Abstract
Background
Proton radiotherapy (RT) is an attractive tool to deliver local therapy with minimal dose to uninvolved tissue, however, not suitable for all patients. The aim was to explore complications, especially severe late complications (grades 3–4), following proton RT delivered to a complete Swedish cohort of paediatric patients aged <18 years treated 2008–2019.
Material and Methods
Data was downloaded from a national registry. Complications with a possible causation with RT are reported. Proton treatments until July 2015 was performed with a fixed horizontal 172 MeV beam (The Svedberg Laboratory (TSL), Uppsala) in a sitting position and thereafter with gantry-based pencil-beam scanning technique (Skandion Clinic, Uppsala) in a supine position.
Results
219 courses of proton RT (77 at TSL and 142 at Skandion) were delivered to 212 patients (mean age 9.2 years) with various tumour types (CNS tumours 58%, sarcomas 26%, germ cell tumours 7%). Twenty-five patients had severe acute complications (skin, mucous membrane, pharynx/oesophagus, larynx, upper gastrointestinal canal, lower gastrointestinal canal, eyes, ears). Fifteen patients had severe late complications; with increased proportion over time: 4% at 1-year follow-up (FU), 5% at 3-year, 11% at 5-year. Organs affected were skin (1 patient), subcutaneous tissue (4), salivary glands (1), upper GI (1), bone (7), joints (2), CNS (2), PNS (1), eyes (1) and ears (5). Twenty-one of the 28 patients with 10-year FU had at least one late complication grades 1–4 and fourteen of them had more than one (2–5 each).
Conclusion
The most important result of our study is the relatively low proportion of severe late complications, comparable with other proton studies on various tumours. Furthermore, the numbers of late complications are lower than our own data set on a mixed population of photon and proton treated paediatric patients, assuring the safety of using proton therapy also in the clinical practice.
Background
During the last 60 years, cure rates among children and adolescents diagnosed with malignant diseases have increased magnificently to 80–85% [Citation1,Citation2]. Historically, more than 60% of the survivors developed late complications [Citation3–7]. Yet today, there is a huge lack of data on safety and efficacy for proton therapy in the literature. However, since proton RT has not been used in larger scale for long, it is important to evaluate the treatment continuously to identify possible pitfalls. Thorough follow-up of patients receiving proton RT, can help to fill some of that knowledge gap.
In Sweden, the yearly cancer incidence in children/adolescence under age 18 is approximately 350 patients, of which 25% receive RT. Paediatric RT in Sweden is centralized to six university cancer centres, where photon and electron RT can be provided. In addition, proton RT is distributed in Uppsala.
Nearly 100% of all children with cancer, who are Swedish residents, are listed in the National Swedish Childhood Cancer Registry. Since 2008, all children treated with RT are registered in Radtox, a national paediatric RT registry assorted under the above-mentioned registry. This, together with hospital based RT registries, provides an excellent opportunity to identify all children diagnosed and treated for cancer during a defined time period and also yields a population-based follow-up (FU).
The primary aim of this study, based on prospectively recorded registry data, was to explore sequelae following proton RT in a cohort of all Swedish paediatric patients treated with proton RT during 2008–2019, with special focus on severe late complications (i.e. grades 3–4).
Material and methods
National paediatric RT registry (Radtox)
Radtox, approved by the National Board of Health and Welfare, started in January 2008 as part of the Swedish Paediatric Cancer Registry. Parents and patients are informed about the registry before start of RT.
Since the beginning, in 2008, all registration of RT data is performed by one dosimetrist, thus securing an almost complete inclusion as well as high quality. Furthermore, this dosimetrist performs quality controls on entered data to assure consistency. Organs at risk (OARs) in the vicinity of the treatment fields are defined according to a reference guide. Treatment planning, dose volume histograms (DVH), and target coverage are discussed at online conferences held by radiation oncologists from all six centres bi-weekly. These OARs are registered in Radtox after treatment completion.
