Abstract
Background
A diagnostic work-up leading to a lung cancer diagnosis is a severely stressful experience that may impact tumor progression. Yet, prospective data are scarce on psychological and biological components of stress at the time of lung cancer diagnosis. The aim of this study was to assess pre-to-post diagnosis change in psychological distress and urinary excretion of catecholamines in patients with suspected lung cancer.
Methods
Participants were 167 patients within the LUCASS study, recruited at referral for suspected lung cancer to University Hospitals in Iceland and Sweden. Patients completed questionnaires on perceived distress (Hospital Anxiety and Depression Scale, HADS) before and after diagnosis of lung cancer or a non-malignant origin. A subpopulation of 85 patients also provided overnight urine for catecholamine analysis before and at a median of 24 days after diagnosis but before treatment.
Results
A lung cancer diagnosis was confirmed in 123 (73.7%) patients, with a mean age of 70.1 years. Patients diagnosed with lung cancer experienced a post-diagnosis increase in psychological distress (p = 0.010), while patients with non-malignant lung pathology showed a reduction in distress (p = 0.070). Both urinary epinephrine (p = 0.001) and norepinephrine (p = 0.032) levels were higher before the diagnosis among patients eventually diagnosed with lung cancer compared to those with non-malignant lung pathology. We observed indications of associations between pre-to-post diagnosis changes in perceived distress and changes in urinary catecholamine levels.
Conclusion
Receiving a lung cancer diagnosis is associated with an increase in psychological distress, while elevated catecholamine levels are evident already before lung cancer diagnosis.
Introduction
Diagnostic work-up for cancer and receiving a definite cancer diagnosis is a severely stressful experience [Citation1–4]. A significant proportion of patients experience high levels of acute traumatic distress after receiving a definitive cancer diagnosis [Citation1]. This distress can persist for years after diagnosis and in some patients may reach levels meeting the criteria for posttraumatic stress disorder (PTSD) [Citation5,Citation6]. Furthermore, cancer diagnosis is associated with a pronounced rise in the incidence of all major mental disorders [Citation7], suicide, and cardiovascular deaths [Citation8], with higher risk levels reported in cancer types with poor prognosis, such as lung cancer [Citation8].
Acute and chronic psychological distress causes a physiological stress reaction through activation of the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system (SNS) [Citation9]. SNS activation results in increased production of catecholamines including norepinephrine and epinephrine from the adrenal medulla and from sympathetic neurons that innervate organs and tissue [Citation10–13]. Several clinical studies indicate an increase in catecholamine metabolism in patients with cancer either by measurements in plasma or urine [Citation14–19]. Studies in patients with oral and oropharyngeal cancers [Citation14,Citation15] and gastric carcinoma [Citation16] showed an association between mental health symptoms and catecholamine levels. In contrast, Carlson et al. [Citation17] reported significantly higher levels of self-reported distress but no difference in urinary excretion of catecholamines among women diagnosed with breast cancer compared to healthy controls, underscoring the mixed results in the existing literature. A recent study demonstrated increased endogenous catecholamine production, mainly epinephrine, in breast cancer cell lines in comparison with non-tumorigenic cell lines [Citation20]. This elevated catecholamine production may be induced by exposure to exogenic beta-adrenoceptor agonists. Limited data exist on the psychobiological stress responses to lung cancer diagnosis, notwithstanding how severely stressful that diagnosis is for patients. Growing preclinical and clinical evidence from recent literature [Citation10,Citation12,Citation13,Citation21] supports that SNS activation facilitates multiple aspects of tumor progression.
Lung cancer is the third most common cancer in both genders in the Nordic countries [Citation22]. Despite recent advances in diagnosis, staging, and treatment [Citation23–25], newly diagnosed lung cancer patients face a prognosis of 15–30% 5-year survival [Citation23,Citation25–27]; hence, mental distress is frequently reported in this patient group along with elevated risk of stress-related health outcomes [Citation4,Citation8,Citation28]. Yet, to date, no prospective studies have addressed the change in the psychobiological stress responses through the diagnostic process of lung cancer.
To address this, the current study aimed to assess symptoms of psychological distress and urinary catecholamines through the diagnostic process of lung cancer. We quantified the pre- to post-diagnosis change in those parameters in patients diagnosed with lung cancer compared with those diagnosed with non-malignant lung pathology. We also investigated the association between psychological distress and urinary levels of catecholamines. We hypothesized a post-diagnosis rise in psychological distress levels in patients diagnosed with lung cancer and further expected an association between psychological distress and urinary catecholamine levels.
