Abstract
Objective: Perioperative pain management is an important aspect of recovery from total knee arthroplasty (TKA) because severe pain can delay ambulation and hospital discharge. The objective of this retrospective sequential cohort study was to determine the impact of local infiltration analgesia using liposome bupivacaine (ExparelFootnote1) when compared with a continuous femoral nerve block (FNB) following TKA.
Methods: This retrospective cohort study included consecutive patients who underwent TKA between April 2011 and April 2014, and received one of three interventions. Study Group A received adductor canal infiltration with bupivacaine HCl and knee infiltration with liposome bupivacaine. Study Group B received adductor canal infiltration with liposome bupivacaine and knee infiltration with liposome bupivacaine. The control group received a continuous FNB with ropivacaine HCl delivered via an elastomeric pump. Numeric pain rating scores (NPRS), distance walked, length of stay (LOS), and dose of narcotic medication were the key efficacy variables of interest.
Results: A total of 237 patients were included in this study: 98 in Group A, 34 in Group B, and 105 controls. On postoperative day (POD) 0, mean (standard deviation [SD]) NPRSs were similar between Group A (1.8 [1.7]), Group B (2.7 [1.8]), and the control group (2.3 [2.4]). Significantly (p < 0.05) more patients in Group A (58%) and Group B (44%) walked on POD0 than in the control group (0%); almost all patients walked on POD1. The mean (SD) distance walked was also significantly greater (p < 0.05) on POD1 in Group A (193 [203] feet) and Group B (211 [144] feet) than in the control group (46 [73] feet). Mean (SD) LOS was significantly (p < 0.05) shorter in Group B (2.2 [1.2] days), than in the control group (3.2 [0.7] days) and Group A (3.0 [1.7] days).
Conclusions: Local infiltration analgesia using liposome bupivacaine was associated with improved ambulation and shorter LOS following TKA when compared with continuous FNB in this retrospective cohort study.
Introduction
Total knee arthroplasty (TKA) represents about 1% of all inpatient surgical procedures performed in the United States each yearCitation1, and most TKA patients experience moderate-to-severe postsurgical painCitation2,Citation3. Achieving adequate postsurgical pain control after TKA is a critical factor for successful recovery because inadequately treated pain may lead to a delay in ambulation and hospital discharge, have an adverse impact on a patient’s quality of life, and may even result in increased risk of developing chronic painCitation2,Citation4. Furthermore, a patient’s level of satisfaction with pain relief following TKA is highly predictive of their overall satisfaction with the entire surgical experienceCitation5.
Opioids are widely used as the main component of most postsurgical analgesic regimens, including TKACitation6,Citation7. However, opioid use also produces undesirable side effects in many patients. A recent hospital claims based study of 37,031 adults undergoing a primary inpatient surgical procedure showed that 14% (n = 4955) of those receiving opioids for the management of postsurgical pain (n = 36,529) had one or more documented, opioid-related adverse events during their hospital stayCitation8. Patients with opioid-related adverse events had longer mean length of hospital stay (LOS), incurred higher cost of care, and had higher rates of readmission and inpatient mortality compared with patients who did not have an opioid-related adverse eventCitation8.
Opioid-sparing multimodal analgesic regimens should be used whenever possible in order to achieve adequate analgesia while minimizing risk of opioid-related adverse eventsCitation4. Although administration of local anesthetics via continuous femoral nerve block (FNB) is among the preferred opioid-sparing analgesic techniques following TKACitation9, continuous infusion pumps can make it more difficult for patients to ambulate after surgery, and pump use may be associated with increased risk of surgical site related adverse events (e.g., tissue necrosis, surgical wound infection)Citation10. Also, motor weakness associated with continuous peripheral nerve blocks can increase risk of patient fallsCitation11.
Liposome bupivacaine (bupivacaine liposome injectable suspension; ExparelFootnote1) is a prolonged-release formulation of bupivacaine indicated for single-dose administration into the surgical site to produce postsurgical analgesiaCitation12. Liposome bupivacaine consists of microscopic, spherical, multivesicular liposomes (DepoFoamFootnote2 drug delivery technology) containing encapsulated bupivacaineCitation13. The structure of liposome bupivacaine allows for drug release over several days after administration into the surgical siteCitation14,Citation15. In published prospective clinical studies in patients undergoing total knee arthroplastyCitation16, hemorrhoidectomyCitation17,Citation18, breast augmentationCitation19, or bunionectomyCitation20, a single administration of liposome bupivacaine at the surgical site was well tolerated and associated with lower mean cumulative pain scores, comparable or less opioid consumption, and a comparable adverse event profile compared with bupivacaine HCl or placebo.
