?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Treating hypoxic tumours remains a challenge in radiotherapy as hypoxia leads to enhanced tumour aggressiveness and resistance to radiation. As escalating the doses is rarely feasible within the healthy tissue constraints, dose-painting strategies have been explored. Consensus about the best of care for hypoxic tumours has however not been reached because, among other reasons, the limits of current functional in-vivo imaging systems in resolving the details and dynamics of oxygen transport in tissue. Computational modelling of the tumour microenvironment enables the design and conduction of virtual clinical trials by providing relationships between biological features and treatment outcomes. This study presents a framework for assessing the therapeutic influence of the individual characteristics of the vasculature and the resulting oxygenation of hypoxic tumours in a virtual clinical trial on dose painting in stereotactic body radiotherapy (SBRT) circumventing the limitations of the imaging systems.
Material and methods
The homogeneous doses required to overcome hypoxia in simulated SBRT treatments of 1, 3 or 5 fractions were calculated for tumours with heterogeneous oxygenation derived from virtual vascular networks. The tumour control probability (TCP) was calculated for different scenarios for oxygenation dynamics resulting on cellular reoxygenation.
Results
A three-fractions SBRT treatment delivering 41.9 Gy (SD 2.8) and 26.5 Gy (SD 0.1) achieved only 21% (SD 12) and 48% (SD 17) control in the hypoxic and normoxic subvolumes, respectively whereas fast reoxygenation improved the control by 30% to 50%. TCP values for the individual tumours with similar characteristics, however, might differ substantially, highlighting the crucial role of the magnitude and time evolution of hypoxia at the microscale.
Conclusion
The results show that local microvascular heterogeneities may affect the predicted outcome in the hypoxic core despite escalated doses, emphasizing the role of theoretical modelling in understanding of and accounting for the dominant factors of the tumour microenvironment.
Background
This year marks the centenary for Petry’s pioneering work on the correlation between oxygen availability and sensitivity to ionizing radiation in living cells [Citation1]. One hundred years later, hypoxia mitigation in radiotherapy is yet to be part of the clinical routine, despite a plethora of published work on the topic [Citation2]. Not only has it been repeatedly demonstrated that most solid tumours contain hypoxic areas, but several clinical studies have also confirmed the correlation between the presence of pre-treatment hypoxia and poorer outcome [Citation3]. This discrepancy between the apparent interest in the subject and the lack of practical advancement in the clinic has been a topic of discussion in itself [Citation4], and one of the main challenges frequently quoted is the gap between the spatial resolution of current in-vivo imaging methods and the microscale at which the oxygen effect occurs [Citation5,Citation6]. Hence, two parallel tracks of study on the therapeutic effects from tumour hypoxia without apparent possibility of crossing have developed: one on experimental studies of the radiobiological response with the related biological pathway characterizations at cellular level, and the other assessing clinical endpoints of treated patients based on macroscopic imaging of the tumour partial oxygen pressure, pO2, usually through positron emission tomography (PET). With a resolution in modern PET imaging on the order of millimetres, resolving the microenvironment on cellular level where the process of oxygen diffusion and consumption takes place, is not possible. Personalized and adaptive radiotherapy based on PET imaging therefore carries a degree of uncertainty of unknown magnitude with respect to actually increasing the effectiveness of the treatment. Producing the evidence for the therapeutic benefits of PET-based dose-escalation for tumour hypoxia that is necessary before its clinical implementation is therefore difficult. Exploring the difference in the treatment outcome of hypoxic tumours with respect to tumour control if different fractionation schemes and irradiation modalities are used is also a challenge. Previous modelling studies of tumour hypoxia and its dynamics used in conjunction with models for the treatment response have, indeed, indicated the importance of accounting for the effects of fractionating the dose in the attempt to overcome tumour hypoxia and led to questions regarding the use of extremely hypofractionated schedules as employed by stereotactic body radiotherapy (SBRT) in the treatment of hypoxic tumours [Citation7,Citation8]. In this context, computerised modelling of the tumour vasculature, oxygenation, and the radiobiological response to treatment not relying on information retrieved from imaging, could be a powerful tool in bridging the resolution gap and truly take hypoxia mitigation in radiotherapy from bench to bedside.
