Abstract
Background: Sodium glucose co-transporter 2 (SGLT2) inhibitors lower blood glucose levels in patients with type 2 diabetes mellitus (T2DM) by increasing urinary glucose excretion. This review provides a comprehensive summary of preclinical and clinical data on the effects of the SGLT2 inhibitor canagliflozin on mineral balance and bone.
Methods: Published articles and internal study reports through November 2015 were included.
Results: In clinical studies, canagliflozin was not associated with meaningful changes in serum or urine calcium, parathyroid hormone, or vitamin D. Canagliflozin was associated with increases in serum magnesium and phosphate without changes in their urinary excretion. Increases in serum collagen type-1 beta-carboxy-telopeptide (beta-CTX), a bone resorption marker, and osteocalcin, a bone formation marker, were observed with canagliflozin. Decreases in total hip bone mineral density (BMD) of up to 1.2% were seen with canagliflozin after 2 years; no changes in BMD were seen at other skeletal sites. Changes in total hip BMD and serum beta-CTX with canagliflozin correlated with decreases in body weight. In a clinical program-wide analysis, canagliflozin was associated with increased fracture risk that was driven by a higher incidence in the cardiovascular safety study (CANVAS), with no fracture imbalance seen in pooled data from other Phase 3 studies. The fracture imbalance occurred within 12 weeks after initiating treatment, most frequently in the distal portion of the upper and lower extremities.
Conclusions: Across clinical studies, canagliflozin did not meaningfully affect calcium homeostasis or hormones regulating calcium homeostasis. Increases in bone turnover markers and decreases in BMD at the total hip, but not at other sites, that correlated with weight loss were seen with canagliflozin. Canagliflozin was associated with a higher fracture incidence within 12 weeks, primarily in distal extremities. Data from ongoing canagliflozin studies will provide additional information on fracture risk.
Introduction
Sodium glucose co-transporter 2 (SGLT2) inhibitors are a novel class of antihyperglycemic agents (AHAs) developed to treat type 2 diabetes mellitus (T2DM)Citation1,Citation2. In contrast to other classes of AHAs, SGLT2 inhibitors lower blood glucose through an insulin-independent mechanism that targets the kidney to promote urinary glucose excretion (UGE)Citation1–3. Glucose reabsorption in the proximal tubule of the kidney is primarily mediated by the glucose co-transporter protein SGLT2Citation4. SGLT2 inhibition lowers the renal threshold for glucose (RTG) in patients with T2DM, thereby increasing UGE and reducing blood glucose levelsCitation5–7. Increased UGE also results in a net loss of calories and mild osmotic diuresis that may contribute to reductions in body weight and blood pressureCitation5–7.
Based on the potential for inhibition of tubular glucose reabsorption to affect mineral metabolism and possibly bone health, a comprehensive preclinical and clinical program was undertaken to examine the effects of canagliflozin on mineral balance and bone health. This manuscript provides a comprehensive summary of these findings.
SGLT1 and SGLT2 inhibition
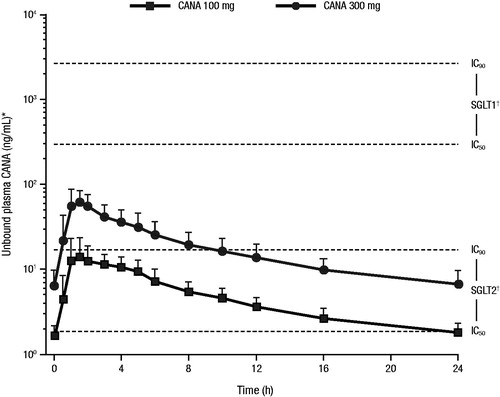
Sodium glucose co-transporter 1 (SGLT1) and SGLT2 are expressed in the proximal renal tubule and are responsible for glucose reabsorption in the kidney, with the majority of glucose reabsorption occurring via SGLT2Citation2,Citation8. SGLT1 is also expressed in the intestine and is the primary mediator of glucose and galactose reabsorptionCitation2,Citation8. To our knowledge, there are no data relating to the expression of SGLT2 in relevant bone-derived cell types relating to bone homeostasis, such as osteoblasts or osteoclasts. However, publications describing expression in bone marrow suggest very low to undetectable levels of expression of SGLT2 in this tissueCitation9–11. Taken together, these data suggest that SGLT2 is unlikely to play a major functional role within bone tissue. Canagliflozin is a high-potency SGLT2 inhibitor and a low-potency inhibitor of SGLT1Citation12,Citation13. The canagliflozin blood levels achieved with the approved doses (100 and 300 mg) relative to the IC50 and IC90 values for SGLT2 and SGLT1 are shown in Citation14. Both the 100 and 300 mg doses maintain plasma canagliflozin levels above the IC50 for SGLT2 inhibition throughout the day, while neither dose achieves levels near the IC50 for SGLT1 inhibition.
Figure 1. Plasma concentration of canagliflozin over 24 hoursCitation14. CANA, canagliflozin; IC, inhibitory concentration; SD, standard deviation; SGLT, sodium glucose co-transporter. *Pharmacokinetic profiles are mean + SD using a free fraction of 1.5%. †IC50 values are mean values from four experiments; IC90 values are assumed to be 9 × IC50 values.
