Abstract
Background
Secondary lymphedema is a known side effect to radiotherapy (RT), but limited information regarding prevalence and risk factors for lower limb edema (LLE) after curative radiotherapy in patients with prostate cancer (PCa) is available. This study provides a descriptive analysis of patient-reported LLE with analysis of risk factors in a cohort of patients with PCa treated with curative RT.
Material and methods
A total of 302 patients with PCa with prospective registration of patient-reported LLE (EORTC QLQ-PR25 (Question 46)) were included. Analysis of LLE was done with the calculation of prevalence rates and Kaplan-Meier statistics. Risk factors for LLE were analyzed multivariate with Cox regression analysis.
Results
At a median follow-up of 15 (3–51) months, the overall crude incidence of patients reporting ‘quite a bit’ or ‘a lot’ of LLE was 49 (16.2%) and 21 (7.0%), respectively. The baseline prevalence rate of ‘quite a bit’ and ‘a lot’ of LLE was 5.0% and 0.8%, respectively. During follow-up the prevalence rate for ‘quite a bit’ or ‘a lot’ of LLE increased significantly and remained constant from 6 months where 11.5% (±1.7%) reported ‘quite a bit’ and 2.9% (±0.5%) reported ‘very much’ LLE (p < 0.001), respectively.
Significant risk factors (p < 0.10) for LLE in univariate analysis included lymph node irradiation (HR:2.325), baseline Body Mass Index (BMI) (HR:1.100), Charlson Comorbidity Index (HR:1.227), Androgen Deprivation Therapy (HR:2,979), and Performance Status (HR:0.594). Only high BMI (HR:1.091) remained significant in multivariate analysis with a three-fold increase in LLE in patients with BMI ≥ 30 compared to normal weight patients.
Conclusion
Severe patient-reported LLE after curative RT for PCa is rare. Significantly more patients with a high BMI report ‘quite a bit’ or ‘very much’ LLE compared to patients with a normal BMI. Obese PCa patients could be offered a rehabilitation program for early detection and management of LLE.
Introduction
Secondary lymphedema (SL) is a well described complication after radiotherapy in patients undergoing treatment for breast- and gynecological cancers [Citation1–3]. This is in contrary to patients diagnosed with prostate cancer (PCa), where only limited information regarding the prevalence as well as risk factors for lower limb edema (LLE) after radiotherapy is available in the literature [Citation4].
The pathological process of SL is due to a disruption of lymphatic drainage which results in the accumulation of lymphatic fluid and plasma proteins in the interstitial tissue compartment, resulting in chronic inflammation and fibrosis. Clinically, SL is associated with a sensation of limb heaviness, abnormal tissue swelling, erythema, pain, and impaired limb function which may significantly impact quality of life [Citation5]. Early diagnosis and treatment of SL is important, because if left untreated, lymphedema may deteriorate over time and become more difficult to treat [Citation4].
An improved understanding of radiotherapy induced LLE in patients with PCa is warranted to select patients at risk for pre-treatment counseling as well as for identification of the potential need for prehabilitation or rehabilitation with post-treatment lymphedema therapies. This study provides a descriptive analysis of LLE in a cohort of patients with PCa treated with primary radiotherapy. Moreover, the impact of elective pelvic lymph node (LN) irradiation as well as other risk factors for LLE are analyzed.
Material and methods
Patients
Between October 2017 and July 2022, a total of 412 consecutive patients with PCa were included in a prospective observational study in the Department of Oncology at Vejle Hospital, Denmark. The protocol was approved by the Regional Committee on Health Research Ethics for Southern Denmark (ID: 2008-28-0035). Patients were informed orally and in writing about the study by the treating physician and invited to participate at the first consultation in the Department of Oncology. Written informed consent was provided by all patients.
The study was designed to gather prospective data on patient-reported outcomes (PRO) and physician assessed morbidity following either curative RT for T1-T3N0M0 prostate cancer, or salvage RT for biochemical recurrence after radical prostatectomy based on validated questionnaires regarding adverse events. Hence, CTCAE v4.0, the Expanded Prostate Cancer Index Composite (EPIC), EORTC QLQ-C30 and a segment of EORTC QLQ-PR25 (question 46) were set up in an electronic database. The study was observational and no power calculations were done.