Computational modelling of the tumour vasculature and oxygenation could be achieved on multiple levels. Starting from the simulation of the vascular structures, the distribution of oxygen partial pressure on microlevel could be realistically calculated based on the diffusion of oxygen from the vessels and the consumption of oxygen by the tissue cells. By modelling the vessels as well as the resulting three-dimensional pO2 map, the dynamic nature of tumour hypoxia can be simulated and hence taken into account in e.g. subsequent modelling of the treatment response on tumour level. Hence, pending the clinical validation of the model, this approach offers the possibility to estimate the results of various treatment schedules in terms of tumour control probability (TCP), enabling the conduction of virtual clinical trials at basically zero expense apart from the computational resources. As an example, an in silico tumour characterized by different sets of vasculatures and therefore oxygenation patterns can serve as its own control when testing various treatment strategies, dose fractionation schedules, etc. This approach has the potential to link the experimental knowledge with the clinical evidence, offering a tool for the identification of what could be the most promising treatment modification strategies which would in turn save economical and temporal resources while possibly reducing the number of animals employed in pre-clinical trials.
It was the purpose of this study to demonstrate one of the possible applications of the concept of in silico clinical trials by simulating a set of tumours treated with a hypoxia dose-painting approach using an SBRT-like strategy and evaluating the treatments with respect to the tumour characteristics on the microscale.
Materials and methods
In this study, the concept of virtual clinical trials was demonstrated by 1) creating a set of realistic in silico tumours with heterogeneous oxygenation; 2) calculating the prescribed doses required to achieve a specified high level of tumour control probability (TCP) in each tumour based on the distribution of radiosensitivity values; and 3) evaluating the resulting dose distributions by calculating the TCP considering the spatial distribution of oxygenation values as well as changes therein from fast reoxygenation between fractions. In the following, these three steps are described in more detail. The entire procedure is summarized in .
Tumour models with heterogeneous and dynamic oxygenation
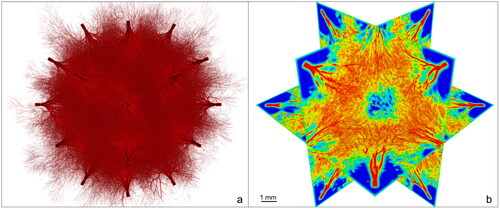
Ten in silico tumours were created by using and further developing a novel model for tumour vasculature and oxygenation [Citation9]. In this model, the spatial positioning of vessels is designed according to fractal principles with a resolution of 10 µm in the resulting oxygenation maps obtained by solving the differential equation of oxygen diffusion and consumption [Citation10]. The tumours were simulated as spheres with a diameter of 1 cm and a central hypoxic target volume (HTV) corresponding to 10% of the total target volume (TV). This can be achieved as the vascular network of vessels perfusing the tumour are generated as vascular trees penetrating the tumour volume from several directions. Variations in the microvasculature between tumours are warranted by allowing the algorithm to pick the angles between consecutive vessels and their respective length randomly from a distribution of values. As a consequence, every tumour presents a unique vasculature, while the overall oxygen distributions and macroscopic quantities, such as the density of vessels, in the HTV and in the TV-HTV are similar in all samples and in line with experimental findings [Citation11–15]. An example of the simulated tumours is shown in .
Figure 1. Computational model of the tumour vasculature. a) Vasculature perfusing a spherical tumour. b) Correspondent oxygenation (red: vessels pO2 = 40 mmHg).
For the subsequent evaluation of the dose distributions, the influence of fast reoxygenation between fractions was considered by simulating slight displacements of the microvessels (range: 0-100 µm in each direction) at every fraction followed by recalculation of the pO2 maps. The resulting oxygenation map thus had a different distribution on the microscale but the same overall level of hypoxia, i.e. no global improvement in the tumour oxygenation.