Preclinical findings and relevance to humans
In rats, SGLT1 inhibition results in carbohydrate malabsorption, increased calcium solubility, enhanced vitamin-D–independent calcium absorption, and hypercalciuriaCitation15–17. Reductions in hormones regulating calcium metabolism and bone turnover, including parathyroid hormone (PTH) and 1,25-dihydroxyvitamin D, were observed in rats treated with canagliflozinCitation18,Citation19. Canagliflozin treatment was associated with hyperostosis (an increase in bone mass) in rats, but not in dogs or miceCitation19. Increases in serum calcium and hyperostosis have also been observed in rats treated with the SGLT2 inhibitors dapagliflozin and empagliflozin and a selective SGLT1 inhibitorCitation15,Citation16,Citation20,Citation21. Avoidance of carbohydrate malabsorption by substituting dietary fructose (transported by glucose transporter 5 [GLUT5] and not by SGLT1Citation22) for glucose prevented canagliflozin-induced hypercalciuria and hyperostosis in ratsCitation18. Thus, canagliflozin-induced carbohydrate malabsorption in rats was likely due to inhibition of intestinal SGLT1-mediated glucose uptakeCitation17–19. There was no evidence of carbohydrate malabsorption in humans treated with canagliflozin (measured by hydrogen breath content and radiolabeled oral glucose ingestion), suggesting that the bone and mineral findings in rats were species-specific and unlikely to be clinically relevantCitation16,Citation17,Citation21.
Clinical findings
Mineral homeostasis
Canagliflozin treatment was not associated with meaningful changes in serum or urine calcium, urine phosphate, urine magnesium, PTH, 1,25-dihydroxyvitamin D, or 25-hydroxyvitamin D (Supplemental Table 1)Citation6,Citation23–29. In a pooled dataset of four placebo-controlled, Phase 3 studies (N = 2215), mean percent changes from baseline in serum calcium were 0.2%, 0.8%, and 1.2% with canagliflozin 100 and 300 mg and placebo, respectively, at Week 26Citation29. Canagliflozin was associated with mean percent increases in serum magnesium (8.1%, 9.3%, and –0.4% with canagliflozin 100 and 300 mg and placebo, respectively) and serum phosphate (3.5%, 5.2%, and 1.4% with canagliflozin 100 and 300 mg and placebo, respectively); despite these increases, serum phosphate and magnesium levels generally remained within the normal limitsCitation29. Similar increases in serum phosphate and magnesium levels have been reported with dapagliflozin in patients with T2DMCitation30,Citation31. No changes in serum phosphate and magnesium have generally been seen with empagliflozin treatmentCitation32–34; however, a recent study in a larger number of patients showed increases in serum phosphate and magnesium similar to those observed with other SGLT2 inhibitorsCitation35.
Bone turnover markers
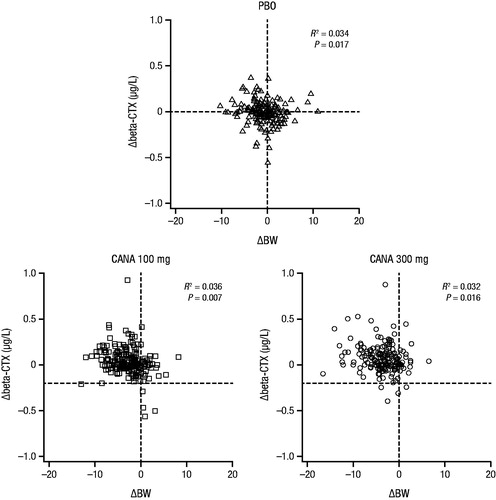
Dose-dependent increases in serum collagen type-1 beta-carboxy-telopeptide (beta-CTX), a bone resorption marker, were seen with canagliflozin treatment; these increases were maximal at 26 weeks and declined thereafter ( and Supplemental Table 1)Citation36. Serum osteocalcin, a bone formation marker, also increased with canagliflozin treatment at 26 weeks and continued to increase through 52 weeksCitation36. There were no significant changes in the bone formation marker amino-terminal type 1 procollagen (P1NP) with canagliflozin treatmentCitation6,Citation37. Increases in beta-CTX were also seen with dapagliflozin (Supplemental Figure 1)Citation38. Note that data for bone turnover markers have not been reported in studies of empagliflozin. Previous studies have shown that weight loss increases bone turnover markers such as beta-CTXCitation39–42. Consistent with findings from weight loss studies, increases in beta-CTX in patients treated with canagliflozin correlated with the degree of weight loss ()Citation36.
Figure 2. Changes from baseline in (A) serum beta-CTX and (B) serum osteocalcin with canagliflozin at Week 52 (study in older patients)Citation36. beta-CTX, serum collagen type-1 beta-carboxy-telopeptide; PBO, placebo; CANA, canagliflozin; LS, least squares; CI, confidence interval. Republished with permission of the Endocrine Society, from Bilezikian et al.Citation36; permission conveyed through Copyright Clearance Center Inc.
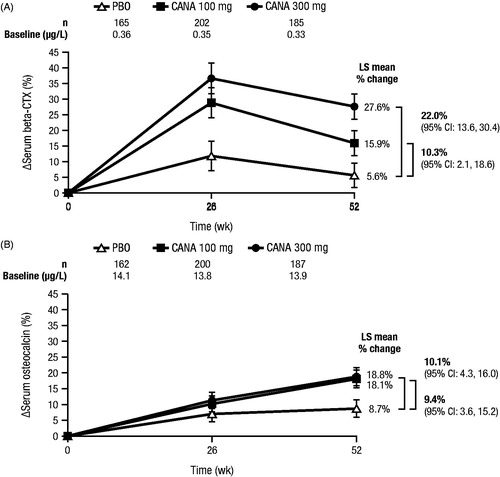
Figure 3. Scatter plot of change in beta-CTX by change in body weight from baseline to Week 52 (study in older patients)Citation36. beta-CTX, collagen type-1 beta-carboxy-telopeptide; BW, body weight; PBO, placebo; CANA, canagliflozin. Republished with permission of the Endocrine Society, from Bilezikian et al.Citation36; permission conveyed through Copyright Clearance Center Inc.
