Abstract
Background
Evidence on the risk of second primary cancer (SPC) following primary laryngeal squamous cell carcinoma (LSCC) is limited, especially in Europe.
Methods
Patients diagnosed with primary LSCC from 1953–2018 were retrieved from the Finnish Cancer Registry. A total of 6241 LSCC patients were identified adding to 49,393 person-years (PY) of follow-up until the end of 2019. Only one patient emigrated and was lost to follow-up. Both standardized incidence ratios (SIR) and excess absolute risk (EAR) per 1000 person-years at risk (PYR) of second primary cancer (SPC) were calculated relative to the general population. Only non-laryngeal SPCs diagnosed six months after diagnosis of primary LSCC were included.
Results
A SPC was diagnosed in 1244 LSCC patients (20% of all LSCC patients) over the 65-year period, predominantly in men (92%, n = 1170). Out of all SPCs, 34% were diagnosed within 0.5 to 5 years and 66% after 5 years from primary LSCC. Among male patients, the overall SIR for SPC at any location was 1.61 (95% CI: 1.52–1.71), corresponding to 9.49 excess SPCs per 1000 PYR (95% CI: 8.19–11). The corresponding SIR for women was 1.47 (95% CI: 1.15–1.84), yielding 4.82 excess SPCs per 1000 PYR (95% CI: 2.36–9.84). The risk remained significant even after 20 years of follow-up (SIR for all 1.73, 95% CI: 1.49–2.01 and EAR 16.8 per 1000 PY, 11.88–23.75). The risk for SPC was also significantly elevated in all age groups, except <40. The highest SIRs were for SPCs arising in the mouth/pharynx (SIR for all 3.08, 95% CI: 2.36–3.95 and EAR 0.80 per 1000 PY, 0.55–1.15) and lungs (3.02, 2.75-3.30 and 5.90 per 1000, 5.13–6.78).
Conclusion
Patients with LSCC as primary cancer have a 60% excess risk for an SPC, especially for tobacco-associated cancers, remaining significantly elevated even decades after treatment.
NOVELTY & IMPACT
Although prior research on the risk of second primary cancer (SPC) among laryngeal squamous cell carcinoma (LSCC) patients has been conducted in other regions, the European perspective remains notably underrepresented. Moreover, studies on the subject focusing especially on LSCC are, even globally, only a few. The present study, with over 6000 LSCC patients followed-up over six decades, consists of the largest reported cohort of LSCC patients in Europe, and with the longest follow-up. Patients with LSCC as a primary cancer have a 60% excess risk for an SPC, which remains significantly elevated even 20 years after the diagnosis of the first primary cancer, especially for those with a tobacco/alcohol-related cancer. Healthcare professionals should be aware of the SPC risk among LSCC survivors who should be counseled about this phenomenon.
Introduction
According to the Global Cancer Observatory database (www.gco.iarc.fr), over 180,000 cases of laryngeal cancer were diagnosed in 2020 and the number of new cases worldwide is anticipated to increase by approximately 50% by year 2040. However, incidence rates vary markedly across countries. For instance, while the incidence rate in men in Finland [age-standardized rate (ASR) 1.7/105] is the lowest in Europe after Sweden (ASR 1.2/105), Southeastern Europe exhibits the world’s highest rates ranging from 4.3 to 12.9. The epidemiological contrasts across regions stem presumably from differences in the prevalence of lifestyle habits such as tobacco smoking and the use of alcohol, which are well-established etiological risk factors [Citation1,Citation2]. Mostly diagnosed between 50 and 70 years and with a five-fold male preponderance, squamous cell carcinomas (SCC) account for over 95% of all laryngeal cancers [Citation3,Citation4].
In Finland, 5-year relative survival during 2015–2019 was 60% (95% CI: 53.9-64.8) and 59% (95% CI: 40.8-72.6) among men and women, respectively [Citation5]. A treatment challenge throughout the follow-up is the emergence of second primary cancers (SPC) [Citation6]. SPCs are considered independent tumors, and not recurrences or metastases of the original primary cancer, occurring synchronously when diagnosed simultaneously or within six months, or metachronously when diagnosed more than six months after the primary tumor [Citation7].