Dose prescription
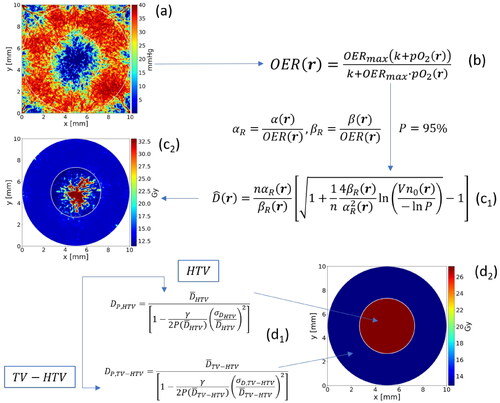
A virtual dose-painting clinical trial was conducted by first calculating the homogeneous doses required in the HTV and TV-HTV respectively to reach a level of control of 95%. This was done by means of a previously proposed radiobiological model taking into account heterogeneous radiosensitivity as introduced by for instance the tumour oxygenation [Citation16,Citation17]. Treatment regimens typical of stereotactic body radiotherapy (SBRT) were considered, consisting of 1, 3 or 5 fractions in accordance with previous studies indicating that hypofractionated schedules should be further explored in order to assess the importance of the choice of fractionation schedule for dose painting approaches [Citation7,Citation8]. In the calculation of the doses to be prescribed is illustrated. First, the pO2 maps derived from the tumour vasculature (a) are transformed into radiosensitivity maps based on the oxygen enhancement ratio (OER) (b), i.e. the dose factor needed to compensate for the increased radioresistance, as expressed by EquationEquation (1)(1)
(1) [Citation18,Citation19]:
(1)
(1)
Figure 2. Process of calculation of the prescribed doses to the HTV and TV-HTV. Starting from the oxygenation map (a), the correspondent radiosensitivity map is produced (b). accounting for an aimed probability of control p = 95% the theoretical dose distribution is computed (c1, c2); the doses are then processed to obtain a constant dose within the volumes if interest (d1, d2), obtaining ideally a TCP close to the aimed one.
Here represents the maximum oxygen enhancement ratio corresponding to the absence of oxygen and
is a reaction constant.
Then, dose maps are calculated based on tumour control probability modelling considering the radiosensitivity values on voxel level (c1). The resulting maps (c2) will be highly heterogeneous and not clinically feasible to deliver. Their ability to achieve a specified level of TCP will also be extremely sensitive to changes in the radiosensitivity on the microscale as could be expected to occur from fast reoxygenation between fractions. The last step of the dose prescription process is therefore to calculate the required homogeneous dose to tumour subvolumes such as the HTV by considering the initial distribution of radiosensitivity in the volume, without regard to the spatial information (d1 and d2). By basing the dose calculation on a distribution of radiosensitivity values, the effect from inter-fraction fast reoxygenation is inherently accounted for by the width of the distribution [Citation17].
Radiobiological evaluation
To evaluate the effectiveness of the prescribed doses in relation to the actual tumour oxygenation and its dynamics throughout the treatment, the surviving fraction (SF) was calculated on voxel level at each fraction by using the linear quadratic model [Citation20,Citation21] including the dose-modifying effect from the oxygenation:
(2)
(2)
where
and
are the radiosensitivity parameters (generic tumour parameters 0.35 Gy–1 and 0.035 Gy–2, respectively, being used in this study),
is the dose prescribed to the voxel
belonging to the target volume (TV-HTV or HTV), and
is the oxygen enhancement ratio at voxel
By remodelling the pO2 distribution on voxel level between fractions as described in 1), the effect from fast reoxygenation on voxel level was included in the calculations. For the sake of comparison, a tumour oxygenation that remained static throughout the treatment was also considered. The prescribed treatments were subsequently evaluated on treatment volume level by using the Poisson-based TCP model:
(3)
(3)
With being the treatment volumes (TV-HTV or HTV) and
being the initial clonogenic density equal to 106 cells/cm3 homogeneously distributed in the tumour.
