Introduction
The global incidence of obesity has tripled since 1975, reflecting an alarming health crisis with more than 1.9 billion adults classified as overweight in 2016 [Citation1]. Parallel to this trend, increasing evidence suggests a compelling association between a high body mass index (BMI ≥ 25 kg/m2) and an array of common malignancies including esophageal, stomach, colon, rectal, liver, gallbladder, pancreatic cancers, post-menopausal breast cancer, cervical and uterine cancers, ovarian cancer, kidney cancer, non-Hodgkin lymphoma, multiple myeloma, and leukemia [Citation2–6].
Furthermore, up to half of all cancer cases could potentially be prevented, given that many oncogenic stimulators, such as overweight and obesity, are modifiable [Citation7,Citation8]. Therefore, understanding the link between elevated BMI and cancer is crucial for effective prevention strategies. Despite several earlier cohort studies confirming this association, the majority of these investigations are over a decade old, suggesting a need for more contemporary analysis.
In light of this, our study aims to provide updated evidence on this relationship by investigating the association between increasing BMI and the incidence of 22 common malignancies. To achieve this, we utilize data from a nationwide cohort with a follow-up duration of up to 55 years.
Methods
We performed a registry-based cohort study, leveraging height and weight measurements from the Norwegian Tuberculosis Screening Program (NTSP) and cancer diagnosis data from the Norwegian Cancer Registry (CRN). The CRN, which mandates reporting of new cancer cases, maintains a high level of data completeness [Citation9]. From 1963 to 1975, the NTSP conducted an unselected nationwide survey, wherein healthcare providers registered participants height and weight [Citation5].
Study population
Out of a population of approximately four million, a total of 1,911,598 individuals (aged 7 to 99 years) participated in the NTSP survey. We utilized the ICD10 lexicon for cancer categorization. Individuals were followed from the NTSP screening date until cancer diagnosis, 75 years of age, emigration, or the end of follow-up (December 31st, 2018).
Our study included all individuals aged 16 to 75 years at the time of NTSP screening. Individuals above the age of 75 were excluded as cancers developed this late are more likely to be sporadic and not attributable to elevated BMI earlier in life. We linked the study cohort to the CRN using personal identification numbers assigned to all Norwegian inhabitants. We obtained data on the first primary diagnosis among 22 common cancers previously reported to be associated with BMI [Citation2].
Exclusions
We excluded individuals younger than 16 or older than 75 years, those with BMI under 15 or over 50 kg/m2, those diagnosed with cancer prior to or within one year after measurement, individuals with short stature (women <150 cm and men <161 cm), those with missing data on height or weight, and those with no follow-up time (Supplementary Figure S1).
Statistical analysis
Standard descriptive statistics were presented, including mean and standard deviations (SD) for continuous variables and absolute and relative frequencies for categorical variables. We modeled the association between BMI and cancer using multivariable Cox proportional hazards regression models, adjusted for sex and age groups at the time of screening. We handled BMI as a continuous variable, assuming log-linearity. This allowed us to present estimated hazard ratios (HR) with 95% confidence intervals (CI) per 5 kg/m2 increase in BMI. We used attained age as the time scale.
A subgroup analysis was conducted on individuals aged 16–29 years (age group 1) to investigate whether individuals with high BMI early in life presented a different risk profile compared to those with high BMI later in life. Sensitivity analyses were performed, including controlling for height, removing underweight individuals, and commencing follow-up two- and five-years post-BMI measurement. These adjustments did not significantly alter the results (data not shown).
The study was approved by the Regional Committee for Medical and Health Research in South-Eastern Norway (REC#: 2018/670), Norwegian Institute of Public Health, Cancer Registry of Norway, Norwegian Tax Administration (which administers the National Population Registry), and Oslo University Hospital data protection officer (SD0759843). The Regional Ethical Committee for Medical and Health Research waived consent for using register data.