Limited data with long follow-up exist in the literature regarding the risk of SPC in patients with LSCC as the index site. Based on the pooled analysis of 13 cancer registries conducted by Chuang et al. [Citation8], head and neck cancer (HNC) patients carry a 20-year cumulative SPC risk of 36%. Almost 20 years ago Gao et al. [Citation9] reviewed 20,074 laryngeal cancer patients and uncovered a cumulative risk for SPC of 26% at 10 years and 47% at 20 years with the highest relative risk (RR) for SPCs in the head and neck (HN) region [RR 4.81, 95% confidence interval (CI): 4.31–5.58] and esophagus (RR 3.99, 95% CI: 3.29–4.83) when compared to the general population. Most SPCs were reported to occur in the HN area, followed by the lungs and esophagus.
In Finland, the risk of SPC following primary LSCC has not yet been evaluated at population level. Additionally, as noted, the literature on the subject is scarce. We aim to assess the risk of SPC among 6241 patients diagnosed with a first primary LSCC in Finland between 1953 and 2018 and examine the influence of age at diagnosis, sex, time elapsed since diagnosis of the first primary cancer, and calendar periods.
Patients and methods
Population. Data on patients diagnosed with LSCC in Finland from 1953–2018 were retrieved from the Finnish Cancer Registry (FCR). Briefly, the FCR includes all new primary cancers diagnosed in Finland since 1953 with data on primary site and histology, as well as complete follow-up until death or emigration. Patients with any previous cancer, except for basal-cell carcinoma of the skin, were excluded. We also excluded patients whose first primary cancer diagnosis and death were recorded simultaneously. A total of 6241 LSCC patients were identified adding to 49,393 person-years (PY) of follow-up until the end of 2019. Only one patient emigrated during the follow-up.
ICD-O-3 topographical and morphological codes (http://www.iacr.com.fr/index.php?Itemid=577) were used to retrieve the LSCC patients from the FCR. Subsequently, all laryngeal SPCs were excluded. Still, some regional, or even distant, recurrences could theoretically, to a minor extent, be misclassified as SPC and vice versa. To minimize the risk of misclassification, only SPCs diagnosed six months after diagnosis of primary LSCC were included, i.e., we excluded all synchronous SPCs diagnosed within six months of diagnosis of the primary LSCC (76 patients).
Statistical analyses. To estimate the SPC risk, standardized incidence ratios (SIRs) and 95% CI were estimated by comparison of the observed number of SPC cases among LSCC patients with the numbers expected, derived from the age, sex, and calendar-specific rates in the Finnish general population using PYs of observations (six months after diagnosis of primary LSCC), and assuming a Poisson distribution for the number of observed SPC cases. The SIR thus estimates the risk of LSCC patients developing an SPC relative to the first occurrence of cancer among the general population. SIR estimates were stratified according to age groups at diagnosis, sex, extent of the primary disease (localized or non-localized) time since primary cancer diagnosis, and calendar period at LSCC diagnosis (1953–1989, 1990–2005, 2005–2018) to assess whether the risk of SPCC differed over the follow-up period. Additionally, we calculated the excess absolute risk (EAR) to explore the excess incidence of overall SPC. EAR is an absolute measure of SPC risk and describes the difference in absolute risk of cancer between the LSCC patient population and the general population (observed cancers – expected cancers) per 1000 PYs at risk. Simply put, the EAR quantifies the absolute number of additional cancers attributable to primary LSCC and thus assesses the burden of SPCs on health care in a given population. All analyses were conducted using R software (The R Project for Statistical Computing) version 3.6.0 and the popEpi and forestplot packages.