Results
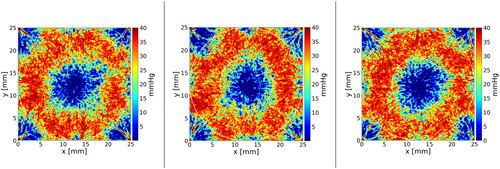
presents the baseline hypoxic fractions for the HTVs and the (TV-HTV)s of the ten tumours, i.e. the fraction of voxels where the pO2 was below 10 mmHg. The average hypoxic fraction resulted to be 44.3% ± 5.7% for the HTV and 1.3% ± 0.3% for the TV-HTV. Examples of cross-sections through the oxygen partial pressure maps for three tumours are shown in . The SBRT doses prescribed to the ten tumours are shown in . The prescribed doses to the HTV were 28.8 Gy ± 1.9 Gy, 41.9 Gy ± 2.8 Gy and 48.3 Gy ± 3.2 Gy for the single, 3-, and 5-fractionated treatments, respectively. The corresponding prescribed doses for the TV-HTV were 18.0 Gy ± 0.0 Gy, 26.5 Gy ± 0.1 Gy and 30.7 Gy ± 0.1 Gy, displaying a lower degree of uncertainty reflecting the more homogeneous radiosensitivity in this volume. The values of the probability of control for the HTV and TV-HTV resulting from delivering these prescribed doses to the ten tumours are depicted in the boxplots of , assuming both static oxygenation and fast reoxygenation between fractions as described in the Materials and Methods.
Figure 4. Prescribed doses (a) and probabilities of control (b, c) for the HTV and the TV-HTV subvolumes of the ten simulated tumours; (b) refers to the case of constant oxygenation between fractions, while (c) includes the assumption of fast reoxygenation at every fraction after the first.
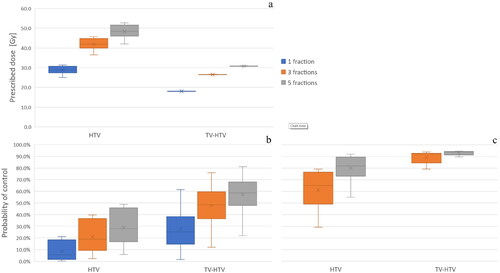
Table 1. Hypoxic fractions for the ten tumours (tum -), for the hypoxic target volume (HTV) and the remainder of the target volume (TV-HTV). pO2 threshold for hypoxia = 10 mmHg.
The radiosurgery case (single-fraction SBRT) yielded a low control for both volumes, while for the 3-fractionated treatment the control probability increases from 20.9% ± 12.9% to 61.3% ± 15.1% and from 47.8% ± 17.4% to 89.2% ± 4.8% for the HTV and the TV-HTV, respectively, when fast reoxygenation is simulated. Similarly, for the 5-fractions case the control probability was raised from 28.9% ± 14.2% to 80.1% ± 10.5% and from 57.3% ± 15.9% to 93.0% ± 1.8% for the HTV and the TV-HTV, respectively.
Discussion
The classical 4th R of radiotherapy, reoxygenation, highlights the long-known importance of the role of oxygen in tumours [Citation1]. The potential gain in tumour control when escalating the dose within the radioresistant subvolumes of tumours has been repeatedly tested in several clinical trials generally based on functional PET imaging [Citation5]. It is expected that the technological progress that led to the development of the hybrid machines such as MR-Linac and PET-Linac, allowing not only tumour tracking during radiation delivery but also imaging tumour features related to resistance to the treatment, would prompt new clinical trials in the future with even better outcomes [Citation22,Citation23]. In the meanwhile, however, while such studies have provided support for a benefit from higher doses to hypoxic tumours, they have also demonstrated the inherent complexity in the analysis of the underlying mechanisms. In support of this difficulty, several studies approached the subject theoretically, using computational and radiobiological modelling of the tumour microenvironment and response [Citation24–26]. The novelty of the present study consists of the use of a complex model of the vasculature and oxygenation on the microscale, while expanding its potential to the realization of a virtual clinical trial and hence merging the advantages of the two approaches.
The dose escalation protocol was based on an initial target control probability of 95%, independent of the inherent technological limits that hinder the delivery of the initially prescribed doses and based solely on the heterogeneous radiosensitivity distribution. In the method employed herein, the subsequent consideration of this limit is reflected by the calculation of uniform doses to tumour subvolumes (hypoxic and normoxic), compensating for the reduction of the probability of control due to those voxels that present higher radioresistance and would receive an insufficient amount of dose [Citation17].
