Abstract
Background
Fatigue is a distressing and prevalent long-term sequela of treatment for childhood cancer, and there is a need for longitudinal studies to investigate the development of fatigue over time. The objective of this study was to calculate growth-curves for the longitudinal development of fatigue after treatment for childhood cancer, and to investigate the effects of biopsychosocial predictors.
Materials and methods
Participants were recruited from a patient monitoring program and data extracted from medical records. Parent-proxy and self-report versions of PedsQLTM Multidimensional Fatigue Scale were used to repeatedly assess fatigue up to 5 years after the end of treatment for childhood cancer. Fatigue was assessed 2440 times for 761 participants (median:3) with proxy-reports (age 2–8 years) and 2657 times for 990 participants with self-reports (above 8 years) (median:2). Mixed models were used to establish growth-curves and to analyze the effect of predictors separately for participants with solid tumors (ST), hemato-oncological malignancies and central nervous system-tumors (CNS).
Results
CNS-tumors were associated with more cognitive fatigue than ST at the end of treatment, for both proxy-reports (−11.30, p<.001) and self-reports (−6.78, p=.002), and for proxy-reports of general fatigue (−6.78, p=.002). The only significant difference in change over time was for self-reports of sleep-rest fatigue. The raw scores for the CNS-group decreased with −0.87 per year (95% CI −1.64; −0.81, p=.031) compared to the ST-group. Parental distress was overall the variable most associated with increased fatigue, while immunotherapy was the most frequent medical predictor. National centralization of childhood cancer care decreased fatigue for the CNS-group, but not for other diagnoses.
Discussion
Children and adolescents treated for CNS-tumors reported more fatigue than other participants after the end of treatment, and this difference remained over time. Results from this study may help to facilitate the early recognition of children with insufficient recovery of fatigue symptoms.
Background
Cancer-related fatigue has repeatedly and consistently been found to be one of the most prevalent and distressing symptoms in survivors of childhood cancer [Citation1]. Despite this, fatigue is frequently overlooked as a long-term sequela of pediatric cancer disease and treatment [Citation1,Citation2]. While most follow-up protocols after cancer treatment do not yet include measurements of fatigue, there is an increased demand for including it in follow-up protocols after both pediatric and adult cancer [Citation3,Citation4]. The National Cancer Institute has published recommendations for high-priority research on cancer-related fatigue in children and adults, and these identify a need for longitudinal studies to uncover the biopsychosocial mechanisms of cancer-related fatigue [Citation5]. The International Late Effects of Childhood Cancer Guidelines Harmonization Group recently published recommendations regarding the surveillance of fatigue after treatment for childhood cancer [Citation6]. These recommendations include regular screenings of fatigue, and also identify a need for longitudinal studies to investigate the change of fatigue patterns over time. Research in adults has demonstrated that fatigue status can change between two assessments [Citation7,Citation8], which further demonstrates the importance of repeated measures of fatigue.
Evidence on risk factors for fatigue after treatment for childhood cancer is limited and contradictory. While previous research has shown that children and adolescents experience more fatigue after pediatric brain tumors than after acute lymphoblastic leukemia (ALL) [Citation4,Citation9,Citation10], the effect of different treatments on fatigue after childhood cancer remains to be elucidated. A study on chronic fatigue in adult survivors of childhood leukemia and lymphomas reported that neither radiotherapy nor chemotherapy predicted fatigue scores [Citation7]. One study showed that surgery in combination with both chemotherapy and radiotherapy increased the risk for severe fatigue in adult survivors of pediatric brain tumors [Citation11], while another study on fatigue in a heterogeneous sample of childhood cancer survivors reported that radiotherapy – but not chemotherapy – increased the risk for fatigue [Citation10]. In adult long-term survivors, the presence of a co-existing health condition requiring medication is associated with more fatigue [Citation12]. In children with chronic diseases, almost three quarters of the variance in fatigue scores has been explained by a biopsychosocial model, and it is recommended to take biological, psychological and social factors into account [Citation13]. Family functioning is also important in childhood cancer, as parents experiencing more psychosocial difficulties have children with lower quality of life (QoL) [Citation14]. It is evident that a diagnosis of childhood cancer is associated with a risk for experiencing fatigue and that patients therefore should be carefully monitored. However, as there is a lack of longitudinal research regarding the development of fatigue over time, it is difficult to predict the course of fatigue after end of treatment. Knowing this course may help early recognition of patients at risk. The aim of this study is therefore: 1) to determine the course in fatigue over time after treatment for childhood cancer, and 2) investigate the effects of biopsychosocial predictors for the course of fatigue.