Results
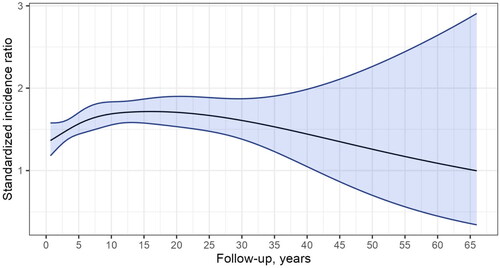
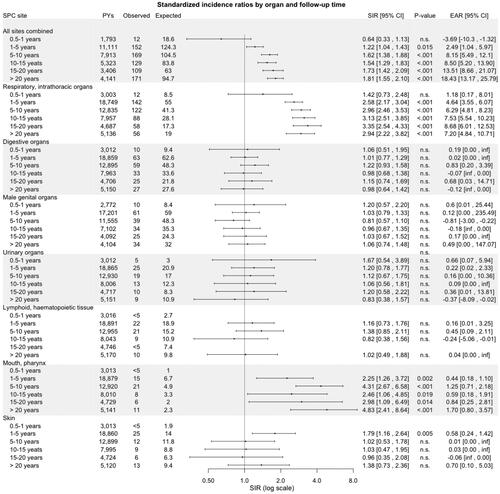
A metachronous SPC was diagnosed in 1244 LSCC patients (20% of all LSCC patients) over the 65-year period, predominantly in men (92%, n = 1170). Out of all SPCs, 34% were diagnosed within 0.5 to 5 years and 66% after 5 years from primary LSCC. The risk of SPC as a function of the follow-up time is presented in . The risk of SPC remained consistently elevated after the first year of follow-up throughout each subsequent follow-up interval of five years, even after 20 years (SIR 1.73, 95% CI: 1.49–2.01).
Figure 1. Standardized incidence ratio during follow-up for second primary cancer among patients with laryngeal squamous cell carcinoma in Finland during 1953–2018.
The SIRs and EARs for SPCs categorically stratified are summarized in . Among male patients with LSCC, the overall SIR for SPC at any location was 1.61 (95% CI: 1.52–1.71, p < .001), corresponding to 9.49 excess SPCs per 1000 PYR (95% CI: 8.19–11). The corresponding SIR for women was 1.47 (95% CI: 1.15–1.84, p < .001), yielding 4.82 excess SPCs per 1000 PYR (95% CI: 2.36–9.84). The risk for SPC was significantly elevated in all age groups, except <40, in comparison with the general population. However, the study comprised only 28 patients aged <40 at the time of primary cancer diagnosis. The risk was also elevated in all three calendar periods of diagnosis of primary LSCC (1953–1989, 1990–2004, and 2005–2018). The extent of the primary disease did not markedly influence the SIR, as both localized and non-localized cases had an increased SIR (1.59 versus 1.86, SIR-ratio 0.86, CI: 0.72–1.02).
Table 1. Standardized incidence Ratios and excess absolute risk per 1000 person-years for any metachronous second primary cancer among 6241 laryngeal squamous cell carcinoma patients diagnosed in Finland during 1953–2018 by period of primary cancer diagnosis and stratified by sex, age at diagnosis of primary tumour, follow-up period, follow-up time, and stage.
Respiratory and intrathoracic organs comprised the largest group of SPCs (38%, n = 478), followed by digestive organs (17%, n = 217) and male genital organs (16%, n = 203), as shown in . For all LSCC patients combined, the highest SIRs were for SPCs arising in the mouth/pharynx (SIR 3.08, 95% CI: 2.36–3.95, p < .001) and in respiratory or intrathoracic organs (2.83, 2.58-3-09, p < .001). Yet, the respiratory organs carried the highest EAR for SPCs (5.90 per 1000 PY, 95% CI: 5.13–6.78), followed by the mouth/pharynx (0.80 per 1000 PY, 0.55–1.15). SIRs in other SPC sites were not elevated among men or women when compared with the general population, except for SPCs of the skin among men (SIR 1.35, 95% CI: 1.04–1.72), p = .002). However, while the overall risk of SPC in the digestive organs was not significantly elevated, when further stratifying by anatomical site, we observed an increased risk (SIR 3.00, 95%-CI: 2.10–4.16) of esophageal SPC (data not shown).
