Abstract
Background
In this study we present the Tracking Accessory 3 (TA3) as an alternative to the commercial gating block (GB) surrogate for the Varian Truebeam™ gating system (TGS). The TGS requires three visible reflectors to track the surrogate, presenting an opportunity for a surrogate to be made with less material and thus smaller dosimetric footprint than the commercial four reflector model.
Materials and methods
Relative dose and depth dose profiles below the TA3 and the GB were measured with radiosensitive film. Accuracy and reproducibility of the detected motion amplitude for three TA3s and one GB were determined using a respiratory phantom with surrogate to determine the camera’s tracking volume. Clinical performance was evaluated prospectively in 10 breast cancer patients treated with deep inspiration breath hold monitored with TA3 and compared to previously published results. Non-parametric statistics were applied to test for significance.
Results and conclusions
Surface doses were increased up to 94% and 187% for the TA3 and GB, respectively, compared to no surrogate. The surface area influenced by at least 25% increase in dose was 12 cm2 and 105 cm2 for the TA3 and GB, respectively. The water equivalent thickness of the surrogates was found to be 1 mm for the TA3 and 3 mm for GB. The difference in measured amplitude were <0.2 mm for TA3 compared to the GB. The TA3s and GB were detected at all extremes of the clinically relevant tracking volume of the TGS. Clinical performance showed no significant differences. The TA3 caused less surface dose increase compared to the commercial GB. In the tested range all surrogates measured motion amplitude within 0.2 mm of reference value, which is not a clinically relevant difference. The TA3 showed no significant differences in clinical performance to similarly positioned surrogates.
Background
In external beam radiotherapy, deep inspiration breath hold (DIBH) is used during treatment of different thoracic and abdominal cancers [Citation1,Citation2]. Specifically, DIBH has proven a valuable method to reduce dose to the heart and lungs when treating breast cancer patients [Citation3–6]. The most common methods for monitoring the thorax position of the patient in DIBH are passive external surrogate tracked by an optical or infrared camera [Citation3,Citation5–11], spirometry [Citation12] or surface monitoring with optical cameras and structured light [Citation13–16]. When using an external surrogate for monitoring breast cancer patients during treatment, the closer the surrogate is positioned to the treated breast in the cranio-caudal and lateral direction the better it represents the motion of the breast [Citation9,Citation17,Citation18]. However, such a surrogate would have to ideally be placed close to or within the path of the treatment beams with the risk of introducing a bolus effect leading to increased risk of erythema and skin irritation [Citation19,Citation20]. Therefore, surrogates for DIBH should be as small and light as possible. Previously, we have shown that it is possible to optimize the external surrogate for the Real-time Position Management (RPM™, Varian Medical Systems Inc, California, USA) system to obtain a more flexible positioning of the surrogate while also reducing the bolus effect [Citation21]. However, the surrogate for the RPM system is not compatible with the Truebeam gating system (Varian Medical Systems Inc, California, USA) necessitating a complete redesign of the surrogate using a minimal amount of material.
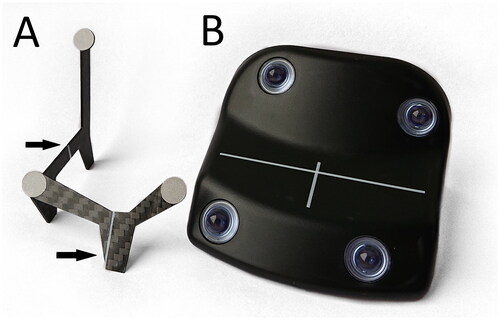
The gating block (GB), see , for the Truebeam gating system has a footprint of 58 cm2. Due to its size and shape it is difficult to position the GB close to the targeted breast. The GB was previously measured to have a water equivalent thickness of 3-4.5 mm [Citation17,Citation22], which causes increased skin dose if the surrogate is placed in the treatment beam. Here we present an alternative to the GB for the Truebeam gating system, the Tracking Accessory 3 (TA3), see , consisting of less material with a smaller footprint compared to the GB. Previously, a prototype of the TA3 was presented [Citation22] and here, a similarevaluation was performed in addition to assessing clinical performance. In this study, we aimed to validate an alternative respiratory surrogate for use with the Varian Truebeam gating system that would reduce skin dose and improve placement flexibility.