Acute and late complications according to RTOG/EORTC forms [Citation8] are assessed by the radiation oncologist and registered at end of RT as well as after 2 months and then after 1, 3, 5 and 10 years, respectively. Thereafter, registration will continue every five to ten years. Thus, in the present study, the same complication for an individual patient can be reported at multiple time-points. It is registered whether the complication is assessed to be related to RT, i.e. the relevant OARs were irradiated to some extent and the symptoms had either presented or worsened after RT. In this study, only complications assessed to be due to RT were reported.
Patients
All patients found suitable are offered referral for proton RT, which is fully subsidized by the Swedish health system. During 2016–2019, only 3 families out of 105 declined referral [Citation9].
This study is a prospective cohort study, including all patients age <18, treated with proton RT during 2008–2019. Last FU date was December 31st 2020. After ethical approval, relevant extracts from the Radtox registry was obtained and then transferred to an IBM© SPSS© Statistics file (version 28.0.1.0 (142)) for compiling the results. For the comparing of proportions (independent samples), Chi2 and Fisher were used. When needed, the patient charts were, in accordance with the ethical approval, reviewed for better understanding of e.g. causality.
Treatment planning and delivery of proton beams at TSL
Treatment planning was carried out with the commercial treatment planning system Helax-TMS©, (Treatment Management System, MDS Nordion Therapy System, Uppsala, Sweden) [Citation10]. Patients underwent magnetic resonance imaging (MRI) for guidance in delineation of the target and OARs in the computed tomography (CT)-series used for treatment planning. Accurate positioning of the patient in the proton beam used implanted fiducial markers in the skull and orthogonal kV imaging in the treatment position. A fixed horizontal 172 MeV proton beam with a maximum diameter of 98 mm was used with passive scattering technique. Treatment was performed in a sitting position with the head fixed to a rotatable treatment chair by an individually-formed helmet and a bite-block. The positioning of the target in the proton beam was done with an accuracy less than +1 mm [Citation11]. Due to the sitting position, targets below the neck were not possible to treat during this era. Exceptions were four patients with spinal/sacral targets, treated lying on their side with one field from the back.
Treatment planning and delivery at the Skandion Clinic
The Skandion Clinic, owned by a municipal association consisting of all seven university hospitals in Sweden, is a modern facility with pencil-beam scanning (PBS) technique [Citation12], equipped with two gantries. All treatment planning is performed at the home clinics (distributed competence [Citation13]) and discussed at national video-conferences before the patients are accepted for proton RT. Individual immobilisation devices are created, CT and MRI in treatment position are performed for delineation. Treatment planning is performed using Eclipse (v.16.0), Varian Medical Systems, Palo Alto, USA, accessed remotely by the home clinics. In this study, for all but craniospinal treatments, comparative photon plans were created, and the most sufficient plan was selected based on target coverage, dose to OARs, integral dose and robustness of RT-delivery.
Dose-data and fractionation
Conventional fractionation once daily with doses appropriate for the various types of tumours according to international or national protocols were used. All doses were stated as biological doses (RBE 1.1).
Dose-volume parameters are stored in the local treatment planning system as well as exported in the so called DICOM-RT format to a central data base. The DICOM data includes the 3D dose matrix, as well as complete information on the treatment plan (beam quality, number of beams and their entry angles, field sizes, field blocking, etc.), shape and volume of the outlined structures (tumour and OARs) as well as CT images used in the treatment planning process.
Results
During 2008–2019, 1072 RT treatment courses were delivered. Of these, 219 were proton RT courses. Seventy-seven treatments took place at the TSL unit and 142 at the Skandion clinic. Seven patients were irradiated more than once (sometimes to a different target due to e.g. metastases) with proton RT during the study period. Thus, 212 patients (mean age 9.2 years, 106 girls, 106 boys) had proton RT and constituted the present study population. Thirty-eight patients (18%) died before last FU, no one had a secondary malignancy. Five patients had no FU for late complications. Thirty study patients were, in addition, irradiated with other modalities (photons, electrons and/or brachytherapy) during the same time period. Two patients had two such retreatments.