However, the method cannot ensure the specified control when considering the spatial heterogeneity in oxygenation in relation to the dose distribution, which is reflected by the drop in control probability for the static oxygenation case (). Indeed, the treatment ensured no control in the scenario with constant oxygenation between fractions with the failure mostly pronounced in the HTV (average control probability below 30%) and particularly evident for the radiosurgical treatment consisting of only one fraction. Given the intrinsically abnormal nature of tumours, some small areas of lower vasculature likely undetectable by in-vivo functional imaging will always endanger tumour control in PET-based dose-painting approaches. As has been repeatedly demonstrated in previous work [Citation7,Citation24] and in the present study as well, this can be mitigated by accounting for fast reoxygenation between fractions which will increase the effective radiosensitivity on the microscale (). Furthermore, the graphs in display a wide range in the control probabilities obtained for the different tumours, an effect from the microscopic variability in the patterns of oxygenation generated. Indeed, despite the prescribed doses to the ten TV-HTV volumes being nearly constant (), their responses depend on the local arrangement of the vessels and their spatial uniformity across the volume. This becomes particularly relevant in the transition areas from hypoxia to normoxia, where a clear boundary as defined by the constant prescribed doses is biologically fictional, but which more realistically would be covered by a dose gradient in stereotactic treatments similar to the red-shell concept proposed for normal tissues [Citation27]. Hence the estimates of the control in the TV-HTV volumes in this study likely constitute an underestimate caused by the hypoxic cells contained in the transition region. However, the results lend support to the importance of choosing the appropriate ‘brush size’ relative to the underlying microphysiology in dose-painting approaches [Citation28,Citation29].
In regard to the range of tumour vasculature patterns presented in this virtual clinical trial, it is remarkable to note that the spread of tumour control probabilities obtained does not reflect the uncertainty related to individual responses to treatment as would be the case in an actual clinical trial. On the contrary, the data presented in this work allows for a direct generalization of the results, under the assumptions of the model. Conversely to the nature of a real-life clinical trial where patients are randomized between the test and the control branches, in an in silico trial every ‘patient’ can be subjected to all treatment branches and all fractionations allowing for a direct assessment of the benefits of the test treatment for the individual tumour vasculature and oxygenation. Furthermore, by simulating not only the oxygenation but also the vessels, this model opens up for considering not only the tumour oxygenation, but also the radiation effects on the vasculature itself e.g. the so-called vascular effect [Citation11,Citation30,Citation31]. Following clinical validation of the model, the transport of not only blood could be simulated in the vessels, but also of radiotracers, radiosensitizers, immunotherapeutic drugs etc., provided accurate parameter values would become available. This offers a unique opportunity to actually model the distribution and uptake of e.g. a PET-hypoxia tracer with full knowledge of the underlying microenvironment with respect to the vasculature and oxygenation of the tumour. It should be noted that while a spherical geometry was assumed for the tumours simulated in this study for simplicity, in principle any shape could be considered by the model. In a full virtual clinical trial, the target volumes as defined on the planning CT would be imported and used. Furthermore, the effect of the escalation of the dose on the normal tissue would also be taken into account in a virtual trial. Thus, normal tissue complication probability calculations for the organs at risk would be included in the assessment of the feasibility of a treatment plan involving the escalation of the dose, and only optimised feasible plans would be actually considered in the virtual trial. Needless to say, these applications critically depend on the clinical validation of the model, which could be done by comparing the outcome predicted by the model with the actual rates of local control in patients treated with the standard protocol, as has been previously discussed [Citation24]. This process would also serve as to refine and ultimately perfect the model parameters to improve its reliability with respect to simulating the clinical scenario.