Materials and methods
Tracking accessory 3
The TA3 consisted of two pieces of 1 mm thick carbon-fiber with three reflective markers, see . The reflective markers had a diameter of 9 mm and were positioned to mimic three of the four reflective spheres of the GB as this was sufficient to track the motion of the surrogate by the infrared camera of the Truebeam gating system (Varian Medical Systems Inc, California, USA). The maximum length, width and height of the TA3 were 7.0 cm, 6.0 cm, and 5.7 cm, respectively, and the total area of the physical footprint in contact with the surface was 0.02 cm2. The reflective markers were elevated 5-10 mm compared to the position of the reflective markers on the GB to improve visibility of the markers to the infrared camera. TA3 had longitudinal and lateral alignment lines similar to those of the GB to help positioning the device relative to the in-room lasers in the treatment room. The TA3 was designed and manufactured by Dennis Langhoff from Langhoff Teknik, Denmark.
Film measurements
Gafchromic EBT-XD dosimetry film (Ashland Advanced Materials, New Jersey, USA) was used to evaluate dose under the surrogates at the surface and at depth. Each film was scanned once in 48 bit colors with positive film settings and analyzed in the green channel.
Two-dimensional profiles were measured with HE Solid Water™ (Gammex Inc, Wisconsin, USA) build-up of 0 mm, 2 mm, and 5 mm for the surrogates as well as without any surrogate. The films were placed on top of 10 cm thick HE Solid Water and were irradiated with a 15 × 15 cm2 6 MV photon beam delivering 2000 MU with an incident angle of 0° and source-to-surface distrance of 100 cm.
Percentage depth-dose curves (PDDs) were measured for each surrogate by placing film vertically under the surrogate between two stacks of HE Solid Water slabs of 5 cm thickness aligning the film horizontally to the top of the HE Solid Water. For the GB, the PDD was obtained below the two reflective spheres on the left of the surrogate seen from the infrared camera. For the TA3, the PDD was obtained where the strongest signal was detected in the surface profile, which was below the front foot seen from the camera. An additional 6 cm backscatter of HE Solid Water was placed below the setup. The setup was irradiated with a 15 × 15 cm2 6 MV photon beam delivering 2000 MU with an incident angle of 0° and source-to-surface distrance of 100 cm.
All films were converted to optical density in Doselab (Varian Medical Systems Inc, California, USA). Planar optical density maps were smoothed in Doselab. Profiles and PDD were generated in ImageJ (U. S. National Institute of Health, Maryland, USA), using a region of interest with a width of approximately 2.5 mm. In case of the film with no surrogate, the central part of the film was analyzed. Dose profiles were measured through the area of the film with maximum signal relative to no surrogate. PDDs from films irradiated with a surrogate were measured below the greatest surface enhancement relative to the edge of the film. Extracted PDDs were exported to R and smoothed. Water equivalent thickness was determined by manual read-out of depth of maximum dose from the smoothed PDD curve relative to no surrogate.
Tracking of the respiratory signal
To compare the tracking capabilities of the TA3 and GB, we used the Truebeam gating system, with a ceiling-mounted infrared camera for tracking. The respiratory motion of a patient was simulated with a QuasarTM phantom (Modus QA, Ontario, Canada) set to 1 cm vertical peak to peak amplitude. Measurements were performed with three different TA3s and one GB. With the GB at the isocenter without any displacements or tilts, the Truebeam gating system measured an amplitude of 1.03 cm; we defined this as the reference amplitude of the motion. The tracking volume of the infrared camera, where the amplitudes of the surrogates were correctly detected, was tested by displacing the phantom, in the vertical (vrt), longitudinal (lng) and lateral (lat) directions as well as rotating in yaw, pitch and roll.
For translational shifts, each surrogate was placed at isocenter and moved in steps of 10 cm until the camera no longer was able to accurately track the motion of the surrogate. For yaw, the couch was rotated in steps of 30°, where positive was counterclockwise. The effect of pitch and roll of the surrogates were measured by placing each surrogate close to the isocenter on a wedge with inclinations of ±10°, ±20°. Each rotational and displacement axis was tested independently.