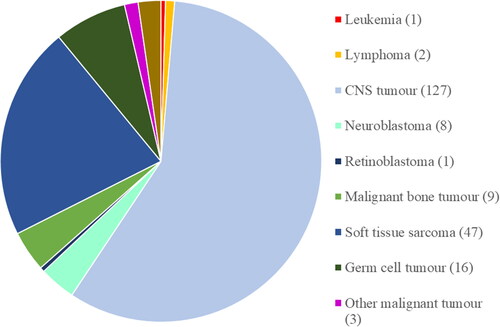
Diagnoses were specified according to International Classification of Childhood Cancer (ICCC-3). More than half (58%) of the treatments were given to patients with CNS tumours, followed by soft tissue sarcomas (21%) and germ cell tumours (7%) ().
Figure 1. Number of patients per diagnosis according to International Classification of Childhood Cancer (ICCC-3). the exact number is given within parentheses. All three patients with ’other malignant disorders’ (ICCC-3 group XI) had parotid cancer. Among the five patients with benign disorders, four had an arteriovenous malformation (AVM) and one had a relapsing choroidal haemangioma.
FU times varies between one and ten years. 50% of the patients had a FU time of at least 3 years.
Acute complications are shown in and late complications in . The patients are divided into ‘CNS tumours (including primary CNS germ-cell tumours)’ and ‘non-CNS tumours’. We report the number of treatments with FU reports for each time-point and the number of treatments with complications grades 1–4 related to RT. Thus, the seven patients with two treatment courses can be reported twice.
Table 1. Acute complications for all treatment courses with a follow-up (FU) registration at end of RT (N = 200).
Table 2. Late complications for all treatment courses. Patients with CNS tumours (primary CNS germ-cell tumours included) and tumours outside CNS are shown separately.
The most usual acute complications were deteriorated performance status, nausea, and reactions of the skin, hair, subcutaneous tissue, mucous membrane, and CNS (). Twenty-five patients had severe (grades 3 or 4) acute complications (data not shown). Four patients had two severe complications and two patients had three severe complications.
Twenty-one of the 28 patients with a 10-year FU had at least one late complication (data not shown) and 14 of them had more than one (2–5 each). Four of the patients, who would have been eligible for a 10-year FU within the study period, died.
Severe late complications increased with longer FU; at 1-year FU 6 of 153 (3.9%) patients had grades 3–4 complications, at 3-year 4 of 82 (4.9%), and at 5-year 6 of 57 (10.5%). At 10-year FU the proportion dropped to 1 of 28 patients (3.6%).
Patients with CNS tumours had more often a deteriorated performance status (p < .05 at acute, 1-year and 3-year FU), while skin and subcutaneous tissue complications were more common in non-CNS tumour patients (p < .05 at acute, 1-year, 3-year and 5 year FU).
In total, 15 patients (7.1%) in the cohort had severe complications, detailed in and Supplementary Table S1. Organs affected were skin, subcutaneous tissue, mucous membrane, salivary glands, teeth, upper GI, muscles, bones, joints, CNS, PNS, eyes and ears. Two of these patients died, two years (no 12) and seven years (no 7) after first course of RT, respectively.
Table 3. Description of all 15 patients with late complications grades 3 or 4. Proton target doses in GyRBE, photon RT and brachytherapy in Gy, fraction doses within parentheses.
The only severe late skin complication was reported for patient number 7, with necrosis and severe telangiectasia after very extensive RT (probably cumulative dose 150–200 Gy during 3 years at some spots). The patient died before 10-year follow-up. The doctor and the patient, including the parents, were aware of the risks and found them justified even after side effects appeared. Four patients had severe fibrosis and/or severely reduced subcutaneous fatty tissue. Two of them (no 1 and 3) had no surgery. Patients number 4 and 7 had extensive surgery, a possible contributing factor.
Patient number 3 had xerostomia grade 3 five years post RT. Patient number 7 had a jaw joint stiffness grade 3 after three years.
Five patients developed severe bone growth inhibition (no 1, 3, 4, 6, and 7), all treated at TSL with ≥45 Gy to the involved area.
Patient number 5, who had surgery with decompression and macroscopically total resection of Ewing sarcoma in a vertebra, had a pathological vertebral fracture without trauma three years after postoperative RT. DEXA scan showed normal bone density. Patient number 10 was extensively investigated because of jaw osteonecrosis one year post RT. Routine dental check-up including radiology prior to RT showed no signs of bone infection, neither did the patient have neurofibromatosis 1 (NF-1) or other known predisposing factors for abnormal RT sensitivity [Citation14].