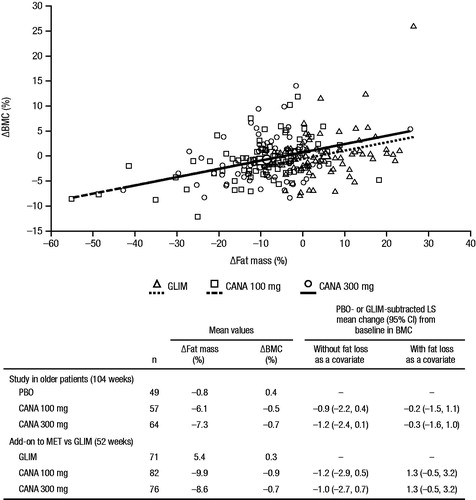
Bone mineral density
Changes in bone mineral density (BMD) were assessed in a 104-week study in patients aged 55 to 80 years with T2DMCitation43. Two-year treatment with canagliflozin 100 and 300 mg was associated with statistically significant decreases in BMD at the total hip (placebo-subtracted changes of –0.9% and –1.2%, respectively), but not at other skeletal sites (i.e. femoral neck, lumbar spine, distal forearm; )Citation36. It is estimated that the total hip BMD reduction would translate into a decrease of approximately 0.1 T-score units or 1% of peak bone mass over 2 years. In a smaller clinical study, dapagliflozin treatment was associated with placebo-subtracted changes of 0.1% in the lumbar spine, –0.6% in the femoral neck, and 0.2% in the total hipCitation38. It is unknown whether changes in BMD occur with empagliflozin treatment, as these data have not been reported.
Figure 4. Change from baseline in BMD with canagliflozin at Week 104 (study in older patients)Citation36. BMD, bone mineral density; CI, confidence interval; PBO, placebo; CANA, canagliflozin. *95% CI for the difference versus PBO excludes 0.
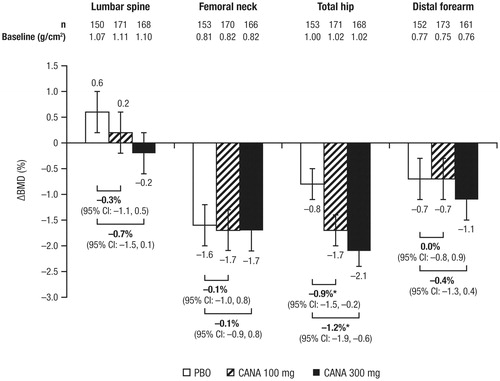
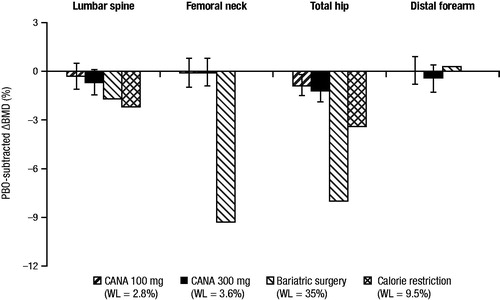
In a post hoc, exploratory analysis, approximately 40% of the reduction in total hip BMD with canagliflozin at 104 weeks was associated with weight loss; the weight change–adjusted difference in BMD with pooled canagliflozin 100 and 300 mg versus placebo was –0.64% compared with the unadjusted difference of –1.05%Citation36. The contribution of weight loss to changes in BMD with canagliflozin is consistent with previous studies that have shown changes in BMD resulting from weight loss interventions (e.g. bariatric surgery, calorie restriction; )Citation44,Citation45. Weight loss has also been shown to be associated with changes in bone mineral content (BMC)Citation39,Citation46,Citation47. In the same 104-week study in older patients and in a 52-week study of canagliflozin versus glimepiride as add-on to metformin, BMC was evaluated using dual-energy x-ray absorptiometry (DXA)Citation43,Citation48. Consistent with the findings of previous weight loss studiesCitation39,Citation46,Citation47, BMC reductions correlated with decreases in fat mass with canagliflozin treatment ().
Figure 5. Changes in BMD associated with canagliflozin (study in older patients) and weight-loss interventionsCitation36,Citation44,Citation45. BMD, bone mineral density; PBO, placebo; CANA, canagliflozin; WL, weight loss.