Clinical performance during treatment
From January 2023, breast cancer patients receiving external beam radiotherapy in DIBH, treated on a Truebeam, were monitored using the TA3 as standard clinical practice at the Department of Oncology and Palliative care, Zealand University Hospital. The first ten consecutively treated patients were included in this study. Patient data was acquired as part of quality assurance in the implementation phase of TA3 and followed regional guidelines for use of patient data including anonymization and data handling. For all patients the respiratory motion during planning CT acquisition was monitored by the RPM system version 1.7 using the surrogate we have previously described [Citation21]. Patient simulation and treatment setup including DIBH was performed following the procedure described by Damkjær et al. [Citation21] Surrogates for both patient groups were placed on the sternum. The difference in points of tracking, for the TA3 and novel surrogate previously published, were accounted for by offsetting the TA3 such that the virtual point at the intersection of the inscribed lines used for alignment (which is the point the Truebeam gating system reports) was in the same position as the front of the novel surrogate which the RPM system reports.
All patients were treated with 3DCRT or VMAT in 15 fractions in DIBH. All patients were aligned using orthogonal 2D-2D kV images acquired at 0˚/180˚ and 270˚. Treatment verification was performed every 5th fraction by either portal MV images of the medial treatment field (3DCRT) or kV images in the approximate primary medial field angle (VMAT).
Portal verification images or kV images acquired of the medial field during the 6th and 11th fractions were registered rigidly in translation only to digitally reconstructed radiographs. 3D information was obtained by applying Pythogoras to the reported lateral, longitudinal and vertical shifts. Two clinically experienced physicists performed the registrations for half of the patients each. The residual shifts were compared to shifts published in Damkjær et al. [Citation21] where the same patient setup, IGRT strategy and treatment planning was used. For these patients, the surrogate was positioned on the sternum as the TA3 was in this study. Patient data with the GB positioned on the sternum were not collected, since it would increase the risk of skin reactions due to the reported bolus equivalent thickness of this surrogate [Citation17,Citation22].
Non-parametric statistics (Mann-Whitney) was used to compare the data and p < 0.05 was considered significant.
Results
Dose profiles below the surrogates
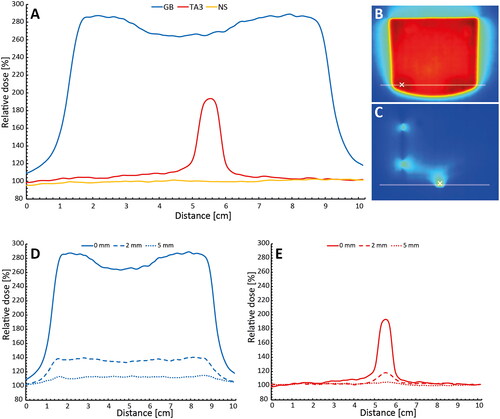
shows profiles of surface dose under the surrogates compared to no surrogate, as well as profiles at 2 mm and 5 mm depth. The relative dose directly under the surrogates in shows profiles in an area of maximum dose increase extracted from the 2D dose films shown for GB in and for TA3 in . For the TA3, the dose at the surface increased by up to 94% and for the GB up to 187% compared to no surrogate. The area of the surface influenced by a 25% increase in dose was 12 cm2 and 105 cm2 for the TA3 and GB, respectively. show the relative dose at depths 0 mm, 2 mm, and 5 mm, respectively, for the TA3 and GB normalized to no surrogate. The TA3 caused an increase in dose by up to 18% and 5% at 2 mm, and 5 mm depths respectively, while the GB increased the dose up to 40% and 14% at the same depths. Overall, the TA3 induced less surface dose in a smaller volume compared to the GB.
Figure 2. Relative dose measured under the TA3 and GB exposed to a 15 x 15 cm2 field of 6 MV photons. (A) Film profiles measured at the surface directly under the TA3 and GB as well as a profile with no surrogate (NS) present. (B,C) 2D dose directly below the GB and TA3, respectively, where the lines represent the position of the respective profiles shown in (a), the ‘x’ in the images represents where the depth dose curves in were measured. (D,E) Profiles at depths 0 mm, 2 mm, and 5 mm for TA3 and GB.