Material and methods
Participants and materials
In 2018, all care for children with cancer in the Netherlands was centralized at the Princess Máxima Center for pediatric oncology in Utrecht. The participants in the study were treated either before or after centralization of care, but all received followed-up care at our center. National centralization of childhood cancer care has led to a more specialized medical team and the opportunity to provide more systematic psychosocial support. Apart from the large psychosocial team, an additional team for supportive care and exercise with a focus on developmentally oriented care is also available. Furthermore, there is a multidisciplinary clinical team in both direct care and at the follow-up clinic, with a broad range of specialists. Improving QoL is part of the core mission, and psychosocial screenings and monitoring of QoL is implemented as a standard of care. Dutch-speaking families are offered to participate in a patient-reported outcome monitoring program called the KLIK program, which includes repeated assessments of patients’ QoL and fatigue [Citation15]. Out of all patients diagnosed at the hospital, approximately 70% of the regularly scheduled psychosocial monitoring and screening questionnaires in clinical care are completed [Citation16].
The national version of the PedsQLTM Multidimensional Fatigue Scale (MFS) was used to assess fatigue in all patients aged 2 years and above, and the questionnaire was filled out through a secured online portal after the end of treatment and then offered in combination with outpatient visits (with a minimum of 3 months between every assessment). The MFS is validated, demonstrates good psychometric properties, and national references are available for healthy populations divided by age and sex [Citation17,Citation18]. Furthermore, the instrument is recommended for the assessment of fatigue after treatment for childhood cancer [Citation6,Citation19]. This 18-item questionnaire consists of three subscale scores: cognitive fatigue, sleep-rest fatigue, and general fatigue. The cognitive fatigue subscale focuses on problems with memory, attention, and processing speed; the sleep/rest fatigue subscale focuses on quality and amount of sleep; and the general fatigue subscale covers problems due to lack of energy (including specific executive difficulties). The occurrence of problems is assessed over the past week on a 5-point Likert scale. Items are rescored to a 0–100 scale. A higher score indicates less fatigue. For children above the age of 8, the self-report was used, and for younger children the parent-proxy report was used.
Additionally, parental distress is also assessed in the monitoring program, with the distress thermometer for parents (DTP). Parents filled out the DTP every 6 months, independently of whether they were proxy-respondents for their young child’s fatigue assessment. The DTP is an instrument for caregivers to self-report their overall distress regarding physical, emotional, social and practical issues on a 0-10 scaled thermometer, with scores of 4 or higher indicating clinical distress [Citation20]. The psychometric properties of the DTP score are good [Citation21].
Data was extracted from the patient-reported outcome monitoring program for all patients for whom informed consent was given. Information regarding the medical variables was collected through the national childhood oncology registry and from medical records. The following independent predictors were included: sex, age at assessment, age at diagnosis, treatment (chemotherapy, radiotherapy, immunotherapy, brain surgery, and allogenic stem cell therapy), treatment period (before or after centralization of care), relapse, and parental distress. Age at diagnosis was a continuous variable, while age at assessment was dichotomous for parent-proxy-reports (2–4 years, or 5–7 years) and trichotomous for self-reports (8–12 years, 13–18 years, or above 18 years). All treatment-related variables, including relapse, were dichotomous. In case of relapse after the end of treatment, the end-date for the final treatment was used for the time variable and the 5 years started after this. The closest assessment with the DTP (clinical or non-clinical), was included as a psychosocial predictor. Parental educational level was defined according to national standards and then divided into low, middle or high educational level.
Statistical methods
The self-reports and the parent-proxy reports were analyzed separately. Linear mixed models were used to estimate growth-curves for the different subscales, using data assessed during the first 5 years after end of treatment. The main independent variable was time since end of treatment, and quadratic and cubic polynomial time variables were included if significant according to the Wald statistic. The models account for within-subject dependency of longitudinal observations using a random intercept and if necessary random slopes for the time variables. Necessity was evaluated by Likelihood ratio tests. After the best fitting growth-curve was defined, differences between the main diagnostic groups were determined. The groups were hemato-oncology (HO), solid tumors (ST), and central nervous system (CNS) tumors. Thereafter, the effects of variables were investigated by adding potential predictors to the best fitting growth-curve model. If p < 0.1 in the univariable models, the variable was selected for the multivariable models. All calculations were made with SPSS version 26.