In the study, patient, disease, and treatment related factors were registered in the database at time of inclusion. All staff-reported morbidity data were entered directly in the database during the consultations. PRO were collected electronically by sending a link out to the patients digital mailbox in advance of each consultation. If the patient did not report PRO electronically, it was also possible to report the data on paper immediately prior to the consultation.
Patients were followed in the study for five years with an assessment of disease status as well as physician-assessed morbidity and PRO at baseline (BL), end of treatment (EOT), and after 1, 3, 6, 9, 12, 18, 24, 30, 36, 48, and 60 months of follow-up (FU).
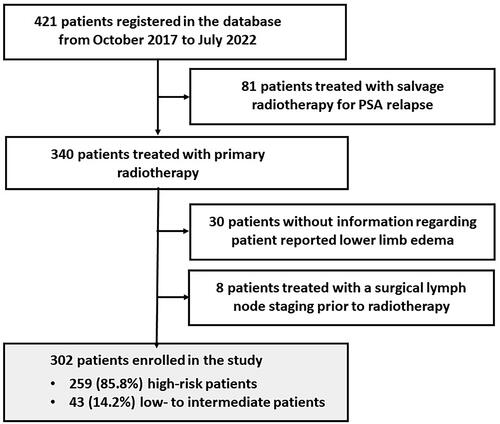
This report aims to investigate the prevalence and risk factors for patient reported LLE after primary radiotherapy for PCa. The study included all patients from the database that were treated with curative RT for T1-T3N0M0 prostate cancer and with at least one reply to the PRO question regarding LLE (N = 310) with the exception of patients (N = 8) who had a surgical staging with a pelvic lymph node dissection (PLND) prior to radiotherapy. The result was a total of 302 patients with PCa included in the present study ().
Diagnosis and treatment
Clinical workup included a clinical examination, blood tests with measurement of prostate specific antigen (PSA), transrectal ultrasound scan (TRUS) with biopsies or MRI-guided biopsies. In all patients, lymph-node staging was done by use of CT and MRI.
Treatment included external beam radiotherapy (EBRT) and 6 or 36 months of androgen deprivation therapy (ADT) in intermediate- and high-risk patients respectively. Low-risk patients were treated with EBRT alone.
EBRT was delivered as volumetric arc therapy. Radiotherapy dose prescription was done according to risk group. Patients with low- or intermediate-risk PCa were treated with 60 Gy in 20 fx or 78 Gy in 39 fx to the prostate alone. Patients with high-risk disease were treated with 56 Gy in 39 fx to the elective LN regions (external and internal ileac, obturator, and pre-sacral lymph nodes (LNs)) at the level of the first and second sacral vertebra (S1-S2)), and to the seminal vesicles plus a simultaneous integrated boost to the prostate alone or prostate and seminal vesicles to 78 Gy in 39 fractions.
Assessment of patient-reported secondary lymphedema
Patient-reported LLE was prospectively recorded at BL, EOT, and at each follow-up by the use of EORTC QLQ-PR25. The assessment of LLE was assigned by question 46 with reporting of symptoms or problems within the past week on a 4-points Likert scale. The patients were asked the question ‘Have you had swelling in your legs or ankles?’ and were given 4 possible answers; ‘not at all’, ‘a little’, ‘quite a bit’ or ‘very much’.
Statistical analysis
The cumulative crude incidence of LLE was evaluated based on the maximum grade reported by each patient during the FU period. Prevalence rates of LLE were calculated at BL, EOT and for each FU (1, 3, 6, 9, 12, 18, 24, and 30 months), as the proportion of patients reporting LLE. Prevalence rates were compared by non-parametric statistics by use of Wilcoxon’s rank sum test.
Time to event analysis for LLE was calculated from the end of treatment by means of actuarial Kaplan-Meier’s estimates. Patients were censored from analysis of morbidity at the date of the last FU or disease recurrence.
Univariate and multivariate analyses of risk factors for LLE were conducted by Cox regression analysis. In the multivariate model, all factors with p-values < 0.1 were included. In previous studies, Pelvic lymph node irradiation and baseline Body Mass Index (BMI) have been associated to LLE. These covariates were included in the analysis [Citation1–3]. Moreover, other clinically relevant risk factors in terms of age, Androgen Deprivation Therapy (ADT), Charlson Comorbidity Index (CCI), and Performance status (PS)) were included as covariates. BMI, CCI, and age were analysed as continuous variables, whereas ADT (yes/no), PS (0/1), and Pelvic lymph node Irradiation (yes/no) were analysed as categorical variables
The SPSS statistical software system v.20 (IBM SPSS Statistics for windows, Version 20.0 Armonk; NY: IBM Corp.) was used for statistical analysis.