We conducted a retrospective, exploratory study to assess the impact of local infiltration analgesia with prolonged-release liposome bupivacaine, either alone or in combination with bupivacaine HCl, compared with continuous FNB with ropivacaine HCl following TKA. Our main purpose was to determine whether either of the single-administration analgesic approaches would result in lower pain intensity scores, less opioid use, greater walk distances, and/or a shorter hospital stay compared with a continuous infusion of local anesthetic in TKA patients followed for at least 3 days after surgery.
Patients and methods
Study design
This was a nonrandomized, retrospective, observational study evaluating three treatment options for TKA implemented by the UnityPoint Health-Proctor in Peoria, Illinois. This study included consecutive patients who received a TKA between April 2011 and April 2014 stratified into three study groups. The patient cohorts were sequentially enrolled in the study according to treatment received; patients in the control group were enrolled between April 2011 and October 2012, and received continuous FNB with ropivacaine HCl administered via an OnQFootnote3 pump, which was the surgeon’s standard of care for TKA during this period. Patients in Group A were enrolled between October 2012 and April 2014, and received adductor canal infiltration with bupivacaine HCl and knee infiltration with liposome bupivacaine, according to the surgeon’s preferred anesthesia technique during this time period. Patients in Group B were enrolled between August 2013 and April 2014, and received adductor canal infiltration with liposome bupivacaine and knee infiltration with liposome bupivacaine, which reflected the surgeon’s preferred anesthesia technique during this time period.
Ethical issues
All patient identifying information was coded, kept separately in a secure location, and maintained by the principal investigator to protect confidentiality. A generic data collection form was used for each patient’s medical record information and demographic information, as well as the patient’s positive screening responses. Patients were only identifiable based on an assigned study identification number. The study protocol was approved by the Peoria Institutional Review Board in the University of Illinois College of Medicine at Peoria.
Inclusion and exclusion criteria
All adult patients who underwent TKA between April 2011 and April 2014 were eligible for inclusion in this study. The final analytical dataset included 237 TKA patients after excluding 4 patients who received bilateral TKA and 1 patient who received a revision TKA. Fifty percent of the patients (119/237) underwent TKA of the right knee.
Treatments
Each patient underwent a cemented, posterior cruciate substituting TKA using a medial parapatellar technique. All surgeries and administration of study drugs were performed by a single surgeon (B.T.M.).
Study Group A (liposome bupivacaine Group A)
Patients received hydrocodone (except in cases where there was a medical contraindication/known allergy) and had an injection of 30 ml of bupivacaine HCl 0.5% (for young or obese patients) or bupivacaine HCl 0.25% with epinephrine (for patients >70 years of age and those with multiple comorbidities) in the adductor canal preoperatively. An injection of 20 ml of liposome bupivacaine 266 mg, ketorolac (30 mg [standard dose used for most patients], 15 mg [used for patients with moderately decreased renal function/elevated serum creatinine level, and those >75 years of age], or none [patients with nonsteroidal anti-inflammatory drug (NSAID) allergy or history of NSAID intolerance and those with significant loss of renal function/elevated serum creatinine]) and 30 ml bupivacaine HCl 0.25% with epinephrine and 40 ml normal saline was placed into the soft tissues about the knee during the procedure. This included the periosteum, the posterior capsule, the retinacular/quadriceps layer, and the subcutaneous tissue. According to the current product labeling for liposome bupivacaine, bupivacaine HCl and liposome bupivacaine may be simultaneously administered in the same syringe and bupivacaine HCl may be injected immediately before liposome bupivacaine as long as the milligram doses of bupivacaine HCl solution and liposome bupivacaine do not exceed a ratio of 1:2Citation12. A tourniquet was used in some of the earlier cases. However, tourniquet use was discontinued during the study due to changes in the standard of care at the study site.