In summary, a novel and complex model of tumour vasculature and oxygenation was incorporated in a response prediction model in order to demonstrate the concept of virtual clinical trials. In line with previous extensive modelling work on the subject, this study demonstrated the challenge in overcoming the radiation resistance in areas with persistent hypoxia, highlighting the importance of allowing for fast reoxygenation by fractionating the treatment of hypoxic tumours. This study furthermore offers an attractive perspective on the use of computational resources, bringing the concept of moving from bench to bedside to a new level by simulating not only the biological features of the individual patient, but also the same standard procedure – the clinical trial – adopted when researching new and potentially more effective treatments. Although those characteristics that often make the specific patient response unpredictable can hardly be programmed within a machine, the use of different virtual and ideal patients in a trial where the study of the effect of only one single variable is permitted could uncover precious information otherwise not achievable. This in turn has the potential to save both money and animal lives in preclinical trials, by identifying promising new treatment strategies on which further resources should be focused, effectively shortening the time to clinical introduction of new treatment regimes. For hypoxic tumours in particular, this could be the virtual step needed in order to finally bring hypoxia-mitigation into the routine clinical setting.
Acknowledgements
The computations were partly enabled by resources provided by the National Academic Infrastructure for Supercomputing in Sweden (NAISS) and the Swedish National Infrastructure for Computing (SNIC) at ‘PDC Center for High Performance Computing, KTH Royal Institute of Technology’ partially funded by the Swedish Research Council through grant agreements no. 2022-06725 and no. 2018-05973.
Disclosure statement
No potential conflict of interest was reported by the authors.
The authors report there are no competing interests to declare.
Data available on request from the authors.
Additional information
Funding
References
- Hall EJ, Giaccia AJ. Radiobiology for the radiologist. 6th ed. Philadelphia (PA): Lippincott Williams & Wilkins; 2006.
- Horsman MR, Overgaard J. The impact of hypoxia and its modification of the outcome of radiotherapy. J Radiat Res. 2016;57(Suppl 1):i90–i98. doi:10.1093/jrr/rrw007.
- Nordsmark M, Bentzen SM, Rudat V, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol. 2005;77(1):18–24. doi:10.1016/j.radonc.2005.06.038.
- Overgaard J. Hypoxic radiosensitization: adored and ignored. J Clin Oncol. 2007;25(26):4066–4074. doi:10.1200/JCO.2007.12.7878.
- Grimes DR, Warren DR, Warren S. Hypoxia imaging and radiotherapy: bridging the resolution gap. Br J Radiol. 2017;90(1076):20160939. doi:10.1259/bjr.20160939.
- Busk M, Overgaard J, Horsman MR. Imaging of tumor hypoxia for radiotherapy: current status and future directions. Semin Nucl Med. 2020;50(6):562–583. doi:10.1053/j.semnuclmed.2020.05.003.
- Lindblom E, Antonovic L, Dasu A, et al. Treatment fractionation for stereotactic radiotherapy of lung tumours: a modelling study of the influence of chronic and acute hypoxia on tumour control probability. Radiat Oncol. 2014;9(1):149. doi:10.1186/1748-717X-9-149.
- Lindblom E, Dasu A, Toma-Dasu I. Optimal fractionation in radiotherapy for non-small cell lung cancer–a modelling approach. Acta Oncol. 2015;54(9):1592–1598. doi:10.3109/0284186X.2015.1061207.
- Schiavo F, Kjellsson Lindblom E, Toma-Dasu I. Towards the virtual tumor for optimizing radiotherapy treatments of hypoxic tumors: a novel model of heterogeneous tissue vasculature and oxygenation. J Theor Biol. 2022;547:111175. doi:10.1016/j.jtbi.2022.111175.
- Daşu A, Toma-Daşu I, Karlsson M. Theoretical simulation of tumour oxygenation and results from acute and chronic hypoxia. Phys Med Biol. 2003;48(17):2829–2842. doi:10.1088/0031-9155/48/17/307.
- Lindblom EK, Hui S, Brooks J, et al. Radiation-induced vascular damage and the impact on the treatment outcome of stereotactic body radiotherapy. Anticancer Res. 2019;39(6):2721–2727. doi:10.21873/anticanres.13398.
- Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26(2):225–239. doi:10.1007/s10555-007-9055-1.
- Kallinowski F, Zander R, Hoeckel M, et al. Tumor tissue oxygenation as evaluated by computerized-pO2-histography. Int J Radiat Oncol Biol Phys. 1990;19(4):953–961. doi:10.1016/0360-3016(90)90018-f.