Figure 2. Standardized incidence ratios and excess absolute risk per 1000 person-years for second primary cancer by site and stratified by Gender among patients with laryngeal squamous cell carcinoma in Finland during 1953–2018. Abbreviations: CI: confidence interval; EAR: excess absolute; PYs: person-years; n.s.: non-significant; SIR: standardized incidence ratio; SPC: second primary cancer.
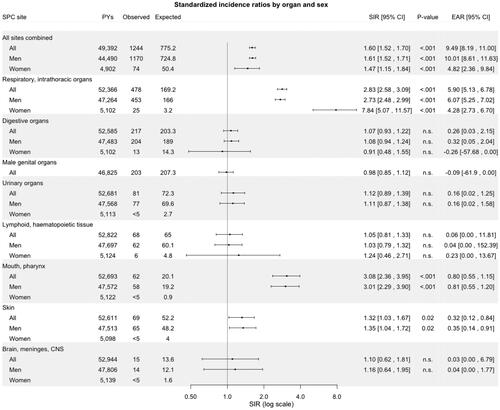
Regarding the respiratory or intrathoracic organs women had a higher SIR than men (7.84, 95% CI: 5.07–11.57, p < .001 versus 2.73, 2.48–2.99, p < .001). Following stratification by site, for all patients, the risk of SPC among the respiratory or intrathoracic organs was increased only for the lungs (SIR 3.03, 95% CI: 2.75–3.30) and specifically for small cell carcinoma (2.93, 2.23–3.78, p < .001), squamous cell carcinoma (4.01, 3.44–4.65, p < .001), and adenocarcinoma (2.05, 1.47–2.78, p < .001).
SIRs for SPCs by organ site and stratified by age, calendar period of diagnosis of primary LSCC (1953–1989, 1990–2004, and 2005–2018), and follow-up time are outlined in , respectively. The risk for SPCs in the mouth/pharynx and respiratory organs was elevated in all age categories, except in <40, and in all three calendar periods of diagnosis of primary LSCC. Furthermore, the risk for these SPCs remained consistently significantly elevated, even after 20 years of follow-up. Patients diagnosed with primary LSCC during 2005–2018 had an increased risk of SPC in digestive organs (SIR 2.08, 95% CI: 1.52–2.77, p < .001).
Figure 3. Standardized incidence ratios and excess absolute risk per 1000 person-years for second primary cancer by site and stratified by age at diagnosis of primary laryngeal squamous cell carcinoma in Finland during 1953–2018. Abbreviations: CI: confidence interval; EAR: excess absolute; PYs: person-years; n.s.: non-significant; SIR: standardized incidence ratio; SPC: second primary cancer.
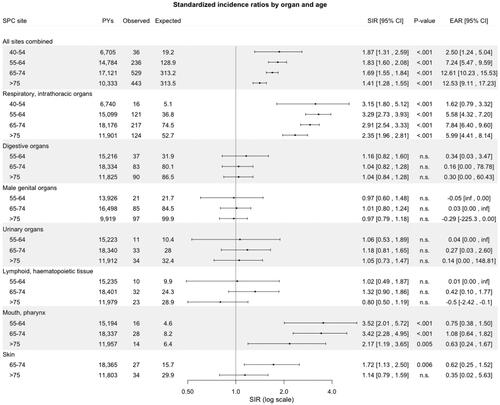
Figure 4. Standardized incidence ratios and excess absolute risk per 1000 person-years for second primary cancer by site and stratified by time period of diagnosis of primary laryngeal squamous cell carcinoma in Finland during 1953–2018. Abbreviations: CI: confidence interval; EAR: excess absolute; PYs: person-years; n.s.: non-significant; SIR: standardized incidence ratio; SPC: second primary cancer.
