Abstract
Objective: To compare the risk of stroke/systemic embolism (S/SE) and major bleeding (MB) of elderly (≥65 years of age) nonvalvular atrial fibrillation (NVAF) patients initiating apixaban vs. rivaroxaban, dabigatran, or warfarin.
Methods: NVAF patients with Medicare Advantage coverage in the US initiating oral anticoagulants (OACs, index event) were identified from the Humana database (1 January 2013–30 September 2015) and grouped into cohorts depending on OAC initiated. Propensity score matching (PSM), 1:1, was conducted among patients treated with apixaban vs. each other OAC, separately. Rates of S/SE and MB were evaluated in the follow-up. Cox regressions were used to compare the risk of S/SE and MB between apixaban and each of the other OACs during the follow-up.
Results: The matched pairs of apixaban vs. rivaroxaban (n = 13,620), apixaban vs. dabigatran (n = 4654), and apixaban vs. warfarin (n = 14,214) were well balanced for key patient characteristics. Adjusted risks for S/SE (hazard ratio [HR] vs. rivaroxaban: 0.72, p = .003; vs. warfarin: 0.65, p < .001) and MB (HR vs. rivaroxaban: 0.49, p < .001; vs. warfarin: 0.53, p < .001) were significantly lower during the follow-up for patients treated with apixaban vs. rivaroxaban and warfarin. Adjusted risks for S/SE (HR: 0.78, p = .27) and MB (HR: 0.82, p = .23) of NVAF patients treated with apixaban vs. dabigatran trended to be lower, but did not reach statistical significance.
Conclusions: In the real-world setting after controlling for differences in patient characteristics, apixaban is associated with significantly lower risk of S/SE and MB than rivaroxaban and warfarin, and a trend towards better outcomes vs. dabigatran among elderly NVAF patients in the US.
Introduction
Nonvalvular atrial fibrillation (NVAF) is a common cardiac rhythm disorder and has long been identified as a significant risk factor for disabling or fatal ischemic stroke and systemic embolism (SE)Citation1,Citation2. The prevalence of NVAF increases with age as does NVAF-related stroke riskCitation1,Citation3. In 2010 it was estimated that AF affected 5.2 million persons in the United StatesCitation4. With the increasing incidence of AF and the growing number of the elderly population, the prevalence of AF is estimated to increase to 12.1 million by 2030Citation4.
For more than 50 years, warfarin has been used to prevent the risk of stroke among patients with NVAF, although its use has been associated with several limitationsCitation5,Citation6. In recent years, direct oral anticoagulants (DOACs) have become available in the US market providing alternative options to warfarin for anticoagulation therapy among NVAF patients. The DOACs, apixaban, rivaroxaban, and dabigatran, have all been shown in large randomized clinical trials to be superior or noninferior to warfarin for the prevention of stroke, as well as to have lower or similar rates of bleeding among patients with NVAFCitation7–9. Furthermore, the DOACs do not require frequent monitoring and lack many of the other limitations associated with warfarin therapyCitation10.
Although some observational studies have shown that the effectiveness and safety of apixaban, rivaroxaban, and dabigatran relative to warfarin are similar to that reported in clinical trialsCitation11–13, there are no direct comparisons of the DOACs in randomized controlled trials, and only a few in the real-world settingCitation14–17. This is particularly true for apixaban, which entered the market later than rivaroxaban and dabigatran. Further real-world assessment of apixaban is warranted, especially among the older population who are at higher risk of stroke and bleedingCitation2,Citation18. The objectives of this study were to evaluate and compare the risk of stroke/systemic embolism (S/SE) and major bleeding (MB) among oral anticoagulant (OAC) treatment naïve elderly NVAF patients in a US population who initiated treatment with apixaban vs. rivaroxaban, dabigatran, and warfarin.
Patients and methods
Study design and data source
This study was an observational, retrospective, cohort analysis conducted using the Humana Research Database. The database comprises claims from more than 20 million members with Medicare Advantage coverage in the US. The database is an integrated source of managed care medical and pharmacy claims and eligibility files containing information on diagnostic and therapeutic services rendered in both inpatient and outpatient settings, prescription drugs dispensed, and demographic characteristics.