Study Group B (liposome bupivacaine Group B)
Patients received hydrocodone (if there were no medical contraindications/known allergies) and had infiltration of the adductor canal with 10 ml liposome bupivacaine 133 mg and 20 ml of bupivacaine HCl 0.5% with epinephrine (for young or obese patients) or bupivacaine HCl 0.25% with epinephrine (for patients >70 years of age and those with multiple comorbidities) preoperatively. During the procedure, a mixture of 10 ml liposome bupivacaine 133 mg, 30 ml bupivacaine HCl 0.25% with epinephrine and ketorolac (30 mg, 15 mg, or none dependent on the patient’s age and renal status) was injected into the posterior capsule of the knee. As noted for Group A, a tourniquet was used in some of the early cases.
Control group
Patients received hydrocodone preoperatively (if there were no medical contraindications/known allergies) and then an elastomeric pump (OnQFootnote3) delivering ropivacaine HCl 0.2% (continuous infusion rate was 6 ml/hour with an on-demand bolus of 5 ml available every 30 minutes). A femoral nerve catheter was placed preoperatively in the adductor canal at mid-thigh level using an ultrasound-guided approach; the catheter remained in place until the morning of postoperative day (POD) 2. A mixture of 30 ml 0.25% bupivacaine HCl and ketorolac (30 mg, 15 mg, or none dependent on the patient’s age and renal status) was injected into the posterior capsule of the knee intraoperatively. A knee immobilizer was used postoperatively to protect against falls due to quadriceps weakness until quadriceps function returned to a safe level.
All groups
The postsurgical protocol in all groups was similar except that there was no immobilizer and no femoral catheter used in the liposome bupivacaine groups. Oral opioids, NSAIDs, and intravenous fentanyl were available to all patients postsurgically, as needed. All patients were followed for the duration of their hospital stay.
Efficacy measures
To evaluate the effectiveness of treatment regimens for TKA, the following efficacy variables were analyzed: numeric pain rating scores (NPRS), distance walked, LOS, and dose of narcotic medication. In accordance with the postsurgical nursing standards at the hospital, pain was measured every 6 to 8 hours or more frequently if needed. Pain with movement and pain at rest were not differentiated in the records, and pain intensity was not measured at the same time points for all patients. The daily mean of NPRS in the hospital was calculated for each patient. The distance walked in feet was recorded twice a day in the hospital. Postsurgical physical therapy criteria remained consistent throughout the study period, and discharge readiness for all patients was determined by the same surgeon (B.T.M.). The LOS was calculated by subtracting the day of admission from the day of discharge. However, persons entering and leaving the hospital on the same day were reported as having an LOS of 1. The narcotic medications included morphine, codeine/acetaminophen, oxycodone, oxycodone/acetaminophen, hydrocodone/acetaminophen, and meperidine hydrochloride; doses were converted to oral morphine equivalents.
Covariates were the patient-level demographic variables: age (years), gender, body mass index, health insurance (Medicare, Medicaid, and private), complications (yes/no), discharge disposition (assisted/nursing/religious home, home, home with outpatient physical therapy [OPPT], and home with home health services [HH]), and comorbidity. A total of 19 comorbidities were identified based on patients’ past medical histories.
Statistical analysis
Demographics were compared among the three groups using either chi-square test or analysis of variance for categorical or continuous variables, respectively. Because Study Group B had a small sample size, we employed univariate analyses to evaluate the efficacy variables of interest among the three groups. One-way analysis of variance was used to compare the NPRS and the LOS, whereas a generalized linear model with a gamma distribution and a log link was applied for the distance walked and the dose of narcotic medication because of a significant skewed distribution.
A power analysis based on a one-way analysis of variance was conducted after the data was collected. The study had a >85% power to detect a difference in mean pain scores and walk distance on day of discharge between Groups A and B compared with the control group, a 99% power to detect a difference in mean LOS in Group B compared with the control group, and a 99% power to detect a difference in mean opioid usage in Group A compared with the control group at an alpha level of 0.05.
Descriptive statistic indicators included mean, standard deviation (SD), median, range, frequency, and percentage. All data management and statistical analyses were conducted on SASFootnote4 version 9.4. The level of statistical significance was set at 0.05.
Results
Patient profile
A total of 237 patients were included in this study: 105 in the control group, 98 in Study Group A, and 34 in Study Group B. Of the 237 patients, approximately half were female (54%) and the mean (SD) age was 70 [8.4] years with a range of 41–91 years. The majority (70%) were covered by Medicare. Poor health conditions such as hypertension (68%), body mass index ≥30 (60%), history of cancer (27%), sleep apnea (25%), cardiovascular disease (21%), and diabetes (21%) were common. More than half of the patients (53%) were discharged to home OPPT. As shown in , no significant difference in demographics was found among the three groups.