Fractures
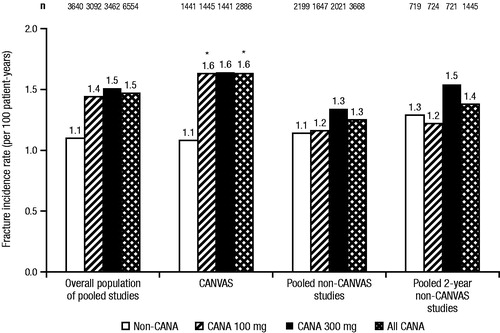
The incidence of fracture adverse events (AEs) was assessed in a pooled analysis of data from nine placebo- and active-controlled studies of canagliflozin in patients with T2DM, including an interim analysis from the CANagliflozin cardioVascular Assessment Study (CANVAS) in patients with a prior history or high risk of cardiovascular diseaseCitation49. Separate analyses assessed fracture risk in a pooled population of eight non-CANVAS studies (N = 5867 patients; 95 patients with fractures) and in CANVAS alone (N = 4327 patients; 151 patients with fractures)Citation49. In the overall population, canagliflozin 100 and 300 mg were associated with a non–dose-dependent numerical increase in fractures compared with non-canagliflozin (incidence per 100 patient-years of 1.4, 1.5, and 1.1, respectively; ), with the increase in fractures observed only in the upper extremitiesCitation49,Citation50. The incidence of fractures was similar with canagliflozin 100 and 300 mg and non-canagliflozin in the pooled non-CANVAS studies (incidence per 100 patient-years of 1.2, 1.3, and 1.1, respectively). The increase in the overall population was driven by a higher incidence of fractures with canagliflozin 100 and 300 mg compared with placebo in CANVAS (incidence per 100 patient-years of 1.6, 1.6, and 1.1, respectively). Although not increased relative to placebo, the fracture incidence rate in another large study (N = 7020) of patients with cardiovascular disease or at risk for cardiovascular events with empagliflozin was similar to that seen with canagliflozin (incidence per 100 patient-years of 1.6, 1.5, and 1.6, for empagliflozin 10 and 25 mg and placebo, respectively)Citation35.
Figure 7. Fracture incidence rate with canagliflozin in the overall population of pooled studies, CANVAS, and pooled non-CANVAS studiesCitation49. CANVAS, CANagliflozin cardioVascular Assessment Study; CANA, canagliflozin; CI, confidence interval. *95% CI for the hazard ratio versus non-CANA excludes 1.
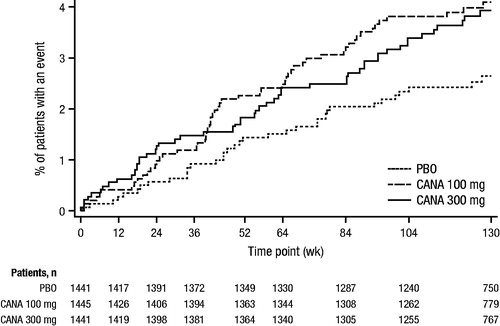
While an increase in upper extremity fractures was only seen with canagliflozin versus non-canagliflozin in the overall populationCitation51, most fractures in CANVAS and the imbalance between canagliflozin and placebo were in the distal portions of both the upper (i.e. hand and wrist) and lower (i.e. foot and ankle) extremitiesCitation49. The imbalance in fractures occurred with canagliflozin, relative to placebo, within the first 12 weeks after randomization, with the relative fracture risk remaining generally constant over the remaining observation period (); this was not seen in the non-CANVAS studies (Supplemental Figure 2)Citation49.
Figure 8. Kaplan-Meier plot of time to first fracture AE with canagliflozin (CANVAS)Citation49. AE, adverse event; CANVAS, CANagliflozin cardioVascular Assessment Study; PBO, placebo; CANA, canagliflozin. Republished with permission of the Endocrine Society, from Watts et al.Citation49; permission conveyed through Copyright Clearance Center Inc.
The fracture incidence with canagliflozin 100 and 300 mg and placebo over 52 weeks in a dedicated study in patients with T2DM and moderate renal impairment (N = 269) was 1.1%, 1.1%, and 2.2%, respectivelyCitation26. While no imbalance in fractures was reported in the dapagliflozin clinical development program, an increased risk of fractures was seen in patients with T2DM and moderate renal impairment in a 104-week study (6.0% and 9.4% with dapagliflozin 5 and 10 mg; no patients in the placebo group experienced a fracture)Citation31.
The incidence of falls reported as spontaneous AEs was low with canagliflozin 100 and 300 mg and non-canagliflozin/placebo across studies (1.5%, 2.1%, and 1.3%, respectively, in the overall population of pooled studies; 1.9%, 3.3%, and 1.5%, respectively, in CANVAS; and 1.2%, 1.3%, and 1.1%, respectively, in the pooled non-CANVAS studies)Citation49. A dose-related increase in AEs possibly related to volume depletion was seen with canagliflozin in the pooled population of eight studies that included CANVAS reported in the product label (2.3%, 3.4%, and 1.5% with canagliflozin 100 and 300 mg and non-canagliflozin, respectively)Citation50. The incidence of AEs related to volume depletion was higher with canagliflozin in a dose-related fashion in CANVAS (2.8%, 4.6%, and 1.9% with canagliflozin 100 and 300 mg and placebo, respectively) and in the pooled non-CANVAS studies (2.6%, 3.1%, and 2.0% with canagliflozin 100 and 300 mg and non-canagliflozin, respectively), with higher absolute incidence in CANVASCitation49. The increase in volume-depletion–related AEs with the 300 mg dose, relative to the 100 mg dose and non-canagliflozin/placebo, occurred during the first 12 weeks of initiating treatment in the overall pooled population, in CANVAS alone, and in the pooled non-CANVAS studies; after 12 weeks, the incidence of volume-depletion–related AEs plateaued with canagliflozin treatmentCitation49. Volume depletion–related AEsCitation49 and hypoglycemic episodes (data on file) were not temporally associated with fracture.