Methods
Procedure and study population
The LUng CAncer, Stress, and Survival (LUCASS) study is a prospective cohort study to investigate the psychobiological stress response in patients undergoing a diagnostic work-up for suspected lung cancer. The measurements include a comprehensive self-assessment of psychological distress with ascertainment of various biomarkers and detailed documentation of the patient’s background, clinical factors, and disease course. All patients who had clinical and/or radiographic changes that were suggestive of lung cancer who were referred for further diagnostic work-up were given written information on the study by the responsible physician and afterward invited to participate by a dedicated study nurse. Eligible participants included all individuals 18–86 years old referred to Landspitali University Hospital in Reykjavík, Iceland and Uppsala University Hospital, Uppsala, Sweden. They went through a routine structured diagnostic work-up, including appropriate imaging, pathologic sampling, and other clinical tests, leading to a definite lung cancer diagnosis and staging during a 24-h diagnostic work-up or within a few days thereafter. Between 15 March 2015 and 31 March 2018, 166 patients were recruited at Landspitali University Hospital of whom 130 (78.3%) received a diagnosis of lung cancer, and between 4 October 2018 and 2 December 2020, 120 patients were recruited at Uppsala University Hospital of whom 73 (60.8%) were diagnosed with lung cancer. Eligible participants for the study were given verbal and written information about the study at the first hospital visit, and informed consent was obtained from all participating patients before any study material was collected. All collection of study materials was conducted by dedicated study nurses who gathered information through interviews (e.g., on medical history, current medication, etc.) or the patients’ self-reports, e.g., on mental and physical symptoms (pen and pencil style).
This study and its procedures were approved by the Icelandic Data Protection Authority (VSNb201460025/03.07) and the Regional Ethics Committee of Uppsala (2017/493).
The analysis reported here is confined to 167 patients (Distress analysis) who completed questionnaires on perceived distress (Hospital Anxiety and Depression Scale, HADS) at two time points: before or at the beginning of the diagnostic workup, referred to as “before diagnosis” and at the follow-up visit at a median 15 days (IQR 9–27 days) after final diagnosis of the lung pathology but before any treatment had been initiated, referred to as “after diagnosis”. A subpopulation (n = 85 patients; Catecholamine analysis) also collected overnight urine for catecholamine analysis before and after a clinical evaluation (, Supplementary Figure 1, and ).
Figure 1. Flow chart of the study population. LC, lung cancer; HADS, Hospital Anxiety and Depression Scale.
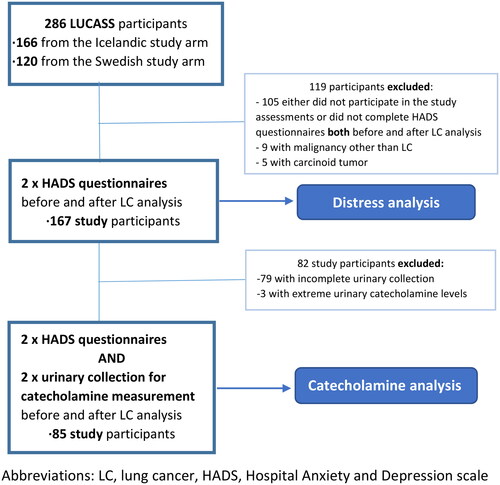
Table 1. Baseline characteristics of study participants by diagnosis of lung cancer.
Diagnosis of lung cancer (non-small cell lung cancer (NSCLC) and small-cell lung cancer (SCLC)) was confirmed by 123 (73.7%) study participants. Patients diagnosed with pulmonary carcinoid tumors and lung metastasis from other cancers was excluded from this analysis (see Supplementary Table 1 for details on patient and tumor characteristics).
Psychosocial assessments
Questionnaire assessment was integrated with the clinical evaluation at two time points: before and after the final diagnosis of the lung pathology. Participants answered questionnaires (pen and paper style) and the information was transferred into the electronic database before statistical analysis.
The patients completed a questionnaire reporting age, education, marital status, work, financial status, smoking, and history of psychiatric morbidities. We considered patients as having a history of psychiatric morbidity if they responded “yes” to “ever have suffered from depression or anxiety for 2 weeks or longer or had ever been treated for psychiatric symptoms”.
The 14-item Hospital Anxiety and Depression Scale (HADS) [Citation29] was used to measure prediagnostic distress, anxiety, and depression during the diagnostic work-up [Citation30]. The HADS scale is widely used as a screening tool for psychological distress in oncology [Citation31,Citation32]. In accordance with guidelines from Singer et al. [Citation30], we used the total score HADS-T ≥ 13 to indicate mental distress of potential clinical significance, the depression score HADS-D ≥ 5 to indicate clinically significant depression, and the anxiety score HADS-A ≥ 7 to indicate clinically significant anxiety [Citation30].