Two patients were registered for severe CNS complications. Patient number 2 developed panhypopituitarism and patient number 8 had a severe stroke due to moyamoya syndrome.
Patient number 6 received chemotherapy before RT (39.7 Gy to the optical nerve, 45 Gy to the frontal medial part of the eye) with partial response and remaining visus 0.6. Extended tumour resection without enucleation resulted in ptos, strabismus and blindness. The role of RT in this complication is uncertain, but a partial causation cannot be excluded.
Six patients had ear complications grade 3. Patient number 3 had constant tinnitus, but no hearing loss five years after RT. Patient number 15, who had external otitis and tympanic membrane perforation one year post RT, was operated on, and had no ear problems at the 3-year FU. A third patient (no 8) had severe hearing loss (>15–20 dB) 5 years after RT (54.7 Gy).
In Supplementary Table S2, late complications for TSL patients compared with Skandion patients are shown. No statistically significant differences were found.
Discussion
Proton RT is growing fast worldwide, especially for treatment of paediatric patients. Still the amount of published data on long-term FU is scarce.
We have follow-up data on a large cohort of paediatric patients receiving proton RT in Sweden during 2008–2019. The aim of the study was to explore the complications following proton RT in this almost complete Swedish cohort of children/adolescents aged <18 years, with special focus on severe late complications (i.e. grades 3–4).
The proportion of patients with at least one late complication grades 1–4 at 10 years FU, was 75% (21 of 28 patients) in our study. Within the whole study population, we found only 15 patients (7.1%) with late complications grades 3–4 according to the RTOG/EORTC forms. However, this proportion may increase with longer FU for more patients. Severe late complications affected skin, subcutaneous tissue, upper GI, bone, joints, CNS, PNS, eyes and ears.
We found differences in performance status, skin and subcutaneous tissue complications between patients with tumours in and outside CNS. A worse performance status among CNS patients might be due to e.g. brain fatigue. The differences in skin/subcutis are more difficult to explain.
Using data from the North American Childhood Cancer Survivor Study (CCSS), 14,359 patients along with 4301 siblings were studied (median FU 24.5 years). The cumulative incidence of a severe, disabling, life-threatening, or fatal health condition according to Common Terminology Criteria for Adverse Events (CTCAE) was greater among patients than siblings (53.6% versus 19.8%) [Citation15].
5223 patients from St. Jude Lifetime Cohort Study (SJLIFE) treated 1962–2012 (FU time ≥8 years), were evaluated using CTCAE21 [Citation16]. They report incidences grades 3–4: cardiovascular disease 7.2%, endocrine disease 16.6%, gastrointestinal disease 12.7%, musculoskeletal disease 10.3%, neurological disease 9.0%, pulmonary disease 5.6%, and renal disease 3.4%. 3.7% of the patients had a subsequent neoplasm.
The reason why we found a lower severe complication incidence at 10-year FU compared to after 5 years is probably the small number of patients eligible for 10-year FU. Another reason could be that only patients treated at TSL were followed for such a long time. For physical reasons those patients had in general smaller targets, mostly limited to the head and neck.
The patient with xerostomia five years post RT had extensive chemotherapy, which could have contributed [Citation17,Citation18]. The patient with a jaw joint stiffness, a complication quite commonly related to RT [Citation19], also had extensive surgery and thus the cause can be multifactorial.
Late complications of bone, including growth inhibition, are well known and can occur after as low doses as 20 Gy to the growth zones, especially before six years of age [Citation20,Citation21]. The most important factors related to the impact of radiation on craniofacial structure is age [Citation22–25] and dose [Citation25,Citation26]. Since radiology is not performed routinely during late FU for patients without evidence of relapse or sequelae, there is a fair amount of missing data on late complications of bone in our registry. Hence, the amount of bone complications might be underestimated. However, severe complications would probably be detected based on anamnesis and physical examination. In addition to the five patients with growth inhibition of the facial skeleton, we found one case of vertebral fracture, a well-known complication especially of those bones under most physiologic stress such as vertebrae [Citation27]. Another patient developed jaw osteonecrosis only one year post RT. There are established relationships between ongoing dental/mandibular problems [Citation28], corticosteroids [Citation29], several chemotherapeutic drugs [Citation30] and non-traumatic osteonecrosis. Ducatman [Citation14] also report NF-1 to cause abnormal RT sensitivity. Our patient had no ongoing jaw infection and no evidence of NF-1, but received a considerable dose of corticosteroids and several types of chemotherapeutic drugs both before and after RT. Thus, it might be more likely that medical drugs, perhaps in the combination with RT, have caused this complication.