Results
Out of all patients that participated in the monitoring program, 94.0% (N = 761) consented to the use of the parent-proxy questionnaires, which resulted in 2440 observations. For the self-reports, 90.2% (N = 990) consented, which resulted in 2657 observations. The total number of participants in both datasets was 1600, with 151 participants participating both before and after 8 years of age. The median number of assessments were 3 for proxy-reports (range 1–11) and 2 for self-reports (range 1–12). The distribution of diagnoses differed between self and proxy-respondents, with ST being the most common diagnosis for the parent-proxy reports (age below 8 years), while HO was the most common for self-reports. Clinical distress was prevalent in less than half of the parents at baseline. The participant demographics are described in for parent-proxy reports and for self-reports. Immunotherapy had been given before 17.8% of the assessments in younger children and 12.3% in older children, and always in combination with chemotherapy. In both groups, the sub diagnoses most commonly treated with immunotherapy were ALL, neuroblastoma, and non-Hodkin lymphoma. This group was further characterized by a higher number of relapse compared to the group not treated with immunotherapy. Fifteen percent of the younger children treated with immunotherapy had relapsed before study start, compared to 8.8% in the group not treated with immunotherapy. Corresponding number for the older children were 29.4% with relapse in the group treated with immunotherapy compared to 29.4%.
Table 1. Patient demographics for the proxy-reports of the PedsQLTM Multidimensional Fatigue Scales.
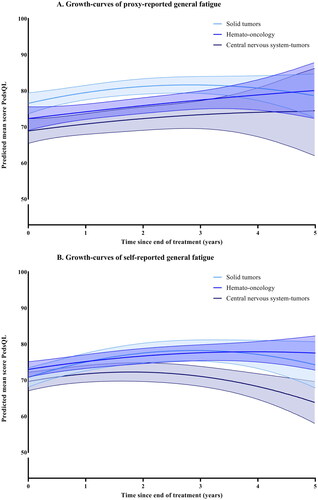
For the total group, cognitive fatigue was stable over time for both younger and older children (no significant change). For proxy-reported sleep-rest fatigue the scores increased over time (p<.001), indicating less problems. For self-reported sleep-rest fatigue (p<.001), proxy reported general fatigue (p=.048), and self-reported general fatigue (p=.010), the scores first improved and then deteriorated. When stratified by main diagnostic group, participants with CNS-tumors had significantly lower scores for cognitive fatigue than ST at the end of treatment. This was significant for both parent-proxy (−11.30, p<.001) and self-reports (−6.78, p=.002), as well as for parent-proxy reports of general fatigue (−6.78, p=.002). Participants treated for HO also scored significantly more cognitive fatigue for parent-proxy reports than ST (−6.63, p<.001). The change over time was only significantly different between groups for self-reports of sleep-rest fatigue, where the CNS-group decreased significantly more over time than ST with −0.87 raw scores per year (95% CI −1.64; −0.81, p=.031). The course of fatigue for the different diagnostic groups are shown in . The best fitting growth-curves for the whole sample, with means and standard deviations, are shown in Supplemental Figures 1–3. In addition, a line indicating the age and sex-weighted mean of cross-sectional norm data was calculated to provide context to the height of the curves for the whole sample in the Supplemental Figures.
Figure 1. (A,B) Growth-curves for cognitive fatigue, with predicted means and 95% confidence intervals. None of the groups have a significant interaction with time.
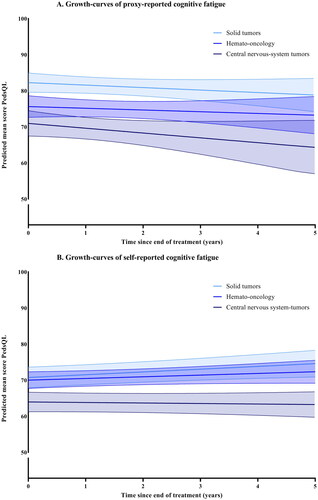
Figure 2. (A,B) Growth-curves for sleep-rest fatigue, with predicted means and 95% confidence intervals. The self-reports for the central-nervous-system group has a significant interaction with time and the scores are decreasing more over time than solid tumors (p=.031).