Discussion
Canagliflozin is a high-affinity SGLT2 inhibitor whose systemic exposures in humans at the 100 and 300 mg approved doses to treat T2DM remain above the SGLT2 IC50 for most of the day and do not reach levels sufficient to result in systemic SGLT1 inhibition. SGLT2 and SGLT1 are both expressed in the kidney with SGLT1 also being expressed in the intestineCitation2,Citation8. SGLT1 and SGLT2 have not been found to be expressed in bone or bone marrowCitation52,Citation53.
In preclinical studies in rats, canagliflozin and other SGLT2 and SGLT1 inhibitors have been associated with increased intestinal calcium absorption, hypercalciuria, decreases in PTH and 1,25-dihydroxyvitamin D levels, and hyperostosisCitation16,Citation18–21. Based on mechanistic studies demonstrating that removal of glucose from the diet in the presence of canagliflozin can prevent these effects, these alterations in calcium homeostasis in rats are likely secondary to glucose malabsorption due to transient inhibition of intestinal SGLT1-mediated glucose uptake. As carbohydrate malabsorption, decreases in PTH and 1,25-dihydroxyvitamin D, and hypercalciuria are not seen in patients treated with canagliflozin or other SGLT2 inhibitors, the hyperostosis and changes in bone-related analytes observed in rats are not clinically relevant in humans.
While not associated with changes in PTH, 1,25-dihydroxyvitamin D, or serum or urine calcium levels in humans, increases in markers of bone resorption and formation and decreases in total hip BMD were seen with canagliflozin treatment. A placebo-adjusted mean BMD decrease of 1.2% at the 300 mg dose was seen at the total hip after 2 years of treatment, yet BMD was not significantly altered at three other anatomic sites, including the key osteoporotic sites of femoral neck and lumbar spine. Increases in markers of bone resorption have also been reported with dapagliflozin treatmentCitation38. As weight loss is associated with decreases in BMD and increases in markers of bone turnoverCitation39–42, canagliflozin-induced weight loss could underlie these alterations. Consistent with this hypothesis is the positive correlation between decreases in BMC (as well as the bone resorption marker beta-CTX) and weight loss with canagliflozin treatment, and a post hoc, exploratory analysis that showed a significant proportion of the total hip BMD decrease with canagliflozin was attributable to weight loss. The changes in total hip BMD with canagliflozin are consistent with findings from weight loss studies demonstrating a correlation between changes in weight and BMD in patients undergoing bariatric surgery (∼8% reduction in total hip BMD with 35% weight loss)Citation45 and in patients on calorie-restricted diets (∼2% reduction in total hip BMD with ∼10% weight loss)Citation44. Weight loss has also been associated with decreases in BMD in previous studies of patients with T2DM. In the Look AHEAD trial, intensive lifestyle modification in patients with T2DM resulted in a weight loss of 9% over 1 year and a placebo-adjusted decrease in total hip BMD of 1%Citation42. Therefore, as weight loss is known to occur with SGLT2 inhibitor treatment, changes in BMD are not unexpected. In studies as monotherapy at 24 to 26 weeks, placebo-subtracted body weight reduction with canagliflozin 100 and 300 mg, dapagliflozin 10 mg, and empagliflozin 10 and 25 mg was –1.9, –2.9, –1.0, –1.9, and –2.2 kg, respectivelyCitation54–56; as add-on to metformin, placebo-subtracted body weight reduction was –2.2, –2.5, –2.0, –1.6, and –2.0 kg, respectively. Weight loss roughly correlates with the reported UGE values of 77–119 g/day with canagliflozin, 70 g/day with dapagliflozin 10 mg, and 64 and 78 g/day with empagliflozin 10 and 25 mg, respectivelyCitation57–59. Given the association of weight loss with UGE, it is possible that SGLT2 inhibitors associated with greater weight loss could also be associated with corresponding differences in BMD changes.
Modest increases in serum phosphate were seen with canagliflozin. Elevated serum phosphate has been shown to stimulate PTH secretion, which can increase bone turnover and fracture riskCitation60–63. Increased PTH secretion results in elevated serum calcium levels and increased synthesis of 1,25-dihydroxyvitamin D, which may potentially stimulate production of fibroblast growth factor 23 (FGF23), a key regulator of phosphorus and vitamin D metabolismCitation60,Citation61. FGF23 has been hypothesized to play a role in the bone and mineral alterations seen with SGLT2 inhibitorsCitation64. Increases in FGF23 can lead to hypophosphatemia, suppressed 1,25-dihydroxyvitamin D levels, elevated PTH levels, and impaired bone and cartilage mineralizationCitation65. While serum phosphate levels increase in response to administration of canagliflozin and other SGLT2 inhibitors, canagliflozin is not associated with hyperphosphaturia, decreased 1,25-dihydroxyvitamin D levels, or increases in PTH In preclinical models, SGLT1 and SGLT2 inhibitors induce hyperostosis, an increase in bone contentCitation15,Citation16,Citation19, rather than the osteomalacia seen in conditions of FGF23 excessCitation66. Elevations in FGF23 levels have been associated with increases in the progression of renal impairment, left ventricular dysfunction, and adverse cardiovascular outcomesCitation67–70. Canagliflozin treatment in patients with T2DM and moderate renal impairment (baseline estimated glomerular filtration rate [eGFR] ≥30 to <50 mL/min/1.73 m2) was associated with a significant reduction in albuminuria; notably, the majority of patients in these studies were on a background of angiotensin-converting enzyme (ACE) inhibitors and/or angiotensin-receptor blockers (ARB)Citation25,Citation26,Citation71. In a recently reported cardiovascular outcome study, empagliflozin treatment was associated with substantial and significant reductions in cardiovascular death and hospitalization for heart failure in patients with T2DM and established atherosclerotic diseaseCitation35. Based on the totality of the preclinical and clinical data, the hypothetical role for FGF23 in underlying bone and mineral findings with SGLT2 inhibitors seems unsubstantiated.