Study population
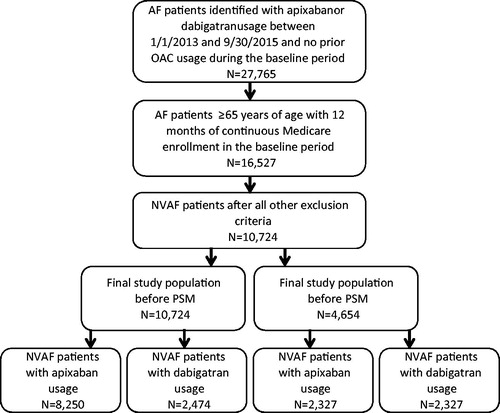
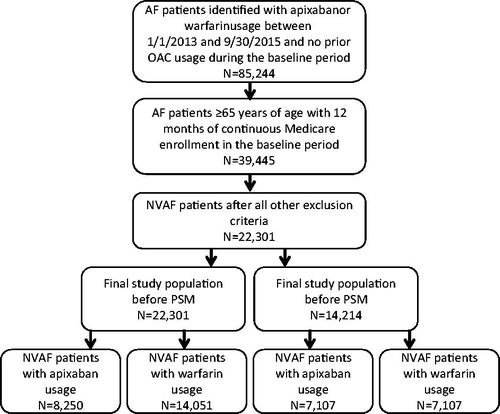
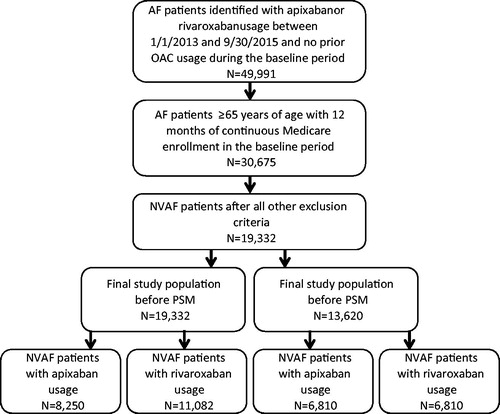
AF patients with Medicare coverage and an age of ≥65 years who had a pharmacy claim of apixaban, rivaroxaban, dabigatran, or warfarin between 1 January 2013 and 30 September 2015 were identified from the data source. Edoxaban was not included in this analysis as its FDA approval was not long before the end of the study period and the patient count was too low for this study. AF patients were identified using ICD-9-CM diagnosis code 427.31, which has been validated to identify AF patients with a median positive predictive value of 89%Citation19. The date of the first pharmacy claim for the specific OAC was defined as index date. Patients were required to have an AF diagnosis within 12 months prior to or on the index date (baseline period), and to have 12 months of continuous health plan enrollment. Patients with claims indicative of diagnoses of valvular heart disease, venous thromboembolism, transient AF, cardiac surgery, hyperthyroidism and thyrotoxicity, or pregnancy during the baseline period were excluded. To ensure patients were OAC treatment naïve, patients were excluded if they had a pharmacy claim for warfarin, apixaban, dabigatran, rivaroxaban, or edoxaban during the baseline period. Patients with claims for >1 type of OAC on the index date were also excluded. Eligible patients were assigned to the apixaban, rivaroxaban, dabigatran, or warfarin cohort based on their newly initiated OAC. The end of the follow-up for patients within the study cohorts was defined as the earliest of the following dates: 90 days after index OAC treatment discontinuation (the end of drug days of supply), date a patient switched from the index OAC treatment to another OAC, date of health plan disenrollment, or 30 September 2015 (end of study period). The selection of patients for study cohorts treated with apixaban, rivaroxaban, dabigatran, and warfarin are shown in .
Figure 1. Selection of patients for study cohorts treated with apixaban and rivaroxaban. AF: atrial fibrillation; NVAF: nonvalvular atrial fibrillation; PSM: propensity score matching.