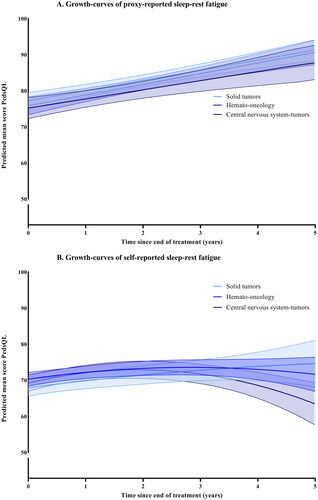
Figure 3. (A,B) Growth-curves for general fatigue, with predicted means and 95% confidence intervals. None of the groups have a significant interaction with time.
The results from the multivariable analyses are reported in for proxy-reports and for self-reports. Neither allogenic stem cell-therapy nor brain surgery were significant in any of the univariable analyses, and sex not for any of the self-reports, and were therefore not included in the corresponding multivariable analyses. For the parent-proxy reports, clinical parental distress was significantly associated with more fatigue for almost all outcomes for all three patient-groups. For the self-reports, the DTP was also significant for all outcomes for the CNS-group. Being diagnosed after the centralization of care was significantly associated with less fatigue after treatment for CNS-tumors for almost all outcomes, but not for any of the outcomes for the other two diagnostic groups. Younger age at diagnosis was not significantly associated with any of the outcomes, while older age at age at assessment frequently predicted more fatigue, especially for self-reports. All associations between treatment and fatigue had a negative direction, meaning that receiving that type of treatment was associated with more fatigue. The self-reports were characterized by immunotherapy being the only significant treatment predictor, with a negative relationship with three of the outcomes. For the parent-proxy reports, chemotherapy, immunotherapy, and radiotherapy had a negative relationship, but only for a few outcomes. Relapse was associated with more sleep-rest fatigue and general fatigue, but not with cognitive fatigue. Due to missing data for many participants, parental educational level could not be included in the analysis.
Discussion
In this study, the development of fatigue after treatment of childhood cancer was explored through repeated assessments from end of treatment up to 5 years later. We provide growth-curves demonstrating the expected trajectory after end of treatment, and investigated biopsychosocial determinants as predictors for fatigue.
We demonstrate that cognitive fatigue was stable over time, with neither increase nor decrease. For proxy-reported sleep-rest fatigue, scores increased over time, indicating less fatigue. For self-reported sleep-rest fatigue and both reports of general fatigue the scores first increased and then decreased. However, the change in raw scores was small compared to confidence intervals and standard deviations (SD). For proxy-reports of sleep-rest fatigue there was an improvement with 14 raw scores (compared to the SD of 10.3), while for the remaining outcomes this change was 4 raw scores or less.
Earlier studies have shown survivors of childhood cancer have fatigue scores that are substantially poorer than healthy norms [Citation4,Citation18]. It is of clinical importance to recognize patients at risk as early as possible, as childhood fatigue has the propensity to become chronic. Approximately one-third of fatigued adolescents in the general population will keep persistent symptoms in adulthood [Citation22], and the duration of fatigue is one of the strongest predictors of treatment success [Citation23]. In children with ALL, it has been shown that fatigue during treatment predicts fatigue one year after the end of therapy [Citation8]. For early recognition, it is essential to know the expected ‘normal’ course of fatigue after childhood cancer. Our study demonstrates that specific oncology curves fulfils an important purpose when evaluating fatigue in individual patients. The next step will be to import these predicted trajectories as reference curves into the monitoring system and use them in clinical practice. Early identification of patients at risk will enable faster access to clinical interventions.
A diagnosis of a CNS-tumor has previously been reported as a risk factor for fatigue in cross-sectional studies [Citation4,Citation9,Citation10,Citation12], and we confirm these results in a longitudinal setting. That HO had significantly lower scores than ST for 2 out of 3 parent-proxy subscales and not for any of the self-reports subscales might indicate that this group recovers over time, whereas no such pattern was seen for the CNS-group. Overall, the difference between diagnostic groups was most evident for cognitive fatigue, while for sleep-rest fatigue there were no differences. Considering that cognitive fatigue is related to lower neuropsychological functioning in survivors of childhood cancer [Citation24,Citation25], it is possible that cognitive deficits in the CNS-group explain these differences. In our current study with data up to 5 years after end of treatment, the difference between the CNS-group and other diagnostic groups did not increase with time for cognitive fatigue.