Results
Patients, disease and treatment characteristics are shown in . Based on risk factors in terms of TNM-stage, PSA, and Gleason score, patients were divided in two risk groups. The first group included 43 (14.2%) patients with low- and intermediate-risk disease treated with radiotherapy to the prostate alone. The second risk group included 259 (85.8%) high risk patients treated with elective radiotherapy to pelvic LNs and seminal vesicles and a simultaneous integrated boost to the prostate alone in 174 (67.2%) patients, or prostate and seminal vesicles in 85 (32.8%) patients.
Table 1. Patient, disease and treatment characteristics at baseline.
A total of 274 (90.7%) patients with intermediate- or high-risk disease were treated with ADT. In 39 (14.2%) patients, ADT was prescribed for 6 months. In the remaining 235 (85.8%) patients, ADT was prescribed for 36 months ().
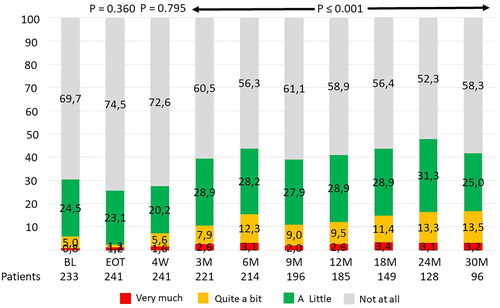
At a median FU of 15 (3–51) months, the overall crude incidence of LLE was as follows: 141 patients (46.7%) had reported ‘a little’ LLE, 49 patients (16.2%) had reported ‘quite a bit’ LLE, while 21 patients (7.0%) reported ‘very much’ LLE.
The baseline prevalence rate of ‘quite a bit’ and ‘very much’ of LLE was 5.0% and 0.8%, respectively. At EOT and 4-weeks FU, no change in LLE was found (p-values: 0.360 and 0.795 respectively). At later FU the prevalence rates for ‘quite a bit’ or ‘very much’ of LLE increased significantly and remained elevated from 6 months and onwards during the remaining FU period with about 11.5% (±1.7%) reporting ‘quite a bit’ and 2.9% (±0.5%) reporting ‘very much’ LLE (p < 0.001) ().
Figure 2. Prevalence rates of patient reported outcome in 302 prostate cancer patients after radiotherapy (EORTC QLQ-PR25) for item 46: ‘have you had swelling in your legs or ankles?’ with answer categories ‘not at all’, ‘a little’, ‘quite a bit’ or ‘very much’.
BL: baseline; EOT: end of treatment; M: months.
Risk factors for LLE were investigated in univariate analysis. All risk factors with p-values < 0.1 in the univariate analysis (ADT (p = 0.084), elective LN irradiation (p = 0.069), BMI (p < 0.001), CCI (p = 0.044), PS (p = 0.074)) were analyzed in multivariate analysis. In the multivariate analysis only BMI (p = 0.002) remained significantly associated with increased risk for LLE after RT ().
Table 2. Univariable and multivariable analysis of prognostic factors for patient reported outcome (EORTC QLQ-PR25) for item 46: ‘have you had swelling in your legs or ankles?’ in 302 prostate cancer patients reporting ‘quite a bit’ or ‘very much’ after definitive radiotherapy.
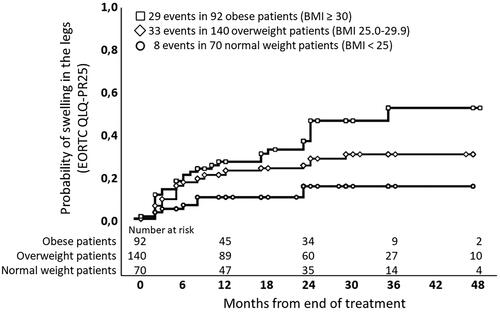
Analysis of patients according to BMI showed that 45.9% (CI: 31.8–60.0) of the obese patients (BMI ≥ 30) reported ‘quite a bit’ or ‘very much’ LLE at 36 months. For overweight (BMI 25.0–29.9) and normal weight patients (BMI < 25), the numbers were 30.2% (CI: 20.8–39.6) and 15.2% (CI: 5.8–24.6), respectively ().
