considered when interpreting the results is that potential factors (e.g., personality, social support) that might moderate the association between BF/PTG and HRQOL were not assessed. Furthermore, there were only two assessment points and the time interval between assessments was long, which may not adequately capture the trajectory of the relationship. Future studies could include other potential moderating factors and assess the outcomes earlier after diagnosis with follow-ups at narrower intervals to analyze the trajectory of the relationship more in detail. The differences in the two conceptualizations of positive growth may be proved by more frequent measurements with narrower intervals, as the more general concept of PTG may depend on other factors than cancer and thus may not be stable enough to predict outcomes many years later. We acknowledge the differences between respondents and non-respondents to the follow-up survey. This potential participation bias may influence the reported mean HRQOL estimates, so as to the relationship with BF/PTG.
Conclusion
In this study, we found negative relationships between BF and HRQOL in general, the association between BF and HRQOL was the same in cross-sectional and prospective analyses. For PTG, there was a positive relationship between PTG and global health status/QOL, and between PTG and role functioning, but these relationships changed to non-significant over time. In general, PTG does not seem to predict later functioning or global health status/QOL. When stratified by sex and cancer type, no clear pattern was found in the longitudinal relationship between BF/PTG and HRQOL. These different HRQOL results between BF and PTG add further evidence that BF and PTG represent two different positive psychological concepts. These findings are important for future research addressing theory building, study comparison, and development of interventions for improving HRQOL of LTCS in the long run.
Ethical approval
This study was approved by the ethics committees of the University of Heidelberg and the cancer registries. All participants provided written informed consent.
Author contributions
Z.L. analyzed the data and wrote the draft. M.S.Y.T. and D.D. reviewed and edited the article. L.K.-G. was the study manager of the CAESAR study. H.Be, A.E., B.H., A.W., S.R.Z., and R.P. contributed to the recruitment of study participants and data collection. H.Br and V.A. are the principal investigators of the CAESAR study. All authors read and approved the article.
Supplemental Material
Download MS Word (56.1 KB)Supplemental Material
Download MS Word (478.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Due to the nature of the research, ethical supporting data are not available.
Additional information
Funding
References
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3.
- Harrison SE, Watson EK, Ward AM, et al. Primary health and supportive care needs of long-term cancer survivors: a questionnaire survey. J Clin Oncol. 2011;29(15):2091–2098. doi: 10.1200/JCO.2010.32.5167.
- Cordova MJ, Cunningham LL, Carlson CR, et al. Posttraumatic growth following breast cancer: a controlled comparison study. Health Psychol. 2001;20(3):176–185. doi: 10.1037/0278-6133.20.3.176.
- Folkman S, Lazarus RS. Stress, appraisal, and coping. New York (NY): Springer; 1984.
- Taylor SE, Aspinwall LG. Mediating and moderating processes in psychosocial stress: appraisal, coping, resistance, and vulnerability. In: Kaplan HB, editor. Psychosocial stress: perspectives on structure, theory, life-course, and methods. San Diego (CA): Academic Press; 1996. p. 71–110.
- Park CL. Making sense of the meaning literature: an integrative review of meaning making and its effects on adjustment to stressful life events. Psychol Bull. 2010;136(2):257–301. doi: 10.1037/a0018301.
- Park CL, Folkman S. Meaning in the context of stress and coping. Rev Gen Psychol. 1997;1(2):115–144. doi: 10.1037/1089-2680.1.2.115.
- Liu Z, Doege D, Thong M, et al. The relationship between posttraumatic growth and health-related quality of life in adult cancer survivors: a systematic review. J Affect Disord. 2020;276:159–168. doi: 10.1016/j.jad.2020.07.044.
- Sears SR, Stanton AL, Danoff-Burg S. The yellow brick road and the emerald city: benefit finding, positive reappraisal coping and posttraumatic growth in women with early-stage breast cancer. Health Psychol. 2003;22(5):487–497. doi: 10.1037/0278-6133.22.5.487.
- Tennen H, Affleck G. Benefit-finding and benefit-reminding. In: Snyder CR, Lopez SJ, editors. Handbook of positive psychology. New York (NY): Oxford University Press; 2002. p. 584–597.