Urinary catecholamines
Overnight urine was collected at two time points: before and after the final diagnosis of the lung pathology. Participants were instructed to thoroughly void before going to bed on the night before collection. A container was provided to collect any nighttime voiding and the first morning void the next day. Overnight urine collection for catecholamine analysis has been shown to be comparable with 24-h urinary collection when using creatinine excretion as a denominator for the catecholamine excretion [Citation33]. The overnight urine collection period, rather than the standard 24-h period, was used to decrease the burden on patients and to increase overall compliance. Collection of adequate urine at the two time points, before and after the final diagnosis of lung pathology, proved difficult for the study population leading to a urine collection rate of 49.1% (). A detailed description of the catecholamine measurement both in laboratories for the Icelandic and the Swedish cohort is in Supplementary Texts 1 and 2 and Supplementary Table 2.
A lung cancer diagnosis was based on cytological and/or histological confirmation according to international guidelines [Citation34]. Lung cancer staging was according to the International Staging System, 7th edition [Citation35].
Statistical analysis
We used summary statistics to describe the demographic and clinical characteristics of the study population. Predictive mean matching was used to impute missing data in the HADS questionnaire (Supplementary Table 3) [Citation36]. The distribution of epinephrine and norepinephrine in the overnight urine collections was markedly skewed. All catecholamine data were logarithmically transformed to normality before statistical analysis. When the distribution of the urinary catecholamines was further analyzed, eight measurements had values more than three times the standard deviation (SD) above the mean. Three of them were deemed noticeably extreme in more than one measure (two measurements of epinephrine and one of norepinephrine) and were excluded from the analysis of the study.
Linear mixed models were used to measure the change of paired measurements and change between assessments before and after lung cancer diagnosis and estimated marginal means for evaluation of linear slopes with an interaction term between assessment waves and the diagnosis (lung cancer vs. not) to test effect modification. We used a random effect for a subject to account for the correlation between measurements. We adjusted for age at entry, sex, and country of participation. We then used multiple linear regression models to estimate the association between the distress levels and urinary catecholamine excretion before and after lung cancer evaluation. Covariates included in the multiple regression models were all factors with a statistically significant effect on the urinary levels of epinephrine and norepinephrine and were as follows: in and : age, sex, and country of participation; in Supplementary Tables 9 and 10: age, sex, education, occupation, smoking, and country of participation. Statistical significance was set at the level of 0.05. We performed all statistical analyses in R, version 4.2.1 (2022-06-23) [Citation37].
Table 2. The estimatedTable Footnotea association between the change of psychological distress (HADS) before and after lung cancer diagnosis and of urinary levels of catecholamines after lung cancer diagnosis (Catecholamine analysis).
Table 3. The estimatedTable Footnotea association between the change of psychological distress (HADS) and the change of urinary levels of catecholamines before and after lung cancer diagnosis (Catecholamine analysis).
Results
Psychological distress before and after diagnostic work-up for suspected lung cancer
Patients who completed the HADS questionnaire at both assessments (Distress analysis) were younger than noncompleters (69.3 vs. 71.6 years, p = 0.036), but otherwise, there were no differences between these groups (Supplementary Table 1).
A total of 123 (73.7%) patients in the Distress analysis were eventually diagnosed with lung cancer ( and Supplementary Table 1). The mean age of the participants was 69.3 years (SD 9.4, range 49–85 years) and 48.5% were women. In comparison with the Icelandic cohort, the Swedish participants were older, more likely to be non- or ex-smokers, less likely to be employed and report suboptimal financial status and more likely to have advanced lung cancer stage (Supplementary Table 4). However, there was not a significant difference in distress measures between the two cohorts. The median time between the two sets of questionnaires (completed before and after lung cancer evaluation) was 26 days (IQR: 13.5–40.5).