The etiology of the non–dose-dependent fracture imbalance in the CANVAS study, but not in the remainder of the clinical development program, is unknown; however, it is likely not due to a direct deleterious effect on bone. In CANVAS, the fracture imbalance occurred within 12 weeks after initiating treatment, with the relative risk remaining constant over the remainder of the observation period. Decreases in BMD seen after 104 weeks of canagliflozin treatment occurred at the total hip and not at other anatomic sites or in the distal radius, a site where fracture imbalance generally occurred (e.g. upper extremities). The early onset of the fracture imbalance at a time when an effect on BMD was not evident or expected suggests that the imbalance in CANVAS could be due to factors extrinsic to bone, such as falls. Hypoglycemia leading to falls would be an unlikely cause of the imbalance in fractures, as the occurrence of hypoglycemic episodes with canagliflozin is low and was not temporally associated with fractures. Falls possibly related to volume depletion–related AEs could be a potential explanation for the fracture imbalance, as imbalances in these events were only seen in CANVAS and not in the pooled analysis of non-CANVAS Phase 3 studies. Attempting to elucidate the relationship between falls and fractures would require more systematic collection of fall data as opposed to spontaneous collection of fall AEs, as was done in the canagliflozin program, which almost certainly led to underreportingCitation72–74.
The US Food and Drug Administration (FDA) recently revised the product label of the SGLT2 inhibitor canagliflozin to include a warning related to the increased fracture risk observed with canagliflozin treatment in patients with T2DMCitation51. Safety information regarding decreases in BMD, seen with canagliflozin in a clinical trial of older patients with T2DM, and a higher risk of falls with canagliflozin based on pooled data was also included in the revised product labelCitation50. The updated fracture incidence, which includes a larger pooling of studies and longer duration of exposure, is generally similar to the incidence reported in the original product label; fracture incidence per 100 patient-years with canagliflozin 100 and 300 mg and non-canagliflozin is 1.4, 1.5, and 1.1, respectively, in the revised label and 1.9, 1.8, and 1.4, respectively, in the original labelCitation50. In the EU Summary of Product Characteristics for canagliflozin, fracture incidence is reported for CANVAS (incidence per 100 patient-years of 1.6, 1.6, and 1.1 with canagliflozin 100 and 300 mg and placebo, respectively); no differences in fracture risk are reported with canagliflozin versus placebo/non-canagliflozin in the pooled non-CANVAS studiesCitation58. As noted earlier, the fracture incidence rate, which adjusts for duration of exposure, in another large cardiovascular outcome study with empagliflozin was similar to that seen in patients treated with canagliflozin in CANVASCitation35.
Notably, the available data on fractures in CANVAS are based on an interim analysis of the ongoing study. Additional information on fractures with canagliflozin use will be provided from multiple ongoing, long-term canagliflozin studies, including CANVAS; CANVAS-R, a study assessing renal outcomes with canagliflozin in patients with T2DM and elevated cardiovascular risk; and the Canagliflozin and Renal Events in Diabetes With Established Nephropathy Clinical Evaluation Trial (CREDENCE), which will assess whether canagliflozin has a renal and vascular protective effect in reducing the progression of renal impairment in patients with T2DM, Stage 2 or 3 chronic kidney disease, and macroalbuminuria.
Summary
Data from clinical studies suggest that canagliflozin treatment was not associated with any meaningful changes in mineral metabolism. Increases in bone turnover markers and small decreases in total hip BMD that correlated with changes in body weight were seen in older patients with T2DM over 104 weeks. Canagliflozin was associated with a higher incidence of fractures in a study including patients with elevated risk or a history of cardiovascular disease, but not in a pooled analysis of other studies in the canagliflozin development program. The etiology of increased fracture risk with canagliflozin treatment is unknown, but is likely related to factors extrinsic to bone. Data from ongoing, long-term outcomes studies will better define the effects of SGLT2 inhibition on bone health and fracture risk.
Transparency
Declaration of funding
This review article reports data from previously published studies funded by Janssen Research & Development, LLC. Medical writing support was funded by Janssen Global Services, LLC.
Declaration of financial/other relationships
M.A., J.X., A.F., and M.D. have disclosed that they are full-time employees of Janssen Research & Development, LLC.
C.M.R.O. peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
supplementary information
Download MS Word (127.5 KB)Acknowledgments
Medical writing support was provided by Kimberly Fuller, PhD, of MedErgy, and was funded by Janssen Global Services, LLC.
Canagliflozin was developed by Janssen Research & Development, LLC, in collaboration with Mitsubishi Tanabe Pharma Corporation.