Results
Our cohort comprised 1,723,692 individuals, of which 894,611 were women (51.9%) (Supplementary Figure S1). Cohort characteristics are presented in . Mean follow-up was 32.5 (SD 14.8) years, extending to a maximum of 55 years. During this period, 481,202 individuals received a diagnosis of a first primary cancer. Follow-up length and age at cancer diagnosis are provided in Supplementary Table S1. At study conclusion, 565,519 individuals remained alive. HR with 95% CI for cancer incidence corresponding to each 5 kg/m2 increase in BMI are given in .
Figure 1. Forest plots illustrating hazard ratios (HR) and corresponding 95% confidence intervals (CI) for the risk of Gastrointestinal cancers across different age groups, considering both sexes combined (a), men (B), and women (C). each plot demonstrates the effect of a 5 kg/m2 increase in body-mass index (BMI) on cancer risk, with BMI modelled as a linear variable. The number of cancer cases are indicated within brackets. Age 16-29 was at time of BMI measurement.
▪ Both sexes.
• Men.
♦ Women.
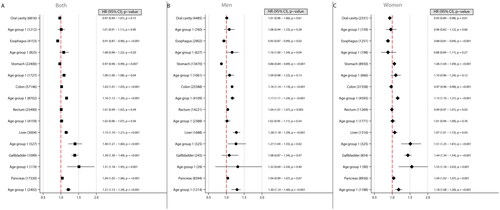
Figure 2. Forest plots illustrating hazard ratios (HR) and corresponding 95% confidence intervals (CI) for the risk of Gender specific cancers across different age groups. The plot demonstrates the effect of a 5 kg/m2 increase in body-mass index (BMI) on cancer risk, with BMI modelled as a linear variable. The number of cancer cases are indicated within brackets. Age 16-29 was at time of BMI measurement.
• Men.
♦ Women.
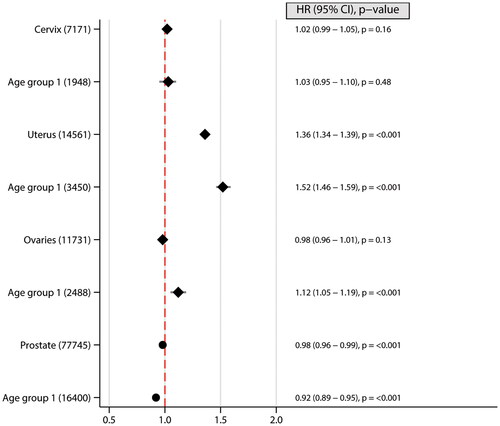
Figure 3. Forest plots illustrating hazard ratios (HR) and corresponding 95% confidence intervals (CI) for the risk of other cancers across different age groups, considering both sexes combined (A), men (B), and women (C). Each plot demonstrates the effect of a 5 kg/m2 increase in body-mass index (BMI) on cancer risk, with BMI modelled as a linear variable. The number of cancer cases are indicated within brackets. Age 16–29 was at time of BMI measurement.
▪ Both sexes.
• Men.
♦ Women.
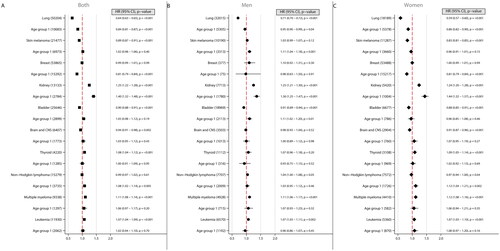
Table 1. Characteristics of the study cohort from the Norwegian tuberculosis screening program 1963–1975.
Gastrointestinal cancers
Liver cancer was associated with an increased risk for each 5 kg/m2 BMI increase in both sexes. This association was strongest in women whose BMI increased in early adulthood (age 16–29 at time of screening). In men, an increased risk was identified for colon cancer, particularly if the BMI increase occurred in early adulthood. Higher BMI was also associated with an increased risk for rectal cancer; however, no statistically significant data were observed for rectal cancer in the youngest male cohort. In women, higher BMI was associated with increased risk of colon cancer confined to those younger than 30 at the time of screening.
Higher BMI in women was associated with an increased risk for stomach, gallbladder, and pancreatic cancer, especially those in early adulthood. Men with increased BMI in early adulthood had an increased risk for pancreatic cancer risk. Higher BMI was negative associated to esophageal and stomach cancer in men, oral cavity and esophageal cancer in women ().