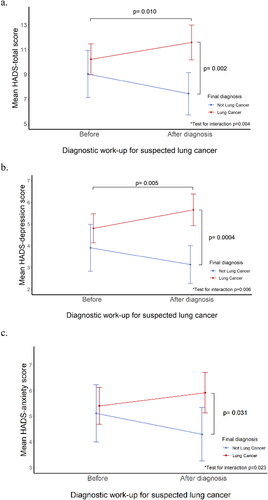
and Supplementary Table 5 show HADS scores among patients diagnosed with lung cancer and those with other causes of their lung pathology. Before diagnosis, there was no difference detected between these groups in either HADS-total scores or depression and anxiety scores. Patients who received a confirmed diagnosis of lung cancer experienced a rise in HADS-total scores (10.2–11.6, p = 0.010) post-diagnosis; there was a corresponding increase in the proportion of patients with HADS-total scores of ≥13 (from 30.1% before diagnosis to 43.1% after the lung cancer diagnosis, p = 0.003). In contrast, those with non-malignant lung pathology showed a reduction in HADS-total scores post-diagnosis (9.0–7.4, p = 0.070), with a nonsignificant decrease of patients with HADS-total scores of ≥13 (from 22.7% before diagnosis to 15.9% after the exclusion of lung cancer diagnosis, p = 0.163). The test for an interaction between the final diagnosis and the change in HADS-total between assessments was statistically significant (p = 0.004). A similar pre-to-post diagnosis change was seen in HADS depression (4.8–5.7, p = 0.005) and anxiety scores (5.4–5.9, p = 0.090) before and after the lung cancer diagnosis (, Supplementary Table 5). The pattern was similar when calculated for other HADS-depression and HADS-anxiety cutoff scores [Citation32] (Supplementary Table 6). No difference was seen in distress levels by tumor stage (Supplementary Table 7).
Figure 2. Distress before and after diagnostic work-up for lung cancer (Distress analysis). These figures show distress measured on HADS (Hospital Anxiety and Depression Scale), mean scores with 95% CI, and p-values corrected for age, sex, and country of participation (a) HADS-Total; (b) HADS-Depression; (c) HADS-Anxiety. *Test for interaction between lung cancer diagnosis and change in HADS measure between assessments.
Biological stress before and after diagnostic work-up for suspected lung cancer
Participants in the Catecholamine analysis did not differ in perceived distress levels at either assessment in comparison with the larger group participating in the Distress analysis (Supplementary Table 8).
The overnight levels of urinary catecholamines among patients diagnosed with lung cancer and those diagnosed with other lung pathologies are shown in . The median time between urinary collection before and after lung cancer evaluation was 24 days (IQR: 13.0–40.0, range: 3–182 days), and 15 participants had a time interval of more than 45 days.
Figure 3. Overnight urinary catecholamine levels before and after diagnostic work-up for lung cancer (Catecholamine analysis), with 95% CI, p-values corrected for age, sex, and country of participation. (a) Overnight urinary epinephrine levels; (b) Overnight urinary norepinephrine levels. *Test for interaction between lung cancer diagnosis and change in urinary levels of catecholamines between assessments.
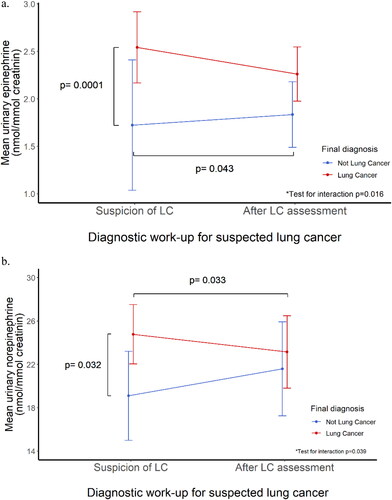
Both urinary epinephrine (2.54 vs. 1.72 nmol/mmol creatinine, p = 0.0001) and norepinephrine (24.8 vs. 19.11 nmol/mmol creatinine, p = 0.032) levels were elevated before the clinical evaluation among patients later diagnosed with lung cancer as compared with those with nonmalignant lung changes. There was no detectable difference in urinary catecholamine levels between these groups after diagnosis had been established. No differences were observed in prediagnosis urinary catecholamine levels by lung cancer stage.
A pre-to-post assessment increase was observed in the urinary levels of epinephrine in patients with non-malignant lung pathology (1.72 to 1.83 nmol/mmol creatinine, p = 0.043) and pre-to-post diagnosis decrease in norepinephrine levels (24.8 to 23.1 nmol/mmol creatinine, p = 0.033) among patients diagnosed with lung cancer. The test for the interaction of the final diagnosis and change in urinary catecholamines was statistically significant (p = 0.016 for epinephrine, p = 0.039 for norepinephrine).
Psychobiological stress before and after diagnostic work-up for suspected lung cancer
No association was detected between psychological distress and urinary levels of catecholamines either at pre- or post- diagnosis assessment (Supplementary Table 9) or pre-to-post diagnosis change in perceived distress and catecholamine levels after diagnosis ().