The effect of tumor group can also be discussed in relation to treatment type. There was no clear pattern regarding type of treatment, but in the case of allogenic stem cell therapy this could have been caused by a very small group receiving this treatment. Immunotherapy was the most frequent treatment-related predictor, and for the self-reports it was the only treatment to be associated with fatigue. It is possible that these participants had received second or third line treatment, and that immunotherapy was therefore a marker of disease severity. More fatigue after treatment with immunotherapy might therefore not be a result of that specific treatment, but of disease components. Research on the association between immunotherapy and fatigue is currently lacking, but will gain importance as the use of these agents is likely to increase over the coming years. Most surprising is perhaps that radiotherapy only predicted lower scores for 1 out of 18 outcomes. A study on chronic fatigue in adult survivors of childhood leukemia and lymphomas reported that neither radiotherapy nor chemotherapy predicted fatigue scores [Citation7]. This is in line with our results, where radiotherapy did not predict any outcome for the HO-group and chemotherapy only one. However, as older research on cognitive deficits in survivors of CNS-tumors showed that radiotherapy was associated with a decrease of 2–4 intelligence quotient points per year per year [Citation26–28], and a recent study by Söderström et al. indicates that it takes 3–5 years after treatment with radiotherapy until the full impact of cognitive deficits are seen [Citation29], it is possible that a longer follow-up time is necessary to fully explore the impact of radiotherapy in the CNS-group.
The centralization of care did not affect the outcomes for ST and HO, but did so for CNS. The CNS-group diagnosed after the centralization of care reported less fatigue for 5 out of 6 outcomes. Since the CNS-group consistently reported more fatigue than HO and ST, it is reasonable that this group benefitted more from the centralization, as this meant a more specialized medical team in addition to both more systematic attention for QoL and fatigue, as well as more multidisciplinary support was offered. The centralization of care further affects the generalizability of the study. While participation rate in KLIK was 70% on average, it is higher after the centralization than it was before it. The participation rate is therefore expected to increase even further in the future. Even though not all patients participate in KLIK, and not all give consent for research, the unique patient monitoring program at the Princess Máxima Centrum provides a robust data set. The longitudinal design in combination with the size of the study sample means that our findings can be applied to the broader population of children treated for childhood cancer. While we had an underrepresentation of parents with low educational level, the clinical patient demographics in our study were in line with what could be expected when comparing the distribution of these variables with the entire Dutch cohort of children diagnosed with cancer [Citation30].
Previous studies on fatigue in survivors of childhood cancer utilizing the MFS have not reported a sex difference [Citation4,Citation8,Citation9,Citation17]. These studies were cross-sectional, and we here report similar results for the development over time.
In a Dutch study on persistent fatigue for more than 6 months in both adult and adolescent survivors of childhood cancer, the prevalence was higher in the adult group than in the adolescent group [Citation12]. This might indicate that the prevalence of fatigue can increase over time. Similarly, we reported older age at assessment to be associated with more fatigue. That older study participants self-report more fatigue can possibly be explained by the higher demands and responsibilities that come with adulthood. A review on transition to adulthood for survivors of childhood cancer concluded that this group has unique medical, developmental, and psychosocial challenges [Citation31]. The combined burden of these can explain why the older participants self-reported more fatigue than younger participants. Lower age at diagnosis did not predict lower scores for any of the outcomes. While no other study has reported growth-curves for fatigue after treatment for childhood cancer before, this can still be considered unexpected due to lower age at diagnosis generally being a risk factor for many different types of outcomes [Citation32–34].
Concerning parental distress, this was overall the most frequent significant predictor. The clinical level of parental distress at baseline in our study was in line with what has previously been reported for ill children [Citation20,Citation21]. Previous studies on fatigue in other patient groups have also reported similar associations [Citation35,Citation36]. This indicates that parental distress should be taken into account when interpreting the results of parent-proxy reports as there is a chance that this might influence their response pattern. However, from our results it is not possible to determine whether parents reported more distress because the children were fatigued, or that they reported that the children were fatigued because they themselves were distressed. Still, it is interesting that the results from the DTP predicted the scores for 8 out of 9 parent-proxy outcomes, and for 4 of the self-report outcomes. There is high-quality evidence that psychosocial assessments should be standard care in pediatric oncology [Citation37], and our results indicate that parental stress also needs to be considered in interventions.
While the presence a co-existing medical condition is related to fatigue in adult long-term survivors of childhood cancer [Citation12], we did not explore this variable. The risk for having health problems after end of treatment generally increases with the duration of follow-up [Citation38], which can explain why the highest prevalence of fatigue has been reported in survivors aged 40–49 years [Citation12]. Cancer treatment might indirectly affect fatigue levels by increasing the likelihood of health conditions (for example heart disease) that are associated with fatigue [Citation39]. However, it is important to acknowledge that cancer treatment in childhood has been hypothesized to induce health problems at a later stage in life that may increase the risk for fatigue [Citation12], and that side effects and health problems that are subclinical at the end of treatment might manifest many years later [Citation40].