Gender-specific cancers
Higher BMI was associated with an increased risk of uterine cancer, particularly when this increase occurred in early adulthood. Similar positive associations were also seen in women in early adulthood for ovarian cancer. In men, however, higher BMI was associated with reduced prostate cancer risk ().
Other cancer types
Higher BMI was associated with an increased risk of kidney cancer, multiple myeloma, and leukemia in both sexes, particularly those in early adulthood in regard to kidney cancer. Men in early adulthood had an increased risk for skin melanoma and urinary bladder cancer with increasing BMI, but no association was found in women. In women, higher BMI was associated with increased risk for thyroid cancer, and non-Hodgkin lymphoma in women in early adulthood (age 16–29 at time of screening). Lung cancer was inversely associated with higher BMI in both genders ().
Discussion
Our study, encompassing an exceptional number of cancer cases, enhances the existing evidence linking BMI to several prevalent malignancies [Citation2–6,Citation10,Citation11]. Fifteen of the 22 cancer entities investigated demonstrated an increased risk with a 5 kg/m2 increase in BMI, which concurs with previously published literature. However, a primary finding of our study is the amplified lifetime cancer risk in individuals whose BMI was high during early adulthood (age 16–29 at time of screening). This augmented risk was observed for colon, liver, gallbladder, pancreatic, and kidney cancers, alongside non-Hodgkin lymphoma in both sexes. Furthermore, the trend held true for uterine and ovarian cancers in women, alongside urinary bladder cancer and skin melanoma in men. The notion of overweight and obesity in early adulthood precipitating a heightened cancer risk in later life, especially in women, aligns with conclusions drawn from earlier meta-analyses [Citation12,Citation13].
While the connection between obesity and cancer risk is not fully explored, several plausible mechanisms have been suggested. These include chronic low-grade inflammation interacting with endocrine homeostasis—encompassing insulin signaling pathways, insulin-like growth factor 1, adipokines, and sex hormones—which are all dysregulated in obesity, potentially fostering an increased cancer risk [Citation10,Citation14]. The heightened risk identified in young individuals with high BMI may be attributed to the extended duration of exposure to these potential oncogenic effects [Citation15].
Sex differences emerged in our findings, revealing distinctive patterns of BMI-associated cancer risk among men and women. These disparities may stem from a complex interplay of hormonal, metabolic, and lifestyle factors [Citation14,Citation16]. For example, estrogen, which is known to influence fat distribution, can have differential effects on cancer development depending on its concentration and the tissue context [Citation17]. In premenopausal women, high BMI is often linked to lower circulating estrogen levels, which may contribute to the reduced risk of certain cancers such as premenopausal breast cancer [Citation3,Citation18,Citation19]. On the other hand, adipose tissue can act as an important source of postmenopausal estrogen, possibly leading to either an increased or decreased risk of certain cancers [Citation20]. It is hypothesized that estrogen might potentially provide protection against colon cancer, a potential explanation to our finding of increased risk of colon cancer only in women with high BMI in early adulthood [Citation5,Citation21].
Lifestyle factors, such as diet and physical activity, which can differ significantly between sexes and cultures, may also play a role [Citation22].
In our study, a decreased risk of lung cancer was observed in association with increasing BMI. This inverse relationship is likely influenced by the lack of data on smoking status [Citation23]. We also found inverse associations for esophageal cancer in both genders, prostate cancer and stomach cancer in men, and cancer of the oral cavity in women. Bhaskaran et al. identified that a low BMI may serve as a risk factor for oral cavity, esophageal and stomach cancer. This relationship was primarily evident in smokers, except in the case of esophageal cancer [Citation2]. Renehan et al. discovered an increased risk for esophageal adenocarcinoma with higher BMI, and a reversed effect for squamous cell carcinoma [Citation6]. Additionally, our findings highlighted a reduced risk of prostate cancer in individuals with high BMI. Potential explanatory factors might include the roles of hormones, such as testosterone and leptin, as well as the influence of smoking [Citation2,Citation24,Citation25]. Our results might have shown different patterns if information on smoking status and histological subtypes were available.