Tests for the interaction of the final diagnosis and pre-to-post diagnosis change in perceived distress and catecholamine levels were not significant. However, multiple regression analysis indicated an association between pre-to-post diagnosis change in both perceived distress and catecholamine levels (; Supplementary Table 10 with extended correlational model). An increase in HADS-depression after lung cancer diagnosis was associated with a rise in norepinephrine levels (β = 1.01; CI: 0.098–1.924; p = 0.034) while, contrary to our prediction, an increase in HADS-anxiety was associated with a reduction in epinephrine levels (β = –0.17; CI: −0.295 to −0.053; p = 0.006).
Discussion
These findings clearly demonstrate that receiving a lung cancer diagnosis is associated with an increase in symptoms of psychological distress. Interestingly, our findings also show that patients who are subsequently diagnosed with lung cancer present with elevated levels of catecholamines even before the diagnosis is established. Although our data do not suggest a clear association between perceived distress and catecholamine levels throughout the process of lung cancer diagnosis, we noted some correlations between pre-to-post diagnosis parameters of distress and catecholamines.
In the present study, we demonstrate varying development of psychological distress in urinary catecholamines across the diagnostic process among patients ultimately diagnosed with lung cancer versus other causes of lung pathology. In a prospective manner, we describe the change in the distress pattern according to the final diagnosis of lung cancer and confirm high levels of acute distress after a definite lung cancer diagnosis. The proportion of patients with clinically significant distress (HADS-total scores of ≥13) went from 30.1% before diagnosis to 43.1% following the confirmation of lung cancer diagnosis.
Psychological distress has previously been reported to be elevated in patients with cancer across cancer sites, particularly in patients diagnosed with cancer with a poor prognosis, including lung cancer (43.4%) [Citation38]. Although psychological distress decreases in the months after the lung cancer diagnosis [Citation2], it remains persistent in around 20% of patients [Citation39] and is associated with premature mortality [Citation13,Citation40].
In this study, we demonstrate an elevation in urinary catecholamine levels prior to diagnosis in patients who were eventually diagnosed with lung cancer in comparison with those who were subsequently diagnosed with nonmalignant lung pathology. The present study is, to our knowledge, the first to report a prediagnostic elevation in catecholamine levels in patients later diagnosed with lung cancer.
It is generally assumed that circulating catecholamines, epinephrine and norepinephrine, have a neuroendocrine origin and that an elevation of catecholamine levels is a physiological marker of psychological distress in both non-oncologic [Citation41] and oncologic settings [Citation15,Citation16,Citation19]. A study on oral neoplasm by Xie et al. [15] similarly reported elevated catecholamines in patients with carcinoma in comparison with nonmalignant oral lesions even before diagnosis. The findings of Amoro et al. [20] on endogenous catecholamine production in breast cancer cell lines combined with our results of increased catecholamine levels before diagnosis of lung cancer may be an indication of neurogenesis, a complex mechanism of nerve-cancer crosstalk associated with tumor progression and dissemination of the malignant disease [Citation42]. The absence of an association between prediagnostic levels of distress and urinary catecholamine levels may further indicate that the observed elevation of urinary catecholamines before confirmation of the lung cancer diagnosis may be related to the tumor biology independent of perceived distress levels. However, this finding calls for further studies, as the results of previous studies have indicated that catecholamines play an important role in cancer progression [Citation12,Citation13,Citation18,Citation43].
We also noted a pre-to-post diagnosis decrease in urinary norepinephrine in those diagnosed with lung cancer and a pre-to-post diagnosis increase in urinary epinephrine among those with nonmalignant lung pathology.
Most previous studies focus on catecholamine levels in patients with cancer in relation to psychosocial factors. We found that increased pre-to-post diagnosis depression levels were associated with a greater increase in norepinephrine levels, which is in accordance with our hypothesis. However, contrary to our expectations, we found an association between a greater pre-to-post diagnosis increase in anxiety with a decrease in pre-to-post diagnosis epinephrine. The literature on the association between perceived distress and catecholamines among patients with cancer remains inconclusive. Several clinical studies indicate that a change occurs in the catecholamine metabolism in patients with cancer [Citation14–19]. Studies on breast [Citation44], gastrointestinal [Citation16,Citation18], renal cell carcinoma, and melanoma [Citation19] demonstrated an association of elevation in either urinary or serum levels of catecholamines with distress. However, other studies demonstrated elevated catecholamine levels in the absence of an association with distress parameters in newly diagnosed patients with cancer [Citation14,Citation17]. These equivocal findings, some studies show significant associations [Citation15,Citation16,Citation19] while others do not [Citation14,Citation17], along with our results, indicate the complexity of this relationship between psychological and biological stress indices. Taken together, more studies are needed to advance our understanding of the complex association between distress and catecholamine levels in cancer patients.