Late complications of CNS are not very well captured by the RTOG/EORTC forms. The definitions of the various grades of complications are blunt, especially concerning neuropsychological evaluation. For this purpose, neuropsychological tests are necessary [Citation31]. Such tests are not recorded in Radtox, but have been reported elsewhere [Citation31]. More Swedish results on this matter are currently under compilation (Söderström, personal communication). One patient developed panhypopituitarism after 52 Gy to the pituitary, which is a known risk factor [Citation32]. We lack hormonal laboratory status for many patients. However, since all patients are asked for current medication at FU, this would probably be detected anyhow, but might be underestimated. One patient treated with 54 Gy due to craniopharyngioma developed a stroke caused by moyamoya syndrome [Citation33]. Others have reported young age as well as NF-1 to be risk factors for development of moyamoya syndrome after receiving RT to the parasellar region [Citation34]. Our patient did not suffer from NF-1.
No studies, including multiple variable analyses, investigating the independent effect of RT on hearing loss were identified in a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCare Consortium [Citation35]. Hence, no safety threshold RT dose could be set. In our study, all the three patients who presented with severe ear complications without having cisplatin treatment had a cochlear dose of more than 50 Gy. One of them had decreased hearing grade 3 five years post RT and one had continuous tinnitus. A third patient had external otitis and perforated tympanic membrane one year post RT, an uncertain RT complication. The additional three patients with severe ear problems had both RT and cisplatin treatment, with for at least two of the patients a temporal relation to cisplatin, a known ototoxic drug [Citation36]. It can, however, not be ruled out that RT was a contributing factor also for them.
Teeth and muscle complications are not included in the RTOG/EORTC forms. Thus, the number of patients with these types of complications are underestimated in our study. In the patient files, we accidentally found two patients with short teeth roots and lack of some permanent teeth (). We also found one patient with a severe muscle atrophy. Different teeth disturbances are well known complications to RT [Citation17,Citation37–39] to which younger age and radiation doses ≥ 20 Gy are risk factors [Citation40–42].
While mortality due to recurrence or tumour progression is decreasing over time, it appears that mortality due to secondary malignancies (SM) is increasing [Citation7,Citation43,Citation44]. In our cohort no SM was reported but the FU time is most likely too short to expect any SM to have occurred.
Unfortunately, it is not possible to calculate the percentage risk for developing a complication by solely using our registry, since grade 0–4 side effects are often reported instead of marking NA even when the OAR is far from the target. Thus, our estimated risks would probably be lower than the actual percentage risks.
Since late complications increase by time it can be argued that the FU time is too short for many of our patients, especially for those treated at the Skandion clinic. We also have too few patients treated below the neck to draw any statistically significant conclusions for each OAR. There is probably an underestimation of bone complications due to lack of routine radiology, and hormonal status is missing for many patients. Neither are there any patient-reported outcome or quality of life measurements.
The strengths of our study are an almost 100% inclusion of patients, RT data and quality control made by one dosimetrist, well documented RTOG/EORTC reporting forms, complications related to RT, and missing data checked manually.
Even though we cannot calculate any reliable incidence figures, the data concerning severe complications of the present study are fairly well in accordance with a 4% non-ocular and 18% ocular severe complication rate in 83 rhabdomyosarcoma patients (median FU 55.5 months) treated with pencil beam RT (median 54 Gy RBE) reported by Leiser et al. [Citation45]. It also probably matches the 5-year ear-toxicity of 16% in a phase 2 proton study of 45 medulloblastoma patients treated with passive scattered proton RT at Massachusetts General Hospital with median FU 7 years [Citation46]. In both these studies, all patients, like most of our patients, received chemotherapy in addition to RT.