References
- Nair S, Wilding JP. Sodium glucose cotransporter 2 inhibitors as a new treatment for diabetes mellitus. J Clin Endocrinol Metab 2010;95:34-42
- Abdul-Ghani MA, Norton L, DeFronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev 2011;32:515-31
- Neumiller JJ, White JR Jr, Campbell RK. Sodium-glucose co-transport inhibitors: progress and therapeutic potential in type 2 diabetes mellitus. Drugs 2010;70:377-85
- Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int 2009;75:1272-7
- DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab 2012;14:5-14
- Rosenstock J, Aggarwal N, Polidori D, et al. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care 2012;35:1232-8
- Wilding JP. The role of the kidneys in glucose homeostasis in type 2 diabetes: clinical implications and therapeutic significance through sodium glucose co-transporter 2 inhibitors. Metabolism 2014;63:1228-37
- Wright EM. Renal Na(+)-glucose cotransporters. Am J Physiol Renal Physiol 2001;280:F10-8
- Chen J, Williams S, Ho S, et al. Quantitative PCR tissue expression profiling of the human SGLT2 gene and related family members. Diabetes Ther 2010;1:57-92
- Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 2011;91:733-94
- Zhou L, Cryan EV, D’Andrea MR, et al. Human cardiomyocytes express high level of Na+/glucose cotransporter 1 (SGLT1). J Cell Biochem 2003;90:339-46
- Liang Y, Arakawa K, Ueta K, et al. Effect of canagliflozin on renal threshold for glucose, glycemia, and body weight in normal and diabetic animal models. PLoS One 2012;7:e30555
- Polidori D, Sha S, Mudaliar S, et al. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo-controlled study. Diabetes Care 2013;36:2154-61
- Devineni D, Curtin CR, Polidori D, et al. Pharmacokinetics and pharmacodynamics of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in subjects with type 2 diabetes mellitus. J Clin Pharmacol 2013;53:601-10
- Kissner T, Doyle N, Samadfam R, et al. Bone as a target tissue in the toxicological assessment of anti-diabetes drug class of SGLT1 inhibitors. Presented at: 49th Annual Meeting of the Society of Toxicology; March 7–11, 2010; Salt Lake City, Utah. Abstract 117.
- Tirmenstein M, Dorr TE, Janovitz EB, et al. Nonclinical toxicology assessments support the chronic safety of dapagliflozin, a first-in-class sodium-glucose cotransporter 2 inhibitor. Int J Toxicol 2013;32:336-50
- Ways K, Johnson MD, Mamidi RNVS, et al. Successful integration of non-clinical and clinical findings in interpreting the clinical relevance of rodent neoplasia with a new chemical entity. Toxicol Pathol J 2015;43:48-56
- Mamidi RNVS, Proctor J, De Jonghe S, et al. Carbohydrate malabsorption mechanism for tumor formation in rats treated with the SGLT2 inhibitor canagliflozin. Chem Biol Interact 2014;221:109-18
- De Jonghe S, Proctor J, Vinken P, et al. Carcinogenicity in rats of the antidiabetic SGLT2 inhibitor canagliflozin. Chem Biol Interact 2014;224:1-12
- Food and Drug Administration. FDA Briefing Document. NDA 202293 Dapagliflozin tablets, 5 and 10 mg. Endocrinologic and Metabolic Drugs Advisory Committee Meeting. 19 July 2011. Available at: http://www.fda.gov/downloads/AdvisoryCommittee/UCM262994.pdf [Last accessed 11 January 2016]
- European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP). European public assessment report (EPAR) for Jardiance (empagliflozin). EMEA/H/C/002677/0000. 20 March 2014. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002677/WC500168594.pdf [Last accessed 11 January 2016]
- Barone S, Fussell SL, Singh AK, et al. Slc2a5 (Glut5) is essential for the absorption of fructose in the intestine and generation of fructose-induced hypertension. J Biol Chem 2009;284:5056-66
- Sha S, Devineni D, Ghosh A, et al. Pharmacodynamic effects of canagliflozin, a sodium glucose co-transporter 2 inhibitor, from a randomized study in patients with type 2 diabetes. PLoS One 2014;9:e105638
- Bode B, Stenlöf K, Sullivan D, et al. Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: a randomized trial. Hosp Pract (1995) 2013;41:72-84
- Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab 2013;15:463-73
- Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab 2014;16:1016-27
- Inagaki N, Kondo K, Yoshinari T, et al. Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, 12-week study. Diabetes Obes Metab 2013;15:1136-45
- Iijima H, Kifuji T, Maruyama N, Inagaki N. Pharmacokinetics, pharmacodynamics, and safety of canagliflozin in Japanese patients with type 2 diabetes mellitus. Adv Ther 2015;32:768-82
- Weir MR, Kline I, Xie J, et al. Effect of canagliflozin on serum electrolytes in patients with type 2 diabetes in relation to estimated glomerular filtration rate (eGFR). Curr Med Res Opin 2014;30:1759-68
- List JF, Woo V, Morales E, et al. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 2009;32:650-7
- Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int 2014;85:962-71
- Ferrannini E, Berk A, Hantel S, et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care 2013;36:4015-21
- Haring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care 2014;37:1650-9
- Rosenstock J, Jelaska A, Zeller C, et al. Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78-week randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab 2015;17:936-48
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117-28
- Bilezikian JP, Watts NB, Usiskin K, et al. Evaluation of bone mineral density and bone biomarkers in patients with type 2 diabetes mellitus treated with canagliflozin, a sodium glucose co-transporter 2 inhibitor. J Clin Endocrinol Metab 2016;101:44-51