- Pogue BW, Paulsen KD, O'Hara JA, et al. Estimation of oxygen distribution in RIF-1 tumors by diffusion model-based interpretation of pimonidazole hypoxia and eppendorf measurements. Radiat Res. 2001;155(1):15–25. doi:10.1667/0033-7587(2001)155[0015:EOODIR.2.0.CO;2]
- Daşu A, Toma-Daşu I. Vascular oxygen content and the tissue oxygenation–a theoretical analysis. Med Phys. 2008;35(2):539–545. doi:10.1118/1.2830382.
- Brahme A. Dosimetric precision requirements in radiation therapy. Acta Radiol Oncol. 1984;23(5):379–391. doi:10.3109/02841868409136037.
- Toma-Daşu I, Daşu A, Brahme A. Dose prescription and optimisation based on tumour hypoxia. Acta Oncol. 2009;48(8):1181–1192. doi:10.3109/02841860903188643.
- Alper T, Howard-Flanders P. Role of oxygen in modifying the radiosensitivity of E. coli B. Nature. 1956;178(4540):978–979. doi:10.1038/178978a0.
- Toma-Dasu I, Dasu A. Modelling tumour oxygenation, reoxygenation and implications on treatment outcome. Comput Math Methods Med. 2013;2013:141087–141089. doi:10.1155/2013/141087.
- Barendsen GW. Dose fraction, dose rate and iso-effect relationships for normal tissue responses. Int J Radiat Oncol Biol Phys. 1982;8(11):1981–1997. doi:10.1016/0360-3016(82)90459-x.
- Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62(740):679–694. doi:10.1259/0007-1285-62-740-679.
- Dubec MJ, Buckley DL, Berks M, et al. First-in-human technique translation of oxygen-enhanced MRI to an MR linac system in patients with head and neck cancer. Radiother Oncol. 2023;183:109592. doi:10.1016/j.radonc.2023.109592.
- Oderinde OM, Shirvani SM, Olcott PD, et al. The technical design and concept of a PET/CT linac for biology-guided radiotherapy. Clin Transl Radiat Oncol. 2021;29:106–112. doi:10.1016/j.ctro.2021.04.003.
- Kjellsson Lindblom E, Ureba A, Dasu A, et al. Impact of SBRT fractionation in hypoxia dose painting - Accounting for heterogeneous and dynamic tumor oxygenation. Med Phys. 2019;46(5):2512–2521. doi:10.1002/mp.13514.
- Chvetsov AV, Rajendran JG, Zeng J, et al. Theoretical effectiveness of cell survival in fractionated radiotherapy with hypoxia-targeted dose escalation. Med Phys. 2017;44(5):1975–1982. doi:10.1002/mp.12177.
- Köthe A, Bizzocchi N, Safai S, et al. Investigating the potential of proton therapy for hypoxia-targeted dose escalation in non-small cell lung cancer. Radiat Oncol. 2021;16(1):199. doi:10.1186/s13014-021-01914-2.
- Yang J, Fowler JF, Lamond JP, et al. Red shell: defining a high-risk zone of normal tissue damage in stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2010;77(3):903–909. doi:10.1016/j.ijrobp.2009.12.069.
- Ureba A, Kjellsson Lindblom E, Toma-Dasu I, et al. Assessment of the probability of tumour control for prescribed doses based on imaging of oxygen partial pressure. Adv Exp Med Biol. 2021;1269:185–190.
- Schiavo F, Toma-Dasu I, Lindblom EK. Perfusion-Limited hypoxia determines the outcome of radiation therapy of hypoxic tumours. Adv Exp Med Biol. 2022;1395:249–254. doi:10.1007/978-3-031-14190-4_41.
- Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300(5622):1155–1159. doi:10.1126/science.1082504.
- Lindblom EK, Dasu A, Toma-Dasu I. Hypoxia induced by vascular damage at high doses could compromise the outcome of radiotherapy. Anticancer Res. 2019;39(5):2337–2340. doi:10.21873/anticanres.13350.