- Tedeschi RG, Calhoun LG. Posttraumatic growth: conceptual foundations and empirical evidence. Psychol Inq. 2004;15(1):1–18. doi: 10.1207/s15327965pli1501_01.
- Applebaum AJ, Marziliano A, Schofield E, et al. Measuring positive psychosocial sequelae in patients with advanced cancer. Psychol Trauma. 2021;13(6):703–712. doi: 10.1037/tra0000944.
- Jansen L, Hoffmeister M, Chang-Claude J, et al. Benefit finding and post-traumatic growth in long-term colorectal cancer survivors: prevalence, determinants, and associations with quality of life. Br J Cancer. 2011;105(8):1158–1165. doi: 10.1038/bjc.2011.335.
- Pascoe L, Edvardsson D. Benefit finding in cancer: a review of influencing factors and health outcomes. Eur J Oncol Nurs. 2013;17(6):760–766. doi: 10.1016/j.ejon.2013.03.005.
- Liu Z, Thong MS, Doege D, et al. Prevalence of benefit finding and posttraumatic growth in long-term cancer survivors: results from a multi-regional population-based survey in Germany. Br J Cancer. 2021;125(6):877–883. doi: 10.1038/s41416-021-01473-z.
- Arndt V, Koch-Gallenkamp L, Jansen L, et al. Quality of life in long-term and very long-term cancer survivors versus population controls in Germany. Acta Oncol. 2017;56(2):190–197. doi: 10.1080/0284186X.2016.1266089.
- Doege D, Thong MS, Weißer L, et al. Health-related quality of life in very long-term cancer survivors 14-24 years post-diagnosis compared to population controls: a population-based study. Cancers. 2021;13(11):2754. doi: 10.3390/cancers13112754.
- Mohamed NE, Böhmer S. Die deutsche Version der Benefit Finding Skala: Ihre psychometrischen Eigenschaften bei Tumorpatienten. Z Med Psychol. 2004;13(2):85–91.
- Schroevers MJ, Teo I. The report of posttraumatic growth in Malaysian cancer patients: relationships with psychological distress and coping strategies. Psychooncology. 2008;17(12):1239–1246. doi: 10.1002/pon.1366.
- Fayers P, Aaronson N, Bjordal K, et al. The EORTC QLQ-C30 scoring manual. 3rd ed. Brussels (Belgium): European Organisation for Research and Treatment of Cancer; 2001.
- Stine RA. Graphical interpretation of variance inflation factors. Am Stat. 1995;49(1):53–56.
- Rinaldis M, Pakenham KI, Lynch BM. Relationships between quality of life and finding benefits in a diagnosis of colorectal cancer. Br J Psychol. 2010;101(Pt 2):259–275. doi: 10.1348/000712609X448676.
- Tomich PL, Helgeson VS. Five years later: a cross-sectional comparison of breast cancer survivors with healthy women. Psychooncology. 2002;11(2):154–169. doi: 10.1002/pon.570.
- Brand C, Barry L, Gallagher S. Social support mediates the association between benefit finding and quality of life in caregivers. J Health Psychol. 2016;21(6):1126–1136. doi: 10.1177/1359105314547244.
- Lin Y, Luo X, Li J, et al. The dyadic relationship of benefit finding and its impact on quality of life in colorectal cancer survivor and spousal caregiver couples. Support Care Cancer. 2021;29(3):1477–1486. doi: 10.1007/s00520-020-05602-x.
- Seitz DC, Hagmann D, Besier T, et al. Life satisfaction in adult survivors of cancer during adolescence: what contributes to the latter satisfaction with life? Qual Life Res. 2011;20(2):225–236. doi: 10.1007/s11136-010-9739-9.
- Silva SM, Crespo C, Canavarro MC. Pathways for psychological adjustment in breast cancer: a longitudinal study on coping strategies and posttraumatic growth. Psychol Health. 2012;27(11):1323–1341. doi: 10.1080/08870446.2012.676644.
- Tomich PL, Helgeson VS. Posttraumatic growth following cancer: links to quality of life. J Trauma Stress. 2012;25(5):567–573. doi: 10.1002/jts.21738.