Figure 3. Kaplan-Meier actuarial estimates for patient reported outcome (EORTC QLQ-PR25) for item 46: ‘Have you had swelling in your legs or ankles?’ in 302 prostate cancer patients reporting ‘quite a bit’ or ‘very much’ after definitive radiotherapy. Patients are stratified according to Body Mass Index (BMI) in normal weight patients (BMI < 25), overweight patients (BMI 25.0–29.9) and obese patients (BMI ≥ 30).
Discussion
Cancer therapy-associated SL can be of major concern and result in discomfort, functional impairment, and even cause psychosocial distress among the patients [Citation6]. The risk for LLE is increased after pelvic radiotherapy and surgical LN staging, but has been less investigated in patients with PCa treated with primary radiotherapy and concomitant ADT [Citation2,Citation4].
This study investigates patient-reported LLE in a cohort of patients with PCa. Treatment included radiotherapy in combination with ADT in intermediate- and high-risk patients, while low risk patients were treated with radiotherapy alone. No patients had a surgical LN staging.
During follow-up, the prevalence rate of LLE increased to reach a plateau 6 months after radiotherapy. However, most patients reported ‘a little’ LLE, and only a modest risk for ‘quite a bit’ or ‘very much’ LLE was found ().
Analysis of risk factors for LLE, showed that obesity was associated with a significant risk for LLE with a three-fold increase in ‘quite a bit’ or ‘very much’ LLE in patients with BMI ≥30 compared to normal weight patients ().
Other potential risk factors in terms of ADT, comorbidity, elective LN irradiation and PS did not increase the risk for LLE ().
LLE after PCa radiotherapy has previously been assessed in studies after radiotherapy alone [Citation7–10] or in studies where subgroups of patients underwent staging PLND followed by radiotherapy to the pelvic LNs in case of pathological LN involvement. The surgical procedure with PLND was associated with an increased risk for LLE of 21–29% compared to 0–3% in patients treated without a PLND [Citation11–13].
In studies that report LLE after radiotherapy alone for PCa, assessment of LLE has been done by the staff with a prevalence between 0–8% [Citation7–10]. These results are in contrast to the present study where the prevalence rates for LLE are considerably higher when all grades of LLE are addressed. Three reasons may explain this difference. First, in our study LLE was patient-reported and it is well known that PRO are higher when compared to staff-reported outcomes. This was also found in the LAPPRO trial which investigated the outcome of robot-assisted laparoscopic surgery in PCa patients. In that study, patient-reported LLE was 14% compared to staff-reported LLE of 5% [Citation14]. Second, previous investigations of LLE are mainly retrospective which gives a risk of underestimating especially milder symptoms that may not have been recorded in the medical charts. Finally, when different studies are compared, radiotherapy may vary with regard to doses, target volumes, and technique, and LLE has not been scored uniformly, nor recorded at the same time during follow-up, which makes any direct comparison between studies challenging.
A majority of all LLE events in this study were reported within the first two years after radiotherapy. This finding is in agreement with a study by Najjari et al. where physician-assessed and patient-reported LLE was reported in 1176 patients with cervical cancer after radiotherapy. They found a majority of all lymph edema events during the first 12 to 24 months indicating that LLE has an early manifestation pattern after pelvic radiotherapy [Citation2]. This finding also indicates that the present study with a relatively short FU has diagnosed a substantial proportion of the patients who will develop LLE after radiotherapy for PCa cancer.
Obesity is a known risk factors for LLE [Citation15]. This is due to impairment of the venous drainage from the lower limbs by an extended abdominal mass which results in dilatation of the veins, reduced valvular function, blood accumulation, and leaking of fluid and erythrocytes into the subcutaneous tissue [Citation16]. In previous studies in gynecological malignancies, an association between overweight and LLE after pelvic radiotherapy has been reported. In the prospective study by Najjari et al. obesity was an independent risk factor for LLE after radiotherapy for cervical cancer [Citation2]. In another study by Graf et al. time to LLE decreased as a function of higher BMI after lymphadenectomy among gynecological patients [Citation1]. These results are in agreement with the present study, where analysis of several risk factors found BMI as the only significant risk factor for LLE after radiotherapy ().