Limitations
The strengths of this study is the longitudinal design, with a uniquely large and robust dataset. While this was an extensive study, utilizing both parent-proxy reports and self-reports, and including more than 5000 observations, some limitations must be mentioned. Firstly, it was not possible to extract information on surgery (apart from brain surgery) from the medical records, meaning that surgery could not be included as a predictor in our model. Secondly, the results may not be representative for patients not participating in KLIK. Participation in the monitoring system is a voluntary intervention, and it is possible that this is a protective factor that in itself increases QoL. The monitoring system is (currently) only available to Dutch-speaking families, which means that immigrant families are participating to a lower extent. Additionally, there were very few participants with low parental education, and these numbers were below what could be expected when considering the distribution in the country. It was therefore not possible to include educational level as a measure of socio-economic status and explore its relationship with fatigue.
Conclusion
The main conclusion of this study is that the main diagnosis affects the longitudinal development of fatigue over time. Participants treated for CNS-tumors reported significantly more fatigue than other diagnoses, and this difference was observed in both younger and older children. In accordance with previous studies, older age at assessment was found to be a risk factor. Parental distress was the most frequent predictor for fatigue scores, for both self-reports and parent-proxy reports, implying a strong relationship between psychosocial variables and reported fatigue. The centralization of care, meaning increased psychosocial interventions and support in addition to a multidisciplinary care team and rehabilitation specialists, benefitted those treated for CNS-tumors but not the other two groups. The lower baseline scores for the CNS-group, together with precious research on cognitive decline, indicates that a follow-up time of 5 years might not be enough to fully explore the impact of fatigue in this group. Future studies should therefore focus on longer follow-up time and investigate the outcomes for different types of CNS-tumors. More research regarding the effects of different types of immunotherapy is also necessary.
Table 2. Patient demographics for the self-reports of the PedsQLTM Multidimensional fatigue Scales.
Table 3. Multivariable analysis of variables associated with fatigue development over time for parent-proxy reports, stratified by main diagnosis, N = 761. Unstandardized estimates with 95% confidence intervals.
Table 4. Multivariable analysis of variables associated with fatigue development over time for self-reports, stratified by main diagnosis, N = 990.
Ethical approval
Data was extracted from the patient-reported outcome monitoring program for all patients for whom informed consent was given. Using data collected in care for research questions was approved by the ethics committee of Rotterdam Center (decision number MEC-2016-739). Additionally, the scientific committee of the Princess Máxima Centrum approved of the project.
Supplemental Material
Download MS Word (428.8 KB)Acknowledgements
We acknowledge the efforts of the KLIK-team at the Princess Máxima Centrum for Childhood Oncology, for their dedicated work with implementing KLIK in clinical practice.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Deidentified individual participant data (including data dictionaries) will be made available, in addition to the statistical analysis plan. The data will be made available after publication upon request to researchers who provide a methodologically sound proposal and after a signed data sharing agreement is reached. Proposals should be submitted to [email protected].
Additional information
Funding
References
- Spathis A, Booth S, Grove S, et al. Teenage and young adult cancer-related fatigue is prevalent, distressing, and neglected: it Is time to intervene. A systematic literature review and narrative synthesis. J Adolesc Young Adult Oncol. 2015;4(1):3–17. doi: 10.1089/jayao.2014.0023.
- Walter LM, Nixon GM, Davey MJ, et al. Sleep and fatigue in pediatric oncology: a review of the literature. Sleep Med Rev. 2015;24:71–82. doi: 10.1016/j.smrv.2015.01.001.
- Berger AM, Mitchell SA, Jacobsen PB, et al. Screening, evaluation, and management of cancer-related fatigue: ready for implementation to practice? CA Cancer J Clin. 2015;65(3):190–211. doi: 10.3322/caac.21268.
- Irestorm E, Tonning Olsson I, Johansson B, et al. Cognitive fatigue in relation to depressive symptoms after treatment for childhood cancer. BMC Psychol. 2020;8(1):31. doi: 10.1186/s40359-020-00398-1.
- Barsevick AM, Irwin MR, Hinds P, et al. Recommendations for high-priority research on cancer-related fatigue in children and adults. J Natl Cancer Inst. 2013;105(19):1432–1440. doi: 10.1093/jnci/djt242.