Table 1. Patient profile.
Numeric pain rating scores
During the day of surgery (POD0) and the first day after surgery (POD1), no significant difference in NPRS was found between Groups A and B compared with controls (). However, both Group A and Group B had significantly lower mean (SD) NPRS the second day after surgery compared with the control group (Group A, 3.8 [1.5]; Group B, 3.4 [1.2]; controls, 4.4 [1.7]; p < 0.05 for comparisons of Groups A and B vs. controls). Group A and Group B also had significantly lower mean (SD) NPRS on the day of discharge (Group A, 3.2 [1.6]; Group B, 2.9 [1.7]; controls, 4.0 [1.9]; p < 0.05 for comparison of Groups A and B vs. controls).
Table 2. Comparisons of NPRS among the three groupsTable Footnotea.
Length of stay
The longest LOS was 8, 12, and 6 days in the control group, Group A, and Group B, respectively. Overall, Group B had significantly shorter mean (SD) LOS (2.2 [1.2] days) than the control group (3.2 [0.7] days; p < 0.05 vs. Group B) and Group A (3.0 [1.7] days; p < 0.05 vs. Group B).
Walked distance in feet
On the day of surgery, 58% (57/98) of patients in Group A and 44% (15/34) in Group B ambulated compared with none in the control group (p < 0.05 for comparison of Groups A and B vs. the control group). On the first day after surgery (POD1), nearly all patients in all three treatment groups walked (Group A, 100%; Group B, 97%; controls, 99%). On POD1, patients in both Group A and Group B walked a significantly longer mean (SD) distance than the control group (Group A, 193.0 [202.9] feet; Group B, 210.8 [143.7] feet; controls, 46.3 [72.6] feet; p < 0.05 for comparisons of Groups A and B vs. controls). Significantly longer mean walk distance was also observed in Groups A and B on the discharge day (Group A, 213.7 [155.2] feet; Group B, 241.9 [123.1] feet; controls, 154.5 [126.4] feet; p < 0.05 for comparisons of Groups A and B vs. controls) ().
Table 3. Comparison of distance walked among the three groupsTable Footnotea.
Narcotic medication use
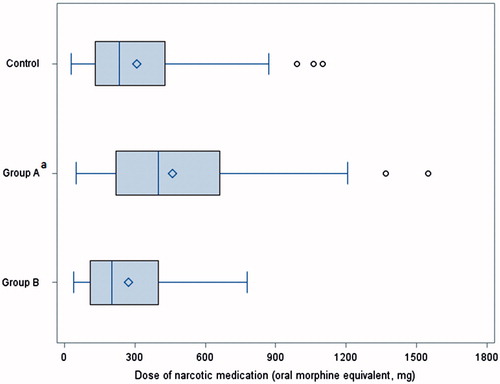
The use of narcotic medication in the first 72 hours was different among the three groups (). Group A used significantly more narcotic medication (median, 400 mg oral morphine equivalent) than both Group B (median, 201 mg; p < 0.05) and the control group (median, 235 mg; p < 0.05).
Figure 1. Comparison of narcotic medication use in the first 72 hours among the three groups. Study Group A: adductor canal infiltration (ACI) with bupivacaine HCl and knee infiltration with liposome bupivacaine (KI/LB); Study Group B: ACI with LB and KI/LB; control group: a continuous femoral nerve block with an elastomeric pump with ropivacaine HCl. Study Group A: adductor canal infiltration (ACI) with bupivacaine and knee infiltration with liposome bupivacaine (KI/LB); Study Group B: ACI with LB and KI/LB; control group: a continuous femoral nerve; with an elastomeric pump and ropivacaine.aThe dose difference between Group A and the control group was statistically significant (p < 0.05) based on generalized linear model with a gamma distribution and a log link. The dose for Group A was also significantly greater than for Group B.