Analysis of other risk factors did not find any association between elective LN irradiation and LLE (). Previously, the value of pelvic irradiation has been investigated in patients with tumors limited to the prostate in the RTOG 77-06. In that study, pelvic LN irradiation was associated with increased risk (p-value 0.03) for LLE with 3 patients developing severe LLE out of 106 patients receiving prostate and pelvic radiotherapy compared to no patients out of 113 patients receiving radiotherapy to the prostate alone [Citation10].
Generally, it is expected that radiotherapy to the elective pelvic LNs increases the risk for LLE. This was also concluded in the study by Najjari et al. where an association between radiotherapy volumes and LLE was found in 1176 patients with cervical cancer [Citation2].
In the present study, elective LN irradiation included radiotherapy to the external- and internal iliac, obturator and the pre-sacral LNs at the level of S1-S2. The result is LN volumes that are smaller compared with cervical cancer radiotherapy, where more LN regions are included and also compared with radiotherapy to the pelvic LNs in the RTOG 77-06 using less advanced imaging and older radiotherapy technique [Citation2,Citation10]. Therefore, it could be speculated that the smaller elective LN volumes in the present study do not increase the risk for LLE to the same extent as to cervical cancer radiotherapy and with prostate cancer radiotherapy, using less advanced imaging and treatment techniques ().
In the present study, LLE was reported as prevalence rates at time of each FU () and with Kaplan-Meier estimates (). Generally, the Kaplan-Meier estimates are higher than the prevalence rates. This is expected because the Kaplan-Meier method assumes that the morbidity event is irreversible after time of diagnosis. This may not be correct in the present setting, because patient-reported LLE can change in magnitude over time and even resolve as a consequence of successful treatment. Therefore, there is a risk for an overestimation of morbidity when Kaplan-Meier estimates are calculated. This is in contrary to the calculation of prevalence rates which allow for changes in magnitude and resolvement during the FU period. Prevalence rates may thereby be a more accurate tool to evaluate the total burden of morbidity in a patient population.
Several limitations of the present study must be taken into account. First, the study is a prospective observational study without a control group not receiving radiotherapy. Therefore, the results can only be compared with previous mainly retrospective studies. Furthermore, in the present study, patient-reported LLE was assessed by the question ‘Have you had swelling in your legs or ankles?’ This question does not cover all aspects of LLE, and especially not the impact that LLE has on the general quality of life in the patients. Furthermore, swelling of the legs and ancles could also be attributed to other factors than LLE.
Apart from patient-reported symptoms, the diagnosis of LLE should also depend on a clinical examination with visual inspection, skin palpation, and measurement of volume differences between the limbs [Citation3].
In spite of these limitations, the strength of the study includes the prospective design with longitudinal assessment of patient-reported LLE in a large cohort of patients that have been treated with modern state of the art radiotherapy for PCa. Furthermore, it is a strength that the assessment of LLE was patient reported because staff-reported outcomes are associated with a risk of underreporting [Citation2,Citation14].
With regard to SL in breast cancer, there are several risk factors (axillary LN dissection, adjuvant radiotherapy, and BMI), and risk models have been developed to predict SL [Citation17,Citation18]. In prostate cancer radiotherapy, we know from previous studies that surgical PLND increases the risk for LLE [Citation11–13]. Furthermore, the present study has found BMI as a risk factor for LLE after radiotherapy for PCa (, ).
For future studies in LLE after radiotherapy for PCa, precise diagnostic criteria for LLE have to be established. Additionally, more prospective data are warranted for the analysis of other risk factors than BMI for LLE and the establishment of risk models. Such a model may serve to select patients with a high risk for LLE for counseling and relevant intervention as part of a rehabilitation program after radiotherapy.
Patients with newly diagnosed intermediate- or high-risk PCa are initially treated with 3 months ADT before the initiation of radiotherapy. Due to the timespan between the two treatment modalities, there may also be a possible role for a prehabilitation program in obese patients. This program could include physical training as a key element in order to reduce weight and thereby also the risk for LLE. However, such a program should be evaluated in a clinical study before the introduction of prehabilitation in routine practice.
Conclusion
Severe patient-reported LLE after curative RT for PCa is rare. However, significantly more patients with a high BMI report ‘quite a bit’ or ‘very much’ LLE compared to patients with a normal BMI. Based on this finding, obese PCa patients could be offered a rehabilitation program after radiotherapy to detect and manage LLE at an early stage.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirms that the data supporting the findings of this study are available within the article. Raw data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
- Graf N, Rufibach K, Schmidt AM, et al. Frequency and risk factors of lower limb lymphedema following lymphadenectomy in patients with gynecological malignancies. Eur J Gynaecol Oncol. 2013;34(1):23–27.