The strength of this study includes the harmonized protocol across the two clinical sites and prospective data collection from a patient population who entered a diagnostic work-up for potential lung cancer in two countries. Because of the prospective design, the patients were naive to their eventual diagnosis, which allowed us to measure the change in distress and catecholamine levels after confirmation of the diagnosis. This is, to our knowledge, the first study to prospectively assess the association of perceived distress and a biological stress measure before and after a lung cancer diagnosis.
Limitations
The main limitation of this study relates to the data collection in a vulnerable population during an extremely difficult period in a person’s life. As in other studies on similarly sensitive populations [Citation2,Citation45], this inevitably affects participation rates and the completeness of data. It is possible that the patients experiencing the highest distress levels were not able to complete the questionnaires or urinary collection on two occasions. This would likely lead to an underestimation of the degree of stress levels in the final analytic sample. Moreover, in addition to frailty and psychological strain in this patient group, logistics in the clinical diagnostic process may affect the time interval elapsing between the two data collections (e.g., catecholamine collection after treatment starts). The diagnosis could not be confirmed until after surgery in six patients; therefore, the second urinary collection took place after treatment initiation in these patients. However, excluding these six patients in a sensitivity analysis did not change our results or conclusion (Supplementary Figure 3). Further, we have no information on whether patients entered the diagnostic process due to symptoms or an incidental finding of a lung nodule. Also, the study participants were referred to the diagnostic work-up after initial assessment by the referring physician and, therefore, may not have been completely naive to the suspicion of lung cancer. We have limited information on the exact number of patients who were approached for the study in the Swedish arm, but the protocol was the same at both study sites; thus, we assume a similar participation rate as in the Icelandic arm (65.6%). Finally, the study population is limited to suspected patients with lung cancer undergoing the harmonized diagnostic work-up within our study clinics in Iceland and Sweden. Therefore, the results may not be readily generalized to other settings, such as participants of lung cancer screening.
In conclusion, this study demonstrates high levels of psychological distress in newly diagnosed patients with lung cancer. Furthermore, we demonstrate that urinary excretion of catecholamines was already elevated before the lung cancer diagnosis. These findings highlight the need for increased clinical awareness of the mental health of patients undergoing lung cancer diagnostics and the need for psychological support at that time and support the need for further research into the causes of the elevated catecholamine levels within this patient group and their potential role in lung cancer progression.
Authors’ contributions
All authors have made significant contributions to (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, and (3) have approved the final version to be submitted for publication.
Ethics approval and patient consent statement
Ethics approval and patient consent statement is included in the main text.
| Abbreviations | ||
| HADS | = | Hospital Anxiety and Depression scale |
| HADS-A | = | Hospital Anxiety and Depression scale – anxiety score |
| HADS-D | = | Hospital Anxiety and Depression scale – depression score |
| HADS-T | = | Hospital Anxiety and Depression scale – total score |
| IQR | = | Interquartile range |
| LUCASS study | = | Lung Cancer, Stress and Survival study |
| NSCLC | = | Non-small cell lung cancer |
| PTSD | = | Post Traumatic Stress Disorder |
| SCLC | = | Small-cell lung cancer |
| SD | = | Standard Deviation |
| SNS | = | the Sympathetic Nervous System |
Supplemental Material
Download MS Word (517 KB)Acknowledgement
We are particularly grateful to all the patients who participated in the study, demanding dedication in collecting the study materials parallel with the load of the lung cancer evaluation studies. Additionally, we thank Sigrún B. Guðmundsdóttir, Hrönn Árnadóttir, and Katarina Nisser for their work in the study.
Data availability statement
The authors have full access to the primary data and can make the data available to the journal or interested researchers upon the approval of the National Bioethics Committee of Iceland and Sweden.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Hardardottir H, Aspelund T, Zhu J, et al. Optimal communication associated with lower risk of acute traumatic stress after lung cancer diagnosis. Support Care Cancer. 2022;30:259–269. doi:10.1007/s00520-021-06138-4.
- Brocken P, van der Heijden EH, Oud KT, et al. Distress in suspected lung cancer patients following rapid and standard diagnostic programs: a prospective observational study. Psychooncology. 2015;24:433–441. doi:10.1002/pon.3660.
- Gould MK, Creekmur B, Qi L, et al. Emotional distress, anxiety, and general health status in patients With small pulmonary nodules shortly After their identification: results From the watch the spot trial. Chest. 2023. doi:10.1016/j.chest.2023.06.022.