Most studies report on complications from RT, chemotherapy and other treatment modalities without separating the different modalities. This probably reflects the huge difficulty in deciding causations, but also makes our comparisons with other studies difficult. One attempt to separate the effect of RT from other treatment modalities was done in a Japanese multicentre study with various diagnoses. The patients were stratified on RT treatment (113 patients) or no RT treatment (72 patients), median FU time >10 years. Late effects grades ≥2 were 68% and 36%, respectively (p = .007) [Citation47]. It was, however, not stated whether RT or chemotherapy were the most likely causes of the complications (more patients in the RT group than in the non-RT group had antracyclines, alkylating agents, etoposide, and stem cell transplantations, while more patients in the non-RT group were operated on).
To conclude, our most important finding was the low proportion of severe late complications, 4% at 1-year FU, 5% at 3-year, 11% at 5-year, and 1 of 28 patients at 10-year FU, compared to our own Radtox registry data for a mixed cohort of 497 patients (brain tumours 39%, sarcoma 23%, leukemia 14%) treated with photon (419 patients) or proton (78 patients) RT from January 2008 to June 2014 with FU ≤5 years; 10% at 1-year FU, 21% at 3-year FU, and 30% at 5-year FU [Citation48]. Even though this cohort included fewer patients with a greater variety of diagnoses, which could influence the statistical significance, proton therapy seems to be a good alternative for treating paediatric patients.
Ethics statement
The study was approved by The Swedish Ethical Review Authority (Dnr 2021-01284) in accordance with the Declaration of Helsinki.
Supplemental Material
Download Zip (65 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Raw data is available from the corresponding author on request.
Additional information
Funding
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073.
- Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2010. Bethesda: National Cancer Institute; 2013.
- Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. doi: 10.1056/NEJMsa060185.
- Geenen MM, Cardous-Ubbink MC, Kremer LCM, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297(24):2705–2715. doi: 10.1001/jama.297.24.2705.
- Kortelainen S, Harju T, Juntti H, et al. Late effects and survival of children with malignant solid tumours in Northern Finland: a single-centre cohort study. Eur J Pediatr. 2022;181(6):2263–2272. doi: 10.1007/s00431-022-04399-7.
- Suh E, Stratton KL, Leisenring WM, et al. Late mortality and chronic health conditions in long-term survivors of early-adolescents and young adult cancers: a retrospective cohort analysis from the childhood cancer survivor study. Lancet Oncol. 2020;21(3):421–435. doi: 10.1016/S1470-2045(19)30800-9.
- Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: a summary from the childhood cancer survivor study. J Clin Oncol. 2009;27(14):2328–2338. doi: 10.1200/JCO.2008.21.1425.
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the radiation therapy oncology group (RTOG) and the european organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341–1346. doi: 10.1016/0360-3016(95)00060-C.
- Martinsson U, Svärd A-M, Taheri Kadkhoda Z, et al. Why are not all paediatric patients treated with protons? A complete national cohort from Sweden 2016–2019. Pediatr Blood Cancer. 2020;67:79.
- Jung B, Montelius A, Dahlin H, et al. The conceptual design of a radiation oncology planning system. Comput Methods Programs Biomed. 1997;52(2):79–92. doi: 10.1016/s0169-2607(96)01783-x.
- Grusell E, Montelius A, Russel KR, et al. Patient positioning for fractionated precision radiation treatment of targets in the head using fiducial markers. Radiother Oncol. 1994;33(1):68–72. doi: 10.1016/0167-8140(94)90088-4.
- Pedroni E, Bacher H, Blattman T, et al. The 200-MeV proton therapy project at the Paul Scherrer institute: conceptual experimental tests of proton beam localization. Med Phys. 1995;22(1):37–53. doi: 10.1118/1.597522.
- Karlsson M, Björk-Eriksson T, Mattsson O, et al. Distributed proton radiation therapy-a new concept for advanced competence support. Acta Oncol. 2006;45(8):1094–1101. doi: 10.1080/02841860600897876.
- Ducatman BS, Scheithauer BW. Postirradiation neurofibrosarco-mas. Cancer. 1983;51(6):1028–1033. doi: 10.1002/1097-0142(19830315)51:6<1028::AID-CNCR2820510610>3.0.CO;2-3.
- Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32(12):1218–1227. doi: 10.1200/JCO.2013.51.1055.
- Howell CR, Bjornard KL, Ness KK, et al. Cohort profile: the St. Jude lifetime cohort study (SJLIFE) for paediatric cancer survivors. Int J Epidemiol. 2021;50(1):39–49. doi: 10.1093/ije/dyaa203.
- Gawade PL, Hudson MM, Kaste SC, et al. A systematic review of dental late effects in survivors of childhood cancer. Pediatr Blood Cancer. 2014;61(3):407–416. doi: 10.1002/pbc.24842.
- Kosuda S, Satoh M, Yamamoto F, et al. Assessment of salivary gland dysfunction following chemoradiotherapy using quantitative salivary gland scintigraphy. Int J Radiat Oncol Biol Phys. 1999;45(2):379–384. doi: 10.1016/s0360-3016(99)00166-2.
- Goldstein M, Maxymiw WG, Cummings BJ, et al. The effects of antitumor irradiation on mandibular opening and mobility: a prospective study of 58 patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88(3):365–373. doi: 10.1016/s1079-2104(99)70044-2.
- Dörr W, Kallfel S, Herrmann T. Late bone and soft tissue sequelae of childhood radiotherapy relevance of treatment age and radiation dose in 146 children treated between 1970 and 1997. Strahlenther Onkol. 2013;189(7):529–534. doi: 10.1007/s00066-013-0361-y.
- Fletcher BD. Effects of pediatric cancer therapy on the musculoskeletal system. Pediatr Radiol. 1997;27(8):623–636. doi: 10.1007/s002470050201.
- Karsila-Tenovuo S, Jahnukainen K, Peltomaki T, et al. Disturbances in craniofacial morphology in children treated for solid tumors. Oral Oncol. 2001;37(7):586–592. doi: 10.1016/s1368-8375(01)00002-1.
- Denys D, Kaste SC, Kun LE, et al. The effects of radiation on craniofacial skeletal growth: a quantitative study. Int J Pediatr Otorhinolaryngol. 1998;45(1):7–13. doi: 10.1016/s0165-5876(98)00028-7.
- Fromm M, Littman P, Raney RB, et al. Late effects after treatment of twenty children with soft tissue sarcomas of the head and neck. Experience at a single institution with a review of the literature. Cancer. 1986;57(10):2070–2076. doi: 10.1002/1097-0142(19860515)57:10<2070::AID-CNCR2820571032>3.0.CO;2-G.
- Sonis AL, Tarbell N, Valachovic RW, et al. Dentofacial development in long-term survivors of acute lymphoblastic leukemia. A comparison of three treatment modalities. Cancer. 1990;66(12):2645–2652. doi: 10.1002/1097-0142(19901215)66:12<2645::AID-CNCR2820661230>3.0.CO;2-S.
- Guyuron B, Dagys AP, Munro IR, et al. Effect of irradiation on facial growth: a 7- to 25-year follow-up. Ann Plast Surg. 1983;11(5):423–427. doi: 10.1097/00000637-198311000-00010.
- Pacheco R, Stock H. Effects of radiation on bone. Curr Osteoporos Rep. 2013;11(4):299–304. doi: 10.1007/s11914-013-0174-z.
- Niewald M, Mang K, Barbie O, et al. Dental status, dental treatment procedures and radiotherapy as risk factors for infected osteoradionecrosis (IORN) in patients with oral cancer – a comparison of two 10 years’ observation periods. Springerplus. 2014;3:263. doi: 10.1186/2193-1801-3-263.
- Weinstein RS. Glucocorticoid-induced osteonecrosis. Endocrine. 2012;41(2):183–190. doi: 10.1007/s12020-011-9580-0.
- Suryani IR, Ahmadzai I, Shujaat S, et al. Non-antiresorptive drugs associated with the development of medication-related osteonecrosis of the jaw: a systematic review and metaanalysis. Clin Oral Investig. 2022;26(3):2269–2279. doi: 10.1007/s00784-021-04331-7.