- Bays HE, Weinstein R, Law G, Canovatchel W. Canagliflozin: effects in overweight and obese subjects without diabetes mellitus. Obesity (Silver Spring) 2014;22:1042-9
- Ljunggren O, Bolinder J, Johansson L, et al. Dapagliflozin has no effect on markers of bone formation and resorption or bone mineral density in patients with inadequately controlled type 2 diabetes mellitus on metformin. Diabetes Obes Metab 2012;14:990-9
- Hyldstrup L, Andersen T, McNair P, et al. Bone metabolism in obesity: changes related to severe overweight and dietary weight reduction. Acta Endocrinol (Copenh) 1993;129:393-8
- Bleicher K, Cumming RG, Naganathan V, et al. The role of fat and lean mass in bone loss in older men: findings from the CHAMP study. Bone 2011;49:1299-305
- Hinton PS, Rector RS, Linden MA, et al. Weight-loss-associated changes in bone mineral density and bone turnover after partial weight regain with or without aerobic exercise in obese women. Eur J Clin Nutr 2012;66:606-12
- Schwartz AV, Johnson KC, Kahn SE, et al. Effect of 1 year of an intentional weight loss intervention on bone mineral density in type 2 diabetes: results from the Look AHEAD randomized trial. J Bone Miner Res 2012;27:619-27
- Bode B, Stenlöf K, Harris S, et al. Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55–80 years with type 2 diabetes. Diabetes Obes Metab 2015;17:294-303
- Villareal DT, Fontana L, Weiss EP, et al. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch Intern Med 2006;166:2502-10
- Fleischer J, Stein EM, Bessler M, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab 2008;93:3735-40
- Skov AR, Haulrik N, Toubro S, et al. Effect of protein intake on bone mineralization during weight loss: a 6-month trial. Obes Res 2002;10:432-8
- Jensen LB, Quaade F, Sorensen OH. Bone loss accompanying voluntary weight loss in obese humans. J Bone Miner Res 1994;9:459-63
- Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 2013;382:941-50
- Watts NB, Bilezikian JP, Usiskin K, et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 2016;101:157-66
- INVOKANA® (canagliflozin) tablets, for oral use [package insert]. Titusville, NJ, USA: Janssen Pharmaceuticals, 2015
- US Food and Drug Administration. FDA Drug Safety Communication: FDA revises label of diabetes drug canagliflozin (Invokana, Invokamet) to include updates on bone fracture risk and new information on decreased bone mineral density. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm461449.htm [Last accessed 11 January 2016]
- Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab Pharmacokinet 2005;20:452-77
- Harada N, Inagaki N. Role of sodium-glucose transporters in glucose uptake of the intestine and kidney. J Diabetes Investig 2012;3:352-3
- Ferrannini E, Ramos SJ, Salsali A, et al. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 2010;33:2217-24
- Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2013;1:208-19
- Stenlöf K, Cefalu WT, Kim KA, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab 2013;15:372-82
- Forxiga (dapagliflozin) 5 mg and 10 mg film-coated tablets [summary of product characteristics]. Middlesex, United Kingdom: Bristol-Myers Squibb/AstraZeneca EEIG, 2014
- INVOKANA (canagliflozin) tablets, for oral use [summary of product characteristics]. Beerse, Belgium: Janssen-Cilag International NV, 2015
- Jardiance (empagliflozin) 10 mg film-coated tablets [summary of product characteristics]. Ingelheim, Germany: Boehringer Ingelheim GmbH, 2014
- Quarles LD. Skeletal secretion of FGF-23 regulates phosphate and vitamin D metabolism. Nat Rev Endocrinol 2012;8:276-86
- Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 2015;100:2849-52
- Rejnmark L, Vestergaard P, Brot C, Mosekilde L. Increased fracture risk in normocalcemic postmenopausal women with high parathyroid hormone levels: a 16-year follow-up study. Calcif Tissue Int 2011;88:238-45
- Hernandez JL, Olmos JM, Pariente E, et al. Influence of vitamin D status on vertebral fractures, bone mineral density, and bone turnover markers in normocalcemic postmenopausal women with high parathyroid hormone levels. J Clin Endocrinol Metab 2013;98:1711-7
- Taylor SI, Blau JE, Rother KI. Possible adverse effects of SGLT2 inhibitors on bone. Lancet Diabetes Endocrinol 2015;3:8-10
- Kovesdy CP, Quarles LD. Fibroblast growth factor-23: what we know, what we don’t know, and what we need to know. Nephrol Dial Transplant 2013;28:2228-36
- Larsson T, Marsell R, Schipani E, et al. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology 2004;145:3087-94
- Arnlov J, Carlsson AC, Sundstrom J, et al. Higher fibroblast growth factor-23 increases the risk of all-cause and cardiovascular mortality in the community. Kidney Int 2013;83:160-6
- Mirza MA, Larsson A, Melhus H, et al. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis 2009;207:546-51
- Wolf M, Molnar MZ, Amaral AP, et al. Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol 2011;22:956-66
- Parker BD, Schurgers LJ, Brandenburg VM, et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med 2010;152:640-8
- Yamout HM, Perkovic V, Davies M, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes and stage 3 nephropathy. Am J Nephrol 2014;40:64-74
- Berlie HD, Garwood CL. Diabetes medications related to an increased risk of falls and fall-related morbidity in the elderly. Ann Pharmacother 2010;44:712-7
- Maurer MS, Burcham J, Cheng H. Diabetes mellitus is associated with an increased risk of falls in elderly residents of a long-term care facility. J Gerontol A Biol Sci Med Sci 2005;60:1157-62
- Schwartz AV, Hillier TA, Sellmeyer DE, et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care 2002;25:1749-54