Depth-dose curves
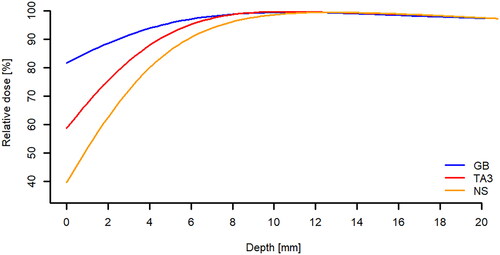
shows the smoothed PDDs for the two surrogates and no surrogate. PDDs were measured below the most absorbing part of the TA3 and through the two reflective spheres in the left side of the GB seen from the infrared camera and with no surrogate. The points where the PDDs were measured are marked in . The TA3 and GB were found to have a water equivalent thickness of 1 mm and 3 mm, respectively. Surface doses from the depth dose curves underestimate the real surface dose as the film has aberrant values at the edge which means dose has to be extrapolated for the outer 1-2 mm.
Figure 3. Smoothed percentage depth dose curves below the surrogates and with no surrogate (NS). The points of measurement were indicated in for the GB and TA3, respectively. The corresponding water equivalent thickness determined by shift in depth of maximum dose was 1 mm and 3 mm for the TA3 and the GB, respectively.
Tracking of the respiratory signal
and show the measured amplitudes and range of tracking with respect to the isocenter for vrt, lng, lat, yaw, pitch and roll. For vrt and lat, the TA3 and GB performed identically. The tracking of TA3 stopped when moved more than 50 cm towards the camera compared to 70 cm for the GB. In yaw, the GB could be tracked while rotated up to ±60° and the TA3 could only be tracked in the range −35° to 40° due to the asymmetry of the reflective markers. Tracking of surrogates with pitch and roll was identical for the TA3 and GB. In all directions and rotations, the absolute difference of the mean amplitude was ≤0.01 cm compared to the reference.
Table 1. Mean amplitudes and range for phantom displacements in vertical, longitudinal and lateral with respect to isocenter.
Table 2. Mean amplitudes and range for phantom rotations in yaw, pitch and roll.
Clinical performance during treatment
Mean 3D vector displacement of the portal verification images was 2.5 mm ± 1.3 mm for the patients monitored with the TA3 versus 3.7 mm ± 1.1 mm for the patients monitored with the novel surrogate published previously [Citation21]. This difference was not found to be statistically significant (p = 0.07). Residual displacements in the patients monitored with TA3 were similar along each cardinal direction at 1.4 mm ± 1.0 mm in vertical, 1.4 mm ± 1.1 mm in longitudinal and 1.1 mm ± 0.8 mm in lateral.
Discussion
In this work we presented an alternative surrogate, the Tracking Accessory 3 (TA3) for the Truebeam gating system. We compared surface dose, tracking performance and water equivalent thickness between the TA3 and the commercial gating block (GB). Clinical performance was investigated by comparing residual shifts of portal verification images ten breast cancer patients monitored with the novel respiratory surrogate [Citation21] to ten patients monitored with TA3.
TA3s narrow feet and tripod design enabled positioning of the surrogate on the sternum, regardless of patient adiposity or chest curvature. The elevation of the reflective markers made the TA3 visible to the Truebeam gating system almost regardless of the shape and adiposity of the patient thus avoiding the use of additional foam material as was used by Lutz et al. [Citation9] The GB, however, pose a greater challenge when positioning it on the sternum due to the larger base.
It has been shown that the closer the surrogate is placed to the breast in cranial-caudal and lateral directions the better it correlates with target motion [Citation9,Citation17]. However, when positioning the surrogate closer to the breast, it is more likely to be in the treatment beam. For the GB it is likely there will be a patch of skin which will be affected by the bolus effect in all treatment fractions due to the size of the increased dose area. However, the TA3 has a very small dosimetric footprint which makes it unlikely to affect the exact same patch of skin, decreasing the risk of additional skin dose [Citation19,Citation20].
The range of tracking for the TA3 was smaller than the GB in longitudinal direction and yaw. If the TA3 is moved more than 50 cm from the isocenter towards the infrared camera the Truebeam gating system may report a value too low because of partial reflector obscuration.