- Christen S, Roser K, Mulder RL, et al. Recommendations for the surveillance of cancer-related fatigue in childhood, adolescent, and young adult cancer survivors: a report from the international late effects of childhood cancer guideline harmonization group. J Cancer Surviv. 2020;14(6):923–938. doi: 10.1007/s11764-020-00904-9.
- Zeller B, Loge JH, Kanellopoulos A, et al. Chronic fatigue in long-term survivors of childhood lymphomas and leukemia: persistence and associated clinical factors. J Pediatr Hematol Oncol. 2014;36(6):438–444. doi: 10.1097/MPH.0000000000000051.
- Irestorm E, Steur LMH, Kaspers GJL, et al. Fatigue trajectories during pediatric ALL therapy are associated with fatigue after treatment: a national longitudinal cohort study. Support Care Cancer. 2022;31(1):1.
- Meeske K, Katz ER, Palmer SN, et al. Parent proxy-reported health-related quality of life and fatigue in pediatric patients diagnosed with brain tumors and acute lymphoblastic leukemia. Cancer. 2004;101(9):2116–2125. doi: 10.1002/cncr.20609.
- Mulrooney DA, Ness KK, Neglia JP, et al. Fatigue and sleep disturbance in adult survivors of childhood cancer: a report from the childhood cancer survivor study (CCSS). Sleep. 2008;31(2):271–281. doi: 10.1093/sleep/31.2.271.
- Puhr A, Ruud E, Anderson V, et al. Self-Reported executive dysfunction, fatigue, and psychological and emotional symptoms in physically well-functioning long-term survivors of pediatric brain tumor. Dev Neuropsychol. 2019;44(1):88–103. 10.1080/87565641.2018.1540007
- van Deuren S, Penson A, van Dulmen‐den Broeder E, et al. Prevalence and risk factors of cancer‐related fatigue in childhood cancer survivors: a DCCSS LATER study. Cancer. 2022;128(5):1110–1121. doi: 10.1002/cncr.33993.
- Nap-van der Vlist MM, Dalmeijer GW, Grootenhuis MA, et al. Fatigue among children with a chronic disease: a cross-sectional study. BMJ Paediatr Open. 2021;5(1):e000958. doi: 10.1136/bmjpo-2020-000958.
- Bakula DM, Sharkey CM, Perez MN, et al. The relationship between parent distress and child quality of life in pediatric cancer: a Meta-Analysis. J Pediatr Nurs. 2020;50:14–19. doi: 10.1016/j.pedn.2019.09.024.
- Haverman L, van Oers HA, Limperg PF, et al. Implementation of electronic patient reported outcomes in pediatric daily clinical practice: the KLIK experience. Clin Pract Pediatr Psychol. 2014;2(1):50–67. doi: 10.1037/cpp0000043.
- van Gorp M, Maurice‐Stam H, Teunissen LC, et al. No increase in psychosocial stress of dutch children with cancer and their caregivers during the first months of the COVID‐19 pandemic. Pediatr Blood Cancer. 2021;68(2):e28827. doi: 10.1002/pbc.28827.
- Varni JW, Burwinkle TM, Katz ER, et al. The PedsQL in pediatric cancer: reliability and validity of the pediatric quality of life inventory generic core scales, multidimensional fatigue scale, and cancer module. Cancer. 2002;94(7):2090–2106. doi: 10.1002/cncr.10428.
- Gordijn M, Cremers EM, Kaspers GJ, et al. Fatigue in children: reliability and validity of the dutch PedsQL Multidimensional Fatigue Scale. Qual Life Res. 2011;20(7):1103–1108. doi: 10.1007/s11136-010-9836-9.
- van Deuren S, Boonstra A, van Dulmen-den Broeder E, et al. Severe fatigue after treatment for childhood cancer. Cochrane Database Syst Rev. 2020;2020(3):CD012681.
- Haverman L, van Oers HA, Limperg PF, et al. Development and validation of the distress thermometer for parents of a chronically ill child. J Pediatr. 2013;163(4):1140–1146.e2. doi: 10.1016/j.jpeds.2013.06.011.
- van Oers HA, Schepers SA, Grootenhuis MA, et al. Dutch normative data and psychometric properties for the distress thermometer for parents. Qual Life Res. 2017;26(1):177–182. doi: 10.1007/s11136-016-1405-4.