Discussion
This single-center, exploratory study showed that patients who received adductor canal and knee infiltration with liposome bupivacaine for postsurgical analgesia after TKA had lower mean pain intensity scores, were able to ambulate further, and had a shorter hospital stay, on average, compared with those who received continuous FNB with ropivacaine HCl administered via an elastomeric pump. Mean pain scores were numerically lower in the two liposome bupivacaine treatment groups versus the continuous FNB group on each day from POD1 until the day of discharge, with the between-group differences between the liposome bupivacaine treated groups and continuous FNB group reaching statistical significance on POD2 and on the day of hospital discharge. Mean walked distance was greater in the liposome bupivacaine groups compared with the continuous FNB group on each day after surgery, with statistically significant differences observed between both of the liposome bupivacaine groups versus the control group on POD1 and on the day of discharge; the mean walked distance was also significantly longer in the bupivacaine HCl/liposome bupivacaine group versus controls on POD2. On average, the group that received adductor canal and knee infiltration with liposome bupivacaine was discharged a full day earlier than the continuous FNB group.
The median amount of postsurgical opioids consumed was nearly two times higher in the group that received adductor canal infiltration with bupivacaine HCl/knee infiltration with liposome bupivacaine (Group A) compared with the other two treatment groups. Interestingly, patients in the group that received adductor canal infiltration with liposome bupivacaine (Group B) were, on average, discharged significantly sooner and consumed less opioids after surgery than the group that received adductor canal infiltration with bupivacaine HCl (Group A). The reasons for this disparity are unclear. The study groups were enrolled in sequential cohorts, so it is possible that the better outcomes observed in Group B could have been associated with improvements in study drug administration techniques that occurred during the course of the study. It is also possible that the duration of analgesia following adductor canal infiltration with bupivacaine HCl could have been shorter than with liposome bupivacaine, leading to greater opioid consumption in Group A, which could have resulted in a longer duration of hospital stay. While the median amount of opioids used was, by far, the highest in Group A, the average pain intensity scores in this group were lower than the scores observed in the control group. The reasons for this disparity are also unclear. It is possible that the significantly greater use of opioids by the patients in Group A could have resulted in lower pain intensity scores. However, the time order of opioid use and assessments of pain intensity were not captured in this study. Clearly, further studies are needed to better define the differential analgesic profiles of these two bupivacaine formulations in this setting.
As with any single-center, retrospective analysis, there are numerous limitations to our study. We could not account for potential confounding factors, such as inclusion of sequential cohorts, differences in sample size across the cohorts, missing data, patient comorbidities (e.g., history of chronic pain, drug abuse), variability in surgical techniques, drug administration techniques, and general perioperative care provided by the surgeon and other medical professionals who took care of the patients in this study. Also, the statistical analyses were not adjusted for multiple comparisons. The efficacy results observed in this relatively small patient population may not be generalizable to larger or more heterogeneous surgical populations, and we cannot be certain that the between-group differences observed were due to liposome bupivacaine rather than some other unmeasured confounding variable or variables. Safety and pharmacoeconomic outcomes were not assessed in this study; therefore, it is unclear whether the apparent efficacy benefits observed in the liposome bupivacaine treated groups might have been qualified by higher or lower incidences of adverse events (e.g., patient fall rates) or meaningful differences in hospital costs across the treatment groups. Also, it should be noted that adductor canal infiltration with liposome bupivacaine has not been studied in well controlled, prospective, randomized studies and administration via adductor canal infiltration is an off-label use of liposome bupivacaine.
Conclusion
The data observed in this retrospective exploratory cohort study, although limited, showed liposome bupivacaine to be a feasible alternative to continuous FNB with ropivacaine HCl in this particular group of TKA patients. These findings need to be confirmed by a larger, prospective study that includes safety and pharmacoeconomic assessments as well as more extensive assessments of analgesic efficacy.
Transparency
Declaration of funding
Data analysis and editorial assistance for this manuscript were funded by Pacira Pharmaceuticals Inc. The authors were fully responsible for the content, editorial decisions, and opinions expressed in the current article. The authors did not receive an honorarium related to the development of this manuscript.
Declaration of financial/other relationships
C.V.A. has disclosed that he is a member of the Health Outcomes and Economics Advisory Board for Pacira Pharmaceuticals Inc. C.S.K., J.R., K.G., P.M., B.M., and B.T.M. have disclosed that they have no significant relationships with or financial interests in any commercial companies related to this study or article.
CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The data analysis was funded by Pacira Pharmaceuticals Inc. Editorial and writing assistance was provided by Peloton Advantage LLC, supported by Pacira Pharmaceuticals Inc.