However, the longitudinal position of the surrogate for breast radiotherapy in DIBH will be closer to the isocenter since the isocenter will not be positioned cranially of the clavicle. For external beam radiotherapy of breast cancer patients, yaw is typically 0° when calculating the treatment plan, however, smaller adjustment of the yaw can be included during IGRT (at our institutions below 10°). Therefore, the performance of the TA3 is identical to the GB within the clinically relevant range. Nielsen et al. [Citation22] observed that the detected amplitude could be incorrect in the case where all three reflective markers could not be tracked during the full breathing cycle. In this study, we did not observe this behavior in the range reported in and , due to the different respiratory phantom design.
The Respiratory Gating for scanners system (RGSC, Varian Medical Systems Inc, California, USA) is a single camera system that requires four reflectors and is therefore not compatible with the TA3. The RGSC can be used with the GB and as skin dose is not a concern for CT scanning to the same extent as treatment the TA3 is not necessary for CT scanning. RPM uses the two-reflector surrogate which is also not compatible with TA3. There is no risk of error in surrogate positioning between RGSC and TGS as the tracking point of the GB and TA3 is the same. There is a risk of tracking point error when using RPM for CT simulation and TA3 or GB for treatment as the tracking point of the RPM is the front of the surrogate and the midpoint of the TA3 or GB, this may cause a difference in amplitude between CT scanning and treatment.
There are limitations in this work. In the analysis of the clinical performance, we compared to data obtained with a different surrogate and different system which may have increased uncertainty. Using 2D images to determine 3D shift has some uncertainties, so residual displacement along each of the tree cardinal directions should be considered approximations. However, this limitation did not affect the total displacement. Since the TA3 only recently has been introduced clinically, our study and clinical experience extends only to the ten included patients.
Conclusion
The Tracking Accessory 3 was found to introduce considerably less bolus effect than the gating block for the Truebeam gating system. The tracking volume of the camera was smaller for the TA3 than the GB, however both were more than adequate for clinical use. The clinical performance, evaluated as residual displacement in portal verification images, for breast cancer patients was on par with a similar setup where the position of surrogate, IGRT and patient positioning were the same. Therefore, we conclude that the Tracking Accessory 3 can be used advantageously for breast cancer patients treated with gated external beam radiotherapy.
Acknowledgments
The authors wish to acknowledge Dennis Langhoff of Langhoff Teknik for providing working prototypes.
Disclosure statement
The authors report no conflicts of interest to disclose.
Data availability statement
The data that support the findings of this study are available from the corresponding author, SMSD, upon reasonable request.
References
- Josipovic M, Aznar MC, Thomsen JB, et al. Deep inspiration breath hold in locally advanced lung cancer radiotherapy: validation of intrafractional geometric uncertainties in the INHALE trail. Br J Radiol. 2019;92(1104):20190569. doi: 10.1259/bjr.20190569.
- Petersen PM, Aznar MC, Berthelsen AK, et al. Prospective phase II trail of image-guided radiotherapy in hodgkin lymphoma: benefit of deep inspiration breath-hold. Acta Oncol. 2015;54(1):60–66. doi: 10.3109/0284186X.2014.932435.
- Bruzzaniti V, Abate A, Pinnarò P, et al. Dosimetric and clinical advantages of deep inspiration breath-hold (DIBH) during radiotherapy of breast cancer. J Exp Clin Cancer Res. 2013;32(1):88. doi: 10.1186/1756-9966-32-88.
- Borst GR, Sonke JJ, den Hollander S, et al. Clinical results of image-guided deep inspiration breath hold breast irradiation. Int J Radiat Oncol Biol Phys. 2010;78(5):1345–1351. doi: 10.1016/j.ijrobp.2009.10.006.
- Pedersen AN, Korreman S, Nyström H, et al. Breathing adapted radiotherapy of breast cancer: reduction of cardiac and pulmonary doses using voluntary inspiration breath-hold. Radiother Oncol. 2004;72(1):53–60. doi: 10.1016/j.radonc.2004.03.012.
- Hjelstuen MHB, Mjaaland I, Vikström J, et al. Radiation during deep inspiration allows loco-regional treatment of left breast and axillary-, supraclavicular- and internal mammary lymph nodes without compromising target coverage or dose restrictions to organs at risk. Acta Oncol. 2012;51(3):333–344. doi: 10.3109/0284186X.2011.618510.