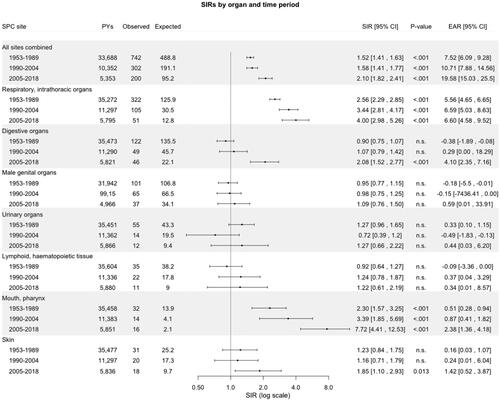
Figure 5. Standardized incidence ratios and excess absolute risk per 1000 person-years for second primary cancer by site and stratified by follow-up time (time elapsed from diagnosis of primary tumour) among patients with laryngeal squamous cell carcinoma in Finland during 1953–2018. Abbreviations: CI: confidence interval; EAR: excess absolute; PYs: person-years; n.s.: non-significant; SIR: standardized incidence ratio; SPC: second primary cancer.
Discussion
The current study confirms that patients with a history of LSCC carry a 60% excess risk of developing an SPC compared to the general Finnish population. Our results also indicate that the SPC risk remains significantly increased even more than 20 years after the diagnosis of primary LSCC, especially for tobacco/alcohol-related cancers. Indeed, besides the aerodigestive organs and skin, the risk of SPC was not elevated for other anatomical sites.
In Finland, the incidence of LSCC has been consistently decreasing in men [Citation5]. However, worldwide the burden of LSCC will continue to increase due to aging population in parallel to global predictions by the Global Cancer Observatory (www.gco.iarc.fr). By analyzing more than 6000 patients with complete follow-up, we were able to evaluate the risk of site-specific SPC in relation to time since the diagnosis of primary LSCC over five decades, age at LSCC diagnosis, and calendar period. Whereas single-institution or pooled-data studies are impeded by cohort size or heterogeneity of patient registries, the nationwide scale and long comprehensive follow-up of the present study allow evaluation of site-specific SPC risk with good statistical power. Underreporting of cancers in the FCR is insignificant, as quality assessment studies have shown high coverage (96% of solid tumors) and accuracy of diagnosis [Citation10]. Additionally, population-based studies minimize the selection bias inherent in hospital or clinical series [Citation11].
The generalizability of our observations regarding the excess of SPCs, particularly affecting the mouth/pharynx, esophagus, and lungs, among LSCC survivors is supported by the available literature. As for HNC in general, a systematic review by Coca-Pelaz et al. [Citation12] published in 2020 and incorporating 456,130 patients from 61 articles with a minimum follow-up of 22 months conveyed a 9.4% rate (95% CI: 7.9–10.9) of metachronous SPCs. Review of the data suggests that the most frequent site of SPTs is the head and neck area, followed by the lung and then, the esophagus. The meta-analysis by Hoxhaj et al. [Citation13], published in 2020, is also supportive of these findings. The most common SPC sites reported by the authors were the oropharynx (pooled SIR 10.19, 95% CI: 2.67–38.95), salivary glands (5.86, 2.57–13.37), esophagus (4.66, 2.16–10.05) and lungs (4.32, 2.15–8.68), mirroring the results of our study.
Vis-à-vis specifically LSCC, in the US, Morris et al. [Citation14] conducted a population-based cohort study and analyzed the data of 25,624 LSCC patients (75,087 HNSCC patients) between 1975 and 2006 with a median follow-up of 69 months. The authors excluded any cancer occurring within five years of primary cancer diagnosis. In alignment with our study, LSCC patients carried a significantly elevated risk of SPC (SIR 1.9; 95% CI: 1.9 to 2.0) when compared to the general population. The highest risk of SPC was observed for SPCs occurring in the oropharynx (11.24, 7.92–15.49), larynx (9.81, 8.09–11.78), and oral cavity/pharynx (7.25, 6.74–7.77). Further supportive of our results is the population-based study by Gao et al. [Citation9]. The authors reviewed 20,074 laryngeal cancer patients surviving at least three months from 1973 to 1996 exploiting the same database as Morris et al. [Citation14] and described an SIR of 1.68 (95% CI: 1.58–1.79) for any SPC. Mirroring further our results, no other organs outside the aerodigestive tract displayed an elevated risk of SPC. In Taiwan, Chen et al. [Citation15] reviewed 6167 laryngeal cancer patients (63,720 HNC patients) and included all lung and esophageal SPCs diagnosed one month after diagnosis of the primary tumor. The authors conveyed an increased risk of lung (SIR 2.05, 95% CI: 1.69–2.45) and esophageal SPC (4.65, 3.37–6.27), which also parallels the increased risk of lung and esophageal SPC we uncovered in our study.