Previous presentation: This data has been previously presented in a poster at the International Society for Pharmacoeconomics and Outcomes Research 20th Annual International Meeting, 16–20 May 2015, Philadelphia, PA, USA.
Notes
Notes
1 Exparel is a registered trade name of Pacira Pharmaceuticals Inc., Parsippany, NJ, USA
2 DepoFoam is a registered trade name of Pacira Pharmaceuticals Inc., Parsippany, NJ, USA
3 OnQ is a registered trade name of Halyard Health Inc., Alpharetta, GA, USA
4 SAS is a registered trade name of SAS Institute Inc., Cary, NC, USA
References
- Centers for Disease Control and Prevention. Inpatient surgery. Available at: http://www.cdc.gov/nchs/fastats/inpatient-surgery.htm [Last accessed 24 March 2015]
- Chan EY, Blyth FM, Nairn L, Fransen M. Acute postoperative pain following hospital discharge after total knee arthroplasty. Osteoarthritis Cartilage 2013;21:1257-63
- Wylde V, Rooker J, Halliday L, Blom A. Acute postoperative pain at rest after hip and knee arthroplasty: severity, sensory qualities and impact on sleep. Orthop Traumatol Surg Res 2011;97:139-44
- American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 2012;116:248-73
- Hamilton DF, Lane JV, Gaston P, et al. What determines patient satisfaction with surgery? A prospective cohort study of 4709 patients following total joint replacement. BMJ Open 2013;3:e002525
- Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic-prescribing rates by specialty, U.S., 2007–2012. Am J Prev Med 2015;49:409-13
- Dalury DF, Lieberman JR, MacDonald SJ. Current and innovative pain management techniques in total knee arthroplasty. J Bone Joint Surg Am 2011;93:1938-43
- Kessler ER, Shah M, Gruschkus SK, Raju A. Cost and quality implications of opioid-based postsurgical pain control using administrative claims data from a large health system: opioid-related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy 2013;33:383-91
- Chan EY, Fransen M, Sathappan S, et al. Comparing the analgesia effects of single-injection and continuous femoral nerve blocks with patient controlled analgesia after total knee arthroplasty. J Arthroplasty 2013;28:608-13
- Brown SL, Morrison AE. Local anesthetic infusion pump systems adverse events reported to the Food and Drug Administration. Anesthesiology 2004;100:1305-7
- Pelt CE, Anderson AW, Anderson MB, et al. Postoperative falls after total knee arthroplasty in patients with a femoral nerve catheter: can we reduce the incidence? J Arthroplasty 2014;29:1154-7
- Exparel (bupivacaine liposome injectable suspension) [prescribing information]. Parsippany, NJ: Pacira Pharmaceuticals Inc., 2015
- Chahar P, Cummings KC III. Liposomal bupivacaine: a review of a new bupivacaine formulation. J Pain Res 2012;5:257-64
- Viscusi ER, Candiotti KA, Onel E, et al. The pharmacokinetics and pharmacodynamics of liposome bupivacaine administered via a single epidural injection to healthy volunteers. Reg Anesth Pain Med 2012;37:616-22
- Hu D, Onel E, Singla N, et al. Pharmacokinetic profile of liposome bupivacaine injection following a single administration at the surgical site. Clin Drug Investig 2013;33:109-15
- Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee 2012;19:530-6
- Gorfine SR, Onel E, Patou G, Krivokapic ZV. Bupivacaine extended-release liposome injection for prolonged postsurgical analgesia in patients undergoing hemorrhoidectomy: a multicenter, randomized, double-blind, placebo-controlled trial. Dis Colon Rectum 2011;54:1552-9
- Haas E, Onel E, Miller H, et al. A double-blind, randomized, active-controlled study for post-hemorrhoidectomy pain management with liposome bupivacaine, a novel local analgesic formulation. Am Surg 2012;78:574-81
- Smoot JD, Bergese SD, Onel E, et al. The efficacy and safety of DepoFoam bupivacaine in patients undergoing bilateral, cosmetic, submuscular augmentation mammoplasty: a randomized, double-blind, active-control study. Aesthet Surg J 2012;32:69-76
- Golf M, Daniels SE, Onel E. A phase 3, randomized, placebo-controlled trial of DepoFoam bupivacaine (extended-release bupivacaine local analgesic) in bunionectomy. Adv Ther 2011;28:776-88