- Latty D, Stuart KE, Wang W, et al. Review of deep inspiration breath-hold techniques for the treatment of breast cancer. J Med Radiat Sci. 2015;62(1):74–81. doi: 10.1002/jmrs.96.
- Damkjær SMS, Aznar MC, Pedersen AN, et al. Reduced lung dose and improved inspiration level reproducibility in visually guided DIBH compared to audio coached EIG radiotherapy for breast cancer patients. Acta Oncol. 2013;52(7):1458–1463. doi: 10.3109/0284186X.2013.813073.
- Lutz CM, Poulsen PR, Fledelius W, et al. Setup error and motion during deep inspiration breath-hold breast radiotherapy measured with continuous portal imaging. Acta Oncol. 2016;55(2):193–200. doi: 10.3109/0284186X.2015.1045625.
- Stranzl H, Zurl B, Langsenlehner T, et al. Wide tangential fields including the internal mammary lymph nodes in patients with left-sided breast cancer. Strahlenther Onkol. 2009;185(3):155–160. doi: 10.1007/s00066-009-1939-2.
- McIntosh A, Shoushtari AN, Benedict SH, et al. Quantifying the reproducibility of heart position during treatment and corresponding delivered heart dose in voluntary deep inhalation breath hold for left breast cancer patients treated with external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2011;81(4):e569–e576. doi: 10.1016/j.ijrobp.2011.01.044.
- Mittauer K, Deraniyagala R, Li JG, et al. Monitoring ABC-assisted deep inspiration breath hold for left-sided breast radiotherapy with an optical tracking system. Med Phys. 2015;42(1):134–143. doi: 10.1118/1.4903511.
- Cerviño LI, Gupta S, Rose MA, et al. Using surface imaging and visual coaching to improve the reproducibility and stability of deep-inspiration breath hold for left-breast-cancer radiotherapy. Phys Med Biol. 2009;54(22):6853–6865. doi: 10.1088/0031-9155/54/22/007.
- Tang X, Zagar TM, Bair E, et al. Clinical experience with 3-dimensional surface matching-based deep inspiration breath hold for left-sided breast cancer radiation therapy. Pract Radiat Oncol. 2014;4(3):e151–e158. doi: 10.1016/j.prro.2013.05.004.
- Kalet AM, Cao N, Smith WP, et al. Accuracy and stability of deep inspiration breath hold in gated breast radiotherapy - A comparison of two tracking and guidance systems. Phys Med. 2019;60:174–181. doi: 10.1016/j.ejmp.2019.03.025.
- Wikström K, Isacsson U, Nilsson K, et al. Reproducibility of heart and thoracic wall position in repeated deep inspiration breath hold for radiotherapy of left-sided breast cancer patients. Acta Oncol. 2018;57(10):1318–1324. doi: 10.1080/0284186X.2018.1490027.
- Conroy L, Guebert A, Smith WL. Technical note: issues related to external marker block placement for deep inspiration breath hold breast radiotherapy. Med Phys. 2017;44(1):37–42. doi: 10.1002/mp.12005.
- Skyttä T, Kapanen M, Laaksomaa M, et al. Improving the reproducibility of voluntary deep inspiration breath hold technique during adjuvant left-sided breast cancer radiotherapy. Acta Oncol. 2016;55(8):970–975. doi: 10.3109/0284186X.2016.1161823.
- Bray FN, Simmons BJ, Wolfson AH, et al. Acute and chronic cutaneous reactions to ionizing radiation therapy. Dermatol Ther (Heidelb). 2016;6(2):185–206. doi: 10.1007/s13555-016-0120-y.
- Ryan JL. Ionizing radiation: the good, the bad, and the ugly. J Invest Dermatol. 2012;132(3 Pt 2):985–993. doi: 10.1038/jid.2011.411.
- Damkjær SMS, Jensen NKJ, Fog LS, et al. A novel surrogate for motion management in external beam radiotherapy of breast cancer patients. Acta Oncol. 2021;60(11):1432–1435. doi: 10.1080/0284186X.2021.1949035.
- Nielsen MMB, Jensen NKJ, Damkjær SMS. Gating device L: an external surrogate for respiratory motion tracking on truebeam. Radiother Oncol. 2022;170(1)::1385–1386.