Future research should aim to unravel the precise mechanisms driving the association between BMI and cancer risk. This includes exploring the roles of adipokines, inflammatory markers, insulin resistance, and sex hormones. Investigating how these factors interact with genetic predispositions and environmental exposures could also be crucial. Additionally, research should focus on dissecting the effects of specific aspects of adiposity, such as visceral vs. subcutaneous fat and the role of weight changes over the life course, to offer more nuanced insights into obesity-related cancer risk. Such studies can help inform targeted prevention strategies, contribute to individualized risk assessment, and potentially identify novel therapeutic targets.
In a broader context, the elevated cancer risk observed in young adults with high BMI holds significant socioeconomic implications and could prove especially pertinent in the development of cancer prevention strategies. This evidence underscores the need for public health initiatives, potentially including preventative programs targeting younger age groups and cancer surveillance protocols tailored for young adults with obesity in an attempt to mitigate the escalating burden of obesity-related cancers. This dual-pronged approach could represent a tangible step towards mitigating the cancer risk associated with high BMI, particularly when it manifests during early adulthood. The effect of such interventions could help not only reduce the individual burden of disease, but also lessen the overall healthcare and societal costs associated with cancer.
Certain limitations must be acknowledged in the interpretation of our study. Absence of data on potential confounding factors like smoking, alcohol consumption, comorbidities, family history of cancer, menopausal status, and medication use may impact our findings. Additionally, we did not consider waist circumference, waist-hip ratio, or histological subtypes.
BMI was measured only once and was extrapolated as a constant proxy for BMI throughout the study period. However, BMI trends show increasing values over time and with age [Citation26]. Early-life obesity is often associated with sustained or increasing weight over time, suggesting an initial high BMI can be a reliable proxy for continuous high BMI.
The obesity rate was 9% during the NTSP screening, compared to the current rate of about 23% in Norway [Citation5]. This discrepancy might lead to an underestimation of obesity-related cancer risks in our study. Our observed associations may not remain significant once adjustments for confounders are applied. Due to the large sample size, even modest effects become statistically significant, which is a factor that needs careful consideration when interpreting our findings.
Despite these limitations, our study possesses several strengths. The extensive data set allowed us to compare normal weight individuals with those having higher BMI for even rare types of cancer. Another strength lies in the lengthy follow-up period of up to 55 years, during which 28% of the study cohort were diagnosed with a cancer. This extensive time-frame strengthens the credibility of our findings and provides valuable insights into the long-term impact of BMI on cancer risk.
Conclusion
This study provides compelling evidence linking high BMI to an elevated risk for 15 of 22 cancers studied. It also emphasizes the association between high BMI in early adulthood and increased lifetime cancer risk for both genders, underlining the importance of healthy weight management from early adulthood as a preventative measure against certain types of cancer.
Supplemental Material
Download MS Word (18.8 KB)Supplemental Material
Download PDF (70.2 KB)Data availability statement
Aggregated anonymous data may be available upon request and approval of Regional Committees for Medical and Health Research Ethics.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- World Health Organization. Obesity and overweight 2021 [cited 2022 Apr 13]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- Bhaskaran K, Douglas I, Forbes H, et al. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5·24 million UK adults. Lancet. 2014;384(9945):755–765. doi:10.1016/S0140-6736(14)60892-8.
- Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer—viewpoint of the IARC working group. N Engl J Med. 2016;375(8):794–798. doi:10.1056/NEJMsr1606602.
- Saeed U, Myklebust T, Robsahm TE, et al. Body mass index and pancreatic adenocarcinoma: a nationwide registry-based cohort study. Scand J Surg. 2022;112(1):11–21. doi:10.1177/14574969221127530.
- Saeed U, Myklebust T, Robsahm TE, et al. Risk and survival in colorectal cancer with increasing BMI: a nationwide population-based cohort study. Colorectal Dis. 2022;25(3):375–385. doi:10.1111/codi.16367.
- Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi:10.1016/S0140-6736(08)60269-X.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660.
- GBD 2019 Cancer Risk Factors Collaborators. The global burden of cancer attributable to risk factors, 2010-19: a systematic analysis for the global burden of disease study 2019. Lancet. 2022;400(10352):563–591.
- Larsen IK, Småstuen M, Johannesen TB, et al. Data quality at the cancer registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009;45(7):1218–1231. doi:10.1016/j.ejca.2008.10.037.
- Sung H, Siegel RL, Torre LA, et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J Clin. 2019;69(2):88–112. doi:10.3322/caac.21499.
- Recalde M, Davila-Batista V, Díaz Y, et al. Body mass index and waist circumference in relation to the risk of 26 types of cancer: a prospective cohort study of 3.5 million adults in Spain. BMC Med. 2021;19(1):10. doi:10.1186/s12916-020-01877-3.
- Hidayat K, Du X, Shi BM. Body fatness at a young age and risks of eight types of cancer: systematic review and meta-analysis of observational studies. Obes Rev. 2018;19(10):1385–1394. doi:10.1111/obr.12705.
- Han X, Stevens J, Truesdale KP, et al. Body mass index at early adulthood, subsequent weight change and cancer incidence and mortality. Int J Cancer. 2014;135(12):2900–2909. doi:10.1002/ijc.28930.
- Argyrakopoulou G, Dalamaga M, Spyrou N, et al. Gender differences in Obesity-Related cancers. Curr Obes Rep. 2021;10(2):100–115. doi:10.1007/s13679-021-00426-0.
- Arnold M, Freisling H, Stolzenberg-Solomon R, et al. Overweight duration in older adults and cancer risk: a study of cohorts in Europe and the United States. Eur J Epidemiol. 2016;31(9):893–904. doi:10.1007/s10654-016-0169-z.
- Kim HI, Lim H, Moon A. Sex differences in cancer: epidemiology, genetics and therapy. Biomol Ther (Seoul). 2018;26(4):335–342. doi:10.4062/biomolther.2018.103.
- Cleary MP, Grossmann ME. Minireview: obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150(6):2537–2542. doi:10.1210/en.2009-0070.
- Hidayat K, Yang CM, Shi BM. Body fatness at a young age, body fatness gain and risk of breast cancer: systematic review and meta-analysis of cohort studies. Obes Rev. 2018;19(2):254–268. doi:10.1111/obr.12627.
- Freeman EW, Sammel MD, Lin H, et al. Obesity and reproductive hormone levels in the transition to menopause. Menopause. 2010;17(4):718–726. doi:10.1097/gme.0b013e3181cec85d.
- Johansson Å, Schmitz D, Höglund J, et al. Investigating the effect of estradiol levels on the risk of breast. J Endocr Soc. 2022;6(8):bvac100.
- Das PK, Saha J, Pillai S, et al. Implications of estrogen and its receptors in colorectal carcinoma. Cancer Med. 2023;12(4):4367–4379. doi:10.1002/cam4.5242.
- Jackson SS, Marks MA, Katki HA, et al. Sex disparities in the incidence of 21 cancer types: quantification of the contribution of risk factors. Cancer. 2022;128(19):3531–3540. doi:10.1002/cncr.34390.
- Sanikini H, Yuan JM, Butler LM, et al. Body mass index and lung cancer risk: a pooled analysis based on nested case-control studies from four cohort studies. BMC Cancer. 2018;18(1):220. doi:10.1186/s12885-018-4124-0.
- Loh NY, Wang W, Noordam R, et al. Obesity, fat distribution and risk of cancer in women and men: a mendelian randomisation study. Nutrients. 2022;14(24):5259. doi:10.3390/nu14245259.
- Suarez Arbelaez MC, Nackeeran S, Shah K, et al. Association between body mass index, metabolic syndrome and common urologic conditions: a cross-sectional study using a large multi-institutional database from the United States. Ann Med. 2023;55(1):2197293.
- WHO. 2020. Obesity and overweight: WHO. Updated 01.04.2020 [cited 2022 Jul 06]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