- Hansen JM, Kjaer TK, Mellemgård A, et al. Association between anxiety and depression symptoms and completion of first-line treatment in newly diagnosed lung cancer patients. Acta Oncol. 2023;62:820–824. doi:10.1080/0284186X.2023.2207745.
- Chan CMH, Ng CG, Taib NA, et al. Course and predictors of post-traumatic stress disorder in a cohort of psychologically distressed patients with cancer: a 4-year follow-up study. Cancer. 2018;124:406–416. doi:10.1002/cncr.30980.
- Tjemsland L, Soreide JA, Malt UF. Posttraumatic distress symptoms in operable breast cancer III: status one year after surgery. Breast Cancer Res Treat. 1998;47:141–151. doi:10.1023/a:1005957302990.
- Lu D, Andersson TM, Fall K, et al. Clinical diagnosis of mental disorders immediately before and after cancer diagnosis: a nationwide matched cohort study in Sweden. JAMA Oncol. 2016;2:1188–1196. doi:10.1001/jamaoncol.2016.0483.
- Fang F, Fall K, Mittleman MA, et al. Suicide and cardiovascular death after a cancer diagnosis. N Engl J Med. 2012;366:1310–1318. doi:10.1056/NEJMoa1110307.
- Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6:240–248. doi:10.1038/nrc1820.
- Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–944. doi:10.1038/nm1447.
- Cole SW, Nagaraja AS, Lutgendorf SK, et al. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. 2015;15:563–572. doi:10.1038/nrc3978.
- Nilsson MB, Le X, Heymach JV. beta-Adrenergic signaling in lung cancer: a potential role for beta-blockers. J Neuroimmune Pharmacol. 2020;15:27–36. doi:10.1007/s11481-019-09891-w.
- Chang A, Sloan EK, Antoni MH, et al. Biobehavioral pathways and cancer progression: insights for improving well-being and cancer outcomes. Integr Cancer Ther. 2022;21:15347354221096081. doi:10.1177/15347354221096081.
- Bastos DB, Sarafim-Silva BAM, Sundefeld M, et al. Circulating catecholamines are associated with biobehavioral factors and anxiety symptoms in head and neck cancer patients. PLoS One. 2018;13:e0202515. doi:10.1371/journal.pone.0202515.
- Xie H, Li B, Li L, et al. Association of increased circulating catecholamine and glucocorticoid levels with risk of psychological problems in oral neoplasm patients. PLoS One. 2014;9:e99179. doi:10.1371/journal.pone.0099179.
- Pan C, Wu J, Zheng S, et al. Depression accelerates gastric cancer invasion and metastasis by inducing a neuroendocrine phenotype via the catecholamine/β(2)-AR/MACC1 axis. Cancer Commun (Lond). 2021;41:1049–1070. doi:10.1002/cac2.12198.
- Carlson LE, Campbell TS, Garland SN, et al. Associations among salivary cortisol, melatonin, catecholamines, sleep quality and stress in women with breast cancer and healthy controls. J Behav Med. 2007;30:45–58. doi:10.1007/s10865-006-9082-3.
- Liu J, Zong G, Zhang C, et al. Anxiety and serum catecholamines as predictors of survival and recurrence in hepatocellular carcinoma. Psychooncology. 2017;26:1347–1353. doi:10.1002/pon.4305.
- Cohen L, de Moor C, Devine D, et al. Endocrine levels at the start of treatment are associated with subsequent psychological adjustment in cancer patients with metastatic disease. Psychosom Med. 2001;63:951–958. doi:10.1097/00006842-200111000-00014.
- Amaro F, Silva D, Reguengo H, et al. β-Adrenoceptor activation in breast MCF-10A cells induces a pattern of catecholamine production similar to that of tumorigenic MCF-7 cells. Int J Mol Sci. 2020;21(21):7968. doi:10.3390/ijms21217968.
- Kaira K, Kamiyoshihara M, Kawashima O, et al. Prognostic impact of beta2 adrenergic receptor expression in surgically resected pulmonary pleomorphic carcinoma. Anticancer Res. 2019;39:395–403. doi:10.21873/anticanres.13125.
- NORDCAN. Association of the Nordic Cancer Registires. 2023 [cited 2023 Aug 23]. Available from: https://nordcan.iarc.fr/en.
- Lindqvist J, Jekunen A, Sihvo E, et al. Effect of adherence to treatment guidelines on overall survival in elderly non-small-cell lung cancer patients. Lung Cancer. 2022;171:9–17. doi:10.1016/j.lungcan.2022.07.006.
- Maity AP, Gangireddy M, Degen KC, et al. Impact of simultaneous circulating tumor DNA and tissue genotyping in the workup of stage IV lung adenocarcinoma on quality of care in an academic community medical center. J Clin Oncol Oncol Pract. 2023;19:620–625. doi:10.1200/OP.22.00405.