Common etiological risk factors could trigger the development of SPCs, as suggested by the significantly elevated risk of SPCs in the aerodigestive tract. Indeed, alcohol drinking [Citation16], together with tobacco smoking [Citation17], well-recognized LSCC risk factors are also associated with other HN and aerodigestive cancers [Citation18,Citation19]. Persistent tobacco smoking or excessive alcohol drinking following treatment of the index cancer may contribute to the risk of SPC [Citation20]. According to Ping Ng et al. [Citation21], patients with an active or even previous history of smoking remain at increased risk of SPC. The authors described cumulative rates of SPC at 15 years of 14, 23, 35, and 32% for never smokers, former smokers with <10-pack year, former smokers with ≥10-pack year, and current smokers, respectively. At odds with these observations, a recent systematic review [Citation22] concluded that smoking cessation in HNC patients was associated with a lower rate of SPCs.
The occurrence of SPCs can also be rationalized by the concept of field cancerization, first introduced by Slaughter et al. [Citation23]. Supportive of this hypothesis is the study of Thomson [Citation24] who collected biopsies from clinically normal-looking mucosa at contralateral anatomical sites from 26 patients affected by unilateral oral cancer or by a premalignant lesion. Cellular atypia, dysplasia, and carcinoma-in-situ (or micro-invasive SCC) were diagnosed in six, seven, and two biopsy specimens, respectively. The carcinogenic effects of tobacco and alcohol on the aerodigestive tract may thus concurrently lead to the development of precancerous lesions, which may partly account for the subsequent elevated SPC risk among these organs.
The significantly elevated SPC risk in the digestive organs observed in our study only for the latest period 2005–2018, may be due to increased surveillance [Citation25] or possibly increased use of radiotherapy as parts of the esophageal tract will then be exposed to radiation during primary treatment [Citation26,Citation27]. Indeed, some studies hint at a potential association between use of radiotherapy and increased risk of SPC [Citation9,Citation27,Citation28]. Gao et al. [Citation9] concluded that radiotherapy was an independent predictor of SPC (RR adjusted for age 1.10, 95% CI: 1.02–1.18). However, radiotherapy was only significantly associated with the risk of developing an SPC in the HN area. Furthermore, the study by Ben Arie et al. [Citation26] uncovered no association between treatment modality and SPC risk among HNC patients. Lastly, the association between radiotherapy and increased overall risk of SPC does not necessarily indicate radiotherapy as the sole culprit, as the potential role of chemotherapy also needs to be accounted for.
Our results should be viewed within the context of the inherent limitations of cancer registry-based studies. Firstly, the lack of data on etiological factors, such as tobacco, alcohol consumption, and HPV status, in addition to primary treatment, particularly radio- and chemotherapy regimens, prevents us from making inferences about the relation of these variables to SPCs. The lack of data on primary cancer stage at diagnosis also hampers us from performing more detailed analysis. The sizable number of comparisons may also yield some statistically significant chance associations. Secondly, our study entails a risk of misclassification, unfortunately, unavoidable in clinical practice. The anatomical vicinity of some SPC sites (such as pharynx) to the primary tumor might have led to a misdiagnosis of local spread or disease recurrence as SPC. Additionally, primary tumor metastases could have also been inaccurately classified as SPCs and vice versa. Still, this potential diagnostic bias cannot be solely accountable for the association between LSCC and increased SPC risk, as the SIR remained elevated even after 20 years following diagnosis of the primary tumor. Lastly, increased surveillance and imaging following primary cancer treatment may have led to an overdiagnosis of indolent tumors that would have not been otherwise detected resulting in an overestimation of the SPC risk when compared to the general population. Nonetheless, our results provide a reasonable assessment of the risk of SPCs following LSCC diagnosis and serve to heighten clinician awareness of this risk.