- Söderström H, Brocki K, Lundin J, et al. Neurocognitive functions Before and After radiotherapy in pediatric brain tumor survivors. Pediatr Neurol. 2022;133:21–29. doi: 10.1016/j.pediatrneurol.2022.05.006.
- Sklar CA, Antal Z, Chemaitilly W, et al. Hypothalamic-Pituitary and growth disorders in survivors of childhood cancer: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(8):2761–2784. doi: 10.1210/jc.2018-01175.
- Suzuki J, Takaku A. Cerebrovascular ’moyamoyá disease: a disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969;20(3):288–299. doi: 10.1001/archneur.1969.00480090076012.
- Desai SS, Paulino AC, Mai WY, et al. Radiation-induced moyamoya syndrome. Int J Radiat Oncol Biol Phys. 2006;65(4):1222–1227. doi: 10.1016/j.ijrobp.2006.01.038.
- Clemens E, van den Heuvel-Eibrink MM, Mulder RL, et al. Recommendations for ototoxicity surveillance for childhood, adolescent, and young adult cancer survivors: a report from the international late effects of childhood cancer guideline harmonization group in collaboration with the PanCare consortium. Lancet Oncol. 2019;20(1):e29–e41. doi: 10.1016/S1470-2045(18)30858-1.
- Meyer AK, Young NM. Auditory late effects of chemotherapy. Cancer Treat Res. 2009;150:195–213.
- Nasman M, Bjork O, Soderhall S, et al. Disturbances in the oral cavity in pediatric long-term survivors after different forms of antineoplastic therapy. Pediatr Dent. 1994;16:217–223.
- Vissink A, Jansma J, Spijkervet FKL, et al. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14(3):199–212. doi: 10.1177/154411130301400305.
- Effinger KE, Migliorati CA, Hudson MM, et al. Oral and dental late effects in survivors of childhood cancer: a children’s oncology group report. Support Care Cancer. 2014;22(7):2009–2019. doi: 10.1007/s00520-014-2260-x.
- Gawade PL, Hudson MM, Kaste SC, et al. Systematic review of selected musculoskeletal late effects in survivors of childhood cancer. Curr Pediatr Rev. 2014;10(4):249–262. doi: 10.2174/1573400510666141114223827.
- Paulino AC, Wen BC, Brown CK, et al. Late effects in children treated with radiation therapy for Wilms’ tumor. Int J Radiat Oncol Biol Phys. 2000;46(5):1239–1246. doi: 10.1016/s0360-3016(99)00534-9.
- van Dijk IW, Oldenburger F, Cardous-Ubbink MC, et al. Evaluation of late adverse events in longterm Wilms’ tumor survivors. Int J Radiat Oncol Biol Phys. 2010;78(2):370–378. doi: 10.1016/j.ijrobp.2009.08.016.
- Bright CJ, Reulen RC, Winter DL, et al. Risks of subsequent primary neoplasms in survivors of adolescents and young adult cancer (teenage and young adult cancer survivor study): a population-based, cohort study. Lancet Oncol. 2019;20(4):531–545. doi: 10.1016/S1470-2045(18)30903-3.
- Palmer, Tsang Tinkle. Late effects of radiation therapy in paediatric patients and survivorship. Pediatr Blood Cancer. 2021;68(suppl 2):e28349.
- Leiser D, Calaminus G, Malyapa R, et al. Tumour control and quality of life in children with rhabdomyosarcoma treated with pencil beam scanning proton therapy. Radiother Oncol. 2016;120(1):163–168. doi: 10.1016/j.radonc.2016.05.013.
- Yock TI, Yeap BY, Ebb DH, et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: a phase 2 single-arm study. Lancet Oncol. 2016;17(3):287–298. doi: 10.1016/S1470-2045(15)00167-9.
- Ishida Y, Sakamoto N, Kamibeppu K, et al. Late effects and quality of life of childhood cancer survivors: part 2. Impact of radiotherapy. Int J Hematol. 2010;92(1):95–104. doi: 10.1007/s12185-010-0611-z.
- Martinsson U, Kristensen I, Swärd A-M. Grade 3 and 4 late side effects after radiotherapy – data from the Swedish RADTOX registry. Edinburgh: ESLCCC (European Symposium on Late Complications after Childhood Cancer); 2014.