- Najjari Jamal D, Potter R, Haie-Meder C, et al. Physician assessed and patient reported lower limb edema after definitive radio(chemo)therapy and image-guided adaptive brachytherapy for locally advanced cervical cancer: a report from the EMBRACE study. Radiother Oncol. 2018;127(3):449–455. doi:10.1016/j.radonc.2018.03.026.
- Rockson SG. Lymphedema after breast cancer treatment. N Engl J Med. 2018;379(20):1937–1944. doi:10.1056/NEJMcp1803290.
- Clinckaert A, Callens K, Cooreman A, et al. The prevalence of lower limb and genital lymphedema after prostate cancer treatment: a systematic review. Cancers (Basel). 2022;14(22):5667. doi:10.3390/cancers14225667.
- Rasmusson E, Gunnlaugsson A, Blom R, et al. Low rate of lymphedema after extended pelvic lymphadenectomy followed by pelvic irradiation of node-positive prostate cancer. Radiat Oncol. 2013;8(1):271. doi:10.1186/1748-717X-8-271.
- Grada AA, Phillips TJ. Lymphedema: diagnostic workup and management. J Am Acad Dermatol. 2017;77(6):995–1006. doi:10.1016/j.jaad.2017.03.021.
- Amdur RJ, Parsons JT, Fitzgerald LT, et al. Adenocarcinoma of the prostate treated with external-beam radiation therapy: 5-year minimum follow-up. Radiother Oncol. 1990;18(3):235–246. doi:10.1016/0167-8140(90)90059-6.
- Aristizabal SA, Steinbronn D, Heusinkveld RS. External beam radiotherapy in cancer of the prostate. The university of Arizona experience. Radiother Oncol. 1984;1(4):309–315. doi:10.1016/s0167-8140(84)80018-3.
- Borghede G, Hedelin H. Radiotherapy of localised prostate cancer. Analysis of late treatment complications. A prospective study. Radiother Oncol. 1997;43(2):139–146. doi:10.1016/s0167-8140(96)01871-3.
- Pilepich MV, Pajak T, George FW, et al. Preliminary report on phase III RTOG studies of extended-field irradiation in carcinoma of the prostate. Am J Clin Oncol. 1983;6(4):485–491. doi:10.1097/00000421-198308000-00017.
- Forman JD, Zinreich E, Lee DJ, et al. Improving the therapeutic ratio of external beam irradiation for carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 1985;11(12):2073–2080. doi:10.1016/0360-3016(85)90086-0.
- Perez CA, Walz BJ, Zivnuska FR, et al. Irradiation of carcinoma of the prostate localized to the pelvis: analysis of tumor response and prognosis. Int J Radiat Oncol Biol Phys. 1980;6(5):555–563. doi:10.1016/0360-3016(80)90382-x.
- Pilepich MV, Perez CA, Walz BJ, et al. Complications of definitive radiotherapy for carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 1981;7(10):1341–1348. doi:10.1016/0360-3016(81)90029-8.
- Thorsteinsdottir T, Stranne J, Carlsson S, et al. LAPPRO: a prospective multicentre comparative study of robot-assisted laparoscopic and retropubic radical prostatectomy for prostate cancer. Scand J Urol Nephrol. 2011;45(2):102–112. doi:10.3109/00365599.2010.532506.
- Gasparis AP, Kim PS, Dean SM, et al. Diagnostic approach to lower limb edema. Phlebology. 2020;35(9):650–655. doi:10.1177/0268355520938283.
- Tobin AM, Ahern T, Rogers S, et al. The dermatological consequences of obesity. Int J Dermatol. 2013;52(8):927–932. doi:10.1111/j.1365-4632.2012.05624.x.
- Kim M, Kim SW, Lee SU, et al. A model to estimate the risk of breast cancer-related lymphedema: combinations of treatment-related factors of the number of dissected axillary nodes, adjuvant chemotherapy, and radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86(3):498–503. doi:10.1016/j.ijrobp.2013.02.018.
- Soran A, Menekse E, Girgis M, et al. Breast cancer-related lymphedema after axillary lymph node dissection: does early postoperative prediction model work? Support Care Cancer. 2016;24(3):1413–1419. doi:10.1007/s00520-015-2933-0.