In summary, patients with LSCC as a primary cancer have a 60% excess risk for an SPC which remains significantly elevated even 20 years after the diagnosis of the first primary cancer. We observed strong associations of first primary LSCC with SPCs of tobacco/alcohol-related cancers – the mouth/pharynx, lungs, and esophagus – suggestive of common risk factors for multiple cancer development. Healthcare professionals should be aware of the SPC risk among LSCC survivors who should be counseled about this phenomenon and encouraged to endorse practices accordant with a healthy lifestyle, including smoking cessation, and be vigilant about symptoms.
Author’s contributions
Rayan Nikkilä, Elli Hirvonen, Aaro Haapaniemi, Laura Tapiovaara, Janne Pitkäniemi, Nea Malila, Antti Mäkitie conceived and designed the study. Elli Hirvonen conceived the data. Rayan Nikkilä and Elli Hirvonen analyzed the data. Rayan Nikkilä devised the first draft of the manuscript. All authors contributed to the revision of the manuscript and had final approval of the submitted and published versions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing not available due to privacy/ethical restrictions
Additional information
Funding
References
- Talamini R, Bosetti C, la Vecchia C, et al. Combined effect of tobacco and alcohol on laryngeal cancer risk: a case-control study. Cancer Causes Control. 2002;13(10):957–964. doi: 10.1023/a:1021944123914.
- Anantharaman D, Marron M, Lagiou P, et al. Population attributable risk of tobacco and alcohol for upper aerodigestive tract cancer. Oral Oncol. 2011;47(8):725–731. doi: 10.1016/j.oraloncology.2011.05.004.
- Nocini R, Molteni G, Mattiuzzi C, et al. Updates on larynx cancer epidemiology. Chin J Cancer Res. 2020;32(1):18–25. doi: 10.21147/j.issn.1000-9604.2020.01.03.
- Ciolofan MS, Vlăescu AN, Mogoantă CA, et al. Clinical, histological and immunohistochemical evaluation of larynx cancer. Curr Health Sci J. 2017;43:367–375.
- Koskinen A, Hemminki O, Försti A, et al. Incidence and survival in laryngeal and lung cancers in Finland and Sweden through a half century. PLOS One. 2022;17(5):e0268922. doi: 10.1371/journal.pone.0268922.
- Haughey BH, Arfken CL, Gates GA, et al. Meta-analysis of second malignant tumors in head and neck cancer: the case for an endoscopic screening protocol. Ann Otol Rhinol Laryngol. 1992;101(2 Pt 1):105–112. doi: 10.1177/000348949210100201.
- Fritz A, Ries L, editors. The SEER Program Code Manual. 3rd ed. Cancer Statistics Branch, Surveillance Program, Division of Cancer Control and Population Sciences, National Cancer Institute, National Institutes of Health, Public Health Service, U.S. Dept. of Health and Human Services; 1998.
- Chuang SC, Scelo G, Tonita JM, et al. Risk of second primary cancer among patients with head and neck cancers: a pooled analysis of 13 cancer registries. Int J Cancer. 2008;123(10):2390–2396. doi: 10.1002/ijc.23798.
- Gao X, Fisher SG, Mohideen N, et al. Second primary cancers in patients with laryngeal cancer: a population-based study. Int J Radiat Oncol Biol Phys. 2003;56(2):427–435. doi: 10.1016/s0360-3016(02)04613-8.
- Leinonen MK, Miettinen J, Heikkinen S, et al. Quality measures of the population-based Finnish Ccancer registry indicate sound data quality for solid malignant tumours. Eur J Cancer. 2017;77:31–39. doi: 10.1016/j.ejca.2017.02.017.
- Tripepi G, Jager KJ, Dekker FW, et al. Selection bias and information bias in clinical research. Nephron Clin Pract. 2010;115(2):c94–99. doi: 10.1159/000312871.
- Coca-Pelaz A, Rodrigo JP, Suárez C, et al. The risk of second primary tumors in head and neck cancer: a systematic review. Head Neck. 2020;42(3):456–466. doi: 10.1002/hed.26016.