- West H, Hu X, Zhang S, et al. Treatment patterns and outcomes in resected early-stage non-small cell lung cancer: an analysis of the SEER-Medicare data. Clin Lung Cancer. 2023;24:260–268. doi:10.1016/j.cllc.2022.12.005.
- Hu S, Zhang W, Guo Q, et al. Prognosis and survival analysis of 922,317 lung cancer patients from the US based on the most recent data from the SEER database (april 15, 2021). Int J Gen Med. 2021;14:9567–9588. doi:10.2147/IJGM.S338250.
- Solberg S, Nilssen Y, Brustugun OT, et al. Increase in curative treatment and survival of lung cancer in Norway 2001-2016. Eur J Epidemiol. 2019;34:951–955. doi:10.1007/s10654-019-00536-z.
- Wright AE, Sheehan E, Qeadan F, et al. Preexisting psychological illness and its association with mortality in lung cancer patients with access to support resources. Clin Respir J. 2022;16:750–755. doi:10.1111/crj.13547.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi:10.1111/j.1600-0447.1983.tb09716.x.
- Singer S, Kuhnt S, Gotze H, et al. Hospital anxiety and depression scale cutoff scores for cancer patients in acute care. Br J Cancer. 2009;100:908–912. doi:10.1038/sj.bjc.6604952.
- Razavi D, Delvaux N, Farvacques C, et al. Screening for adjustment disorders and major depressive disorders in cancer in-patients. Br J Psychiatry. 1990;156:79–83. doi:10.1192/bjp.156.1.79.
- Annunziata MA, Muzzatti B, Bidoli E, et al. Hospital anxiety and depression scale (HADS) accuracy in cancer patients. Support Care Cancer. 2020;28:3921–3926. doi:10.1007/s00520-019-05244-8.
- White IR, Brunner EJ, Barron JL. A comparison of overnight and 24 hour collection to measure urinary catecholamines. J Clin Epidemiol. 1995;48:263–267. doi:10.1016/0895-4356(94)00127-c.
- Travis WD, Brambilla E, Burke AP, et al. Introduction to The 2015 world health organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol. 2015;10:1240–1242. doi:10.1097/JTO.0000000000000663.
- Vallieres E, Shepherd FA, Crowley J, et al. The IASLC lung cancer staging project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:1049–1059. doi:10.1097/JTO.0b013e3181b27799.
- van Buuren SG-OK. Mice: multivariate imputation by chained equation in R. J Stat Software. 2011;45:1–67.
- R Core Team. R: a language and environment for statistical computing. Vienna, Austria: Foundation for Statistical Computing; 2014.
- Zabora J, BrintzenhofeSzoc K, Curbow B, et al. The prevalence of psychological distress by cancer site. Psycho-oncology. 2001;10:19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::AID-PON501>3.0.CO;2-6.
- Dougall AL, Swanson J, Kyutoku Y, et al. Posttraumatic symptoms, quality of life, and survival among lung cancer patients. J Appl Biobehav Res. 2017;22(3):e12065. doi:10.1111/jabr.12065.
- Andersen BL, McElroy JP, Carbone DP, et al. Psychological symptom trajectories and Non-Small cell lung cancer survival: a joint model analysis. Psychosom Med. 2022;84:215–223. doi:10.1097/PSY.0000000000001027.
- Pan X, Kaminga AC, Wen SW, et al. Catecholamines in post-traumatic stress disorder: a systematic review and meta-analysis. Front Mol Neurosci. 2018;11:450. doi:10.3389/fnmol.2018.00450.
- Silverman DA, Martinez VK, Dougherty PM, et al. Cancer-associated neurogenesis and nerve-cancer cross-talk. Cancer Res. 2021;81:1431–1440. doi:10.1158/0008-5472.CAN-20-2793.
- Yazawa T, Kaira K, Shimizu K, et al. Prognostic significance of beta2-adrenergic receptor expression in non-small cell lung cancer. Am J Translational Res. 2016;8:5059–5070.
- Thornton LM, Andersen BL, Blakely WP. The pain, depression, and fatigue symptom cluster in advanced breast cancer: covariation with the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system. Health Psychol. 2010;29:333–337. doi:10.1037/a0018836.
- Akkol-Solakoglu S, Hevey D. Internet-delivered cognitive behavioural therapy for depression and anxiety in breast cancer survivors: results from a randomised controlled trial. Psychooncology. 2023;32:446–456. doi:10.1002/pon.6097.