- Janssens KA, Klis S, Kingma EM, et al. Predictors for persistence of functional somatic symptoms in adolescents. J Pediatr. 2014;164(4):900–905. e2. doi: 10.1016/j.jpeds.2013.12.003.
- Nijhof SL, Priesterbach LP, Uiterwaal CS, et al. Internet-based therapy for adolescents with chronic fatigue syndrome: long-term follow-up. Pediatrics. 2013;131(6):e1788–e1795. doi: 10.1542/peds.2012-2007.
- Irestorm E, Ora I, Linge H, et al. Cognitive fatigue and processing speed in children treated for brain tumours. J Int Neuropsychol Soc. 2021;27(9):865–874. doi: 10.1017/S1355617720001332.
- Cheung YT, Brinkman TM, Mulrooney DA, et al. Impact of sleep, fatigue, and systemic inflammation on neurocognitive and behavioral outcomes in long‐term survivors of childhood acute lymphoblastic leukemia. Cancer. 2017;123(17):3410–3419. doi: 10.1002/cncr.30742.
- Palmer SL, Goloubeva O, Reddick WE, et al. Patterns of intellectual development among survivors of pediatric medulloblastoma: a longitudinal analysis. J Clin Oncol. 2001;19(8):2302–2308. 10.1200/JCO.2001.19.8.2302
- Ris MD, Packer R, Goldwein J, et al. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a children’s cancer group study. J Clin Oncol. 2001;19(15):3470–3476. doi: 10.1200/JCO.2001.19.15.3470.
- Palmer SL, Gajjar A, Reddick WE, et al. Predicting intellectual outcome among children treated with 35-40 gy craniospinal irradiation for medulloblastoma. Neuropsychology. 2003;17(4):548–555. doi: 10.1037/0894-4105.17.4.548.
- Söderström H, Brocki K, Kleberg JL, et al. Neurocognitive functions before and after radiotherapy in pediatric brain tumor survivors. Pediatr Neurol. 2022;133:21–29. doi: 10.1016/j.pediatrneurol.2022.05.006.
- Reedijk AMJ, Kremer LC, Visser O, et al. Increasing incidence of cancer and stage migration towards advanced disease in children and young adolescents in The Netherlands, 1990–2017. Eur J Cancer. 2020;134:115–126. doi: 10.1016/j.ejca.2020.04.011.
- Freyer DR. Transition of care for young adult survivors of childhood and adolescent cancer: rationale and approaches. J Clin Oncol. 2010;28(32):4810–4818. doi: 10.1200/JCO.2009.23.4278.
- Robison LL, Hudson MM, Science and Society. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14(1):61–70. doi: 10.1038/nrc3634.
- Hudson MM, Mulrooney DA, Bowers DC, et al. High-risk populations identified in childhood cancer survivor study investigations: implications for risk-based surveillance. J Clin Oncol. 2009;27(14):2405–2414. doi: 10.1200/JCO.2008.21.1516.
- Dixon SB, Bjornard KL, Alberts NM, et al. Factors influencing risk-based care of the childhood cancer survivor in the 21st century. CA Cancer J Clin. 2018;68(2):133–152. doi: 10.3322/caac.21445.
- van de Putte EM, van Doornen LJP, Engelbert RHH, et al. Mirrored symptoms in mother and child with chronic fatigue syndrome. Pediatrics. 2006;117(6):2074–2079. doi: 10.1542/peds.2005-2307.
- Chalder T, Goodman R, Wessely S, et al. Epidemiology of chronic fatigue syndrome and self reported myalgic encephalomyelitis in 5–15 year olds: cross sectional study. BMJ. 2003;327(7416):654–655. doi: 10.1136/bmj.327.7416.654.
- Kazak AE, Abrams AN, Banks J, et al. Psychosocial assessment as a standard of care in pediatric cancer. Pediatr Blood Cancer. 2015;62(S5):S426–S59. doi: 10.1002/pbc.25730.
- Sieswerda E, Dijkgraaf MGW, Heinen RC, et al. Increased hospitalization rates in survivors of childhood cancer: a cohort study using medical record linkage. Tijdschr Kindergeneeskunde. 2013;81(S1):15–16. doi: 10.1007/s12456-013-0017-y.
- Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. Jama. 2007;297(24):2705–2715. doi: 10.1001/jama.297.24.2705.
- Schwartz CL. Long-term survivors of childhood cancer: the late effects of therapy. Oncologist. 1999;4(1):45–54. doi: 10.1634/theoncologist.4-1-45.