- Hoxhaj I, Hysaj O, Vukovic V, et al. Occurrence of metachronous second primary cancer in head and neck cancer survivors: a systematic review and meta-analysis of the literature. Eur J Cancer Care. 2020;29(5):e13255. doi: 10.1111/ecc.13255.
- Morris LGT, Sikora AG, Patel SG, et al. Second primary cancers after an index head and neck cancer: subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J Clin Oncol. 2011;29(6):739–746. doi: 10.1200/JCO.2010.31.8311.
- Chen MC, Chen PT, Chan CH, et al. Second primary esophageal or lung cancer in patients with head and neck carcinoma in Taiwan: incidence and risk in relation to primary index tumor site. J Cancer Res Clin Oncol. 2011;137(1):115–123. doi: 10.1007/s00432-010-0865-0.
- Islami F, Tramacere I, Rota M, et al. Alcohol drinking and laryngeal cancer: overall and dose-risk relation–a systematic review and meta-analysis. Oral Oncol. 2010;46(11):802–810. doi: 10.1016/j.oraloncology.2010.07.015.
- Zuo JJ, Tao ZZ, Chen C, et al. Characteristics of cigarette smoking without alcohol consumption and laryngeal cancer: overall and time-risk relation. A meta-analysis of observational studies. Eur Arch Otorhinolaryngol. 2017;274(3):1617–1631. doi: 10.1007/s00405-016-4390-x.
- Jayasekara H, MacInnis RJ, Room R, et al. Long-term alcohol consumption and breast, upper aero-digestive tract and colorectal cancer risk: a systematic review and meta-analysis. Alcohol Alcohol. 2016;51(3):315–330. doi: 10.1093/alcalc/agv110.
- Gandini S, Botteri E, Iodice S, et al. Tobacco smoking and cancer: a meta-analysis. Int J Cancer. 2008;122(1):155–164. doi: 10.1002/ijc.23033.
- Tabuchi T, Ozaki K, Ioka A, et al. Joint and independent effect of alcohol and tobacco use on the risk of subsequent cancer incidence among cancer survivors: a cohort study using cancer registries. Int J Cancer. 2015;137(9):2114–2123. doi: 10.1002/ijc.29575.
- Ng SP, Pollard C, Kamal M, et al. Risk of second primary malignancies in head and neck cancer patients treated with definitive radiotherapy. NPJ Precis Oncol. 2019;3(1):22. doi: 10.1038/s41698-019-0097-y.
- von Kroge PR, Bokemeyer F, Ghandili S, et al. The impact of smoking cessation and continuation on recurrence and survival in patients with head and neck cancer: a systematic review of the literature. Oncol Res Treat. 2020;43(10):549–558. doi: 10.1159/000509427.
- Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6(5):963–968. doi: 10.1002/1097-0142(195309)6:5<963::AID-CNCR2820060515>3.0.CO;2-Q.
- Thomson PJ. Field change and oral cancer: new evidence for widespread carcinogenesis? Int J Oral Maxillofac Surg. 2002;31(3):262–266. doi: 10.1054/ijom.2002.0220.
- Voutilainen ME, Juhola MT. The changing epidemiology of esophageal cancer in Finland and the impact of the surveillance of Barrett’s esophagus in detecting esophageal adenocarcinoma. Int J Radiat Oncol Biol Phys. 2005;18:221–225.
- Ben Arie G, Shafat T, Belochitski O, et al. Treatment modality and second primary tumors of the head and neck. ORL J Otorhinolaryngol Relat Spec. 2021;83(6):420–427. doi: 10.1159/000513617.
- Lawson W, Som M. Second primary cancer after irradiation of laryngeal cancer. Ann Otol Rhinol Laryngol. 1975;84(6):771–775. doi: 10.1177/000348947508400607.
- Salminen EK, Pukkala E, Kiel KD, et al. Impact of radiotherapy in the risk of esophageal cancer as subsequent primary cancer after breast cancer. Int J Radiat Oncol Biol Phys. 2006;65(3):699–704. doi: 10.1016/j.ijrobp.2006.01.017.