Propensity score matching
Multivariate logistic regression was used to generate propensity scores with covariates including patient characteristics of age, gender, race, US geographic region, Charlson Comorbidity Index (CCI) score, CHA2DS2-VASc score, HAS-BLED score, follow-up period duration, baseline total healthcare cost, baseline bleeding-related medical cost, baseline stroke-related medical cost, baseline comorbidities (thrombocytopenia, congestive heart failure, diabetes, hypertension, renal disease, myocardial infarction, dyspepsia/stomach discomfort, peripheral vascular disease, transient ischemic attack, coronary artery disease), and baseline medication usage (angiotensin-converting enzyme inhibitors, amiodarone, angiotensin receptor blockers, beta blockers, H2-receptor antagonists, proton pump inhibitors, statins, anti-platelet drugs). Separate 1:1 propensity score matching (PSM) was conducted (using the nearest neighbor algorithm) among patients treated with apixaban and rivaroxaban, those treated with apixaban and dabigatran, and those treated with apixaban and warfarin. The matched apixaban vs. rivaroxaban, apixaban vs. dabigatran, and apixaban vs. warfarin cohorts were inspected to verify that cohorts were well balanced with key patient characteristics not statistically different (p > .05).
Demographics and clinical characteristics
Demographic information and clinical characteristics, including CCI score, CHADS2 score, CHA2DS2-VASc score, HAS-BLED score, and prior bleeding and stroke diagnoses during the 12 month baseline period were determined for each patient in the study cohorts prior to and after PSM.
Outcome measurements
During the follow-up, S/SE and MB events were evaluated, based on hospitalization claims with the corresponding ICD-9-CM codes at the first position among the diagnosis codes associated with any of the inpatient claims. The diagnosis codes used for S/SE and MB were based on a validated administrative-claim-based algorithm as well as the International Society on Thrombosis and Haemostasis’ definition of MB as used in the ARISTOTLE trialCitation7,Citation20,Citation21. The clinical outcomes evaluated included rates of S/SE (any S/SE, with breakdown into following subcategories: ischemic stroke, hemorrhagic stroke, and SE) and MB (any MB with breakdown into the following subcategories; intracranial hemorrhage [ICH], gastrointestinal [GI] MB, and other MBs). One patient may have had more than one subcategories of S/SE or MB and the categories are not mutually exclusive.
Statistical analyses
Descriptive statistics were used to evaluate differences between study cohorts (apixaban vs. rivaroxaban, apixaban vs. dabigatran, and apixaban vs. warfarin) in demographics and clinical characteristics prior to and after PSM; t-tests and chi-square tests were used for the continuous and categorical variables, respectively. Propensity scores were generated using a multivariate logistic regression. Among the propensity score matched populations, Cox proportional hazards models were further used to assess the relationship between anticoagulation treatment (apixaban vs. rivaroxaban, apixaban vs. dabigatran, and apixaban vs. warfarin) and risks for S/SE and MB during the follow-up. No other covariates were used in such regression analyses after the PSM, since patient cohorts had balanced distribution for other covariates. Kaplan Meier analysis was used to illustrate time to events for any S/SE and any MB among matched cohorts. The threshold of p < .05 was used to determine statistical significance. Statistical software SAS version 9.4 (Cary, NC, USA) was utilized to perform all data analyses.
Results
Unmatched study cohorts
shows the baseline demographics and clinical characteristics prior to matching of study cohorts of patients treated with apixaban and other OACs. Of the unmatched study population, 8250 received apixaban, 11,082 received rivaroxaban, 2474 received dabigatran, and 14,051 received warfarin.
Table 1. Baseline demographics and clinical characteristics of study cohorts treated with apixaban vs. other oral anticoagulants prior to matching.
Apixaban vs. rivaroxaban
Relative to those treated with rivaroxaban, NVAF patients treated with apixaban had a higher mean age (78.0 vs. 77.2 years, p < .001), CCI score (3.0 vs. 2.8, p < .001), CHADS2 score (2.7 vs. 2.6, p < .001), CHA2DS2-VASc score (4.6 vs. 4.4, p < .001), and HAS-BLED score (3.1 vs. 3.0, p < .001) at baseline. The proportion of patients with bleeding diagnoses during the baseline period was lower among those treated with apixaban vs. rivaroxaban (18.9% vs. 20.5%, p = .007). Additionally, the mean duration of follow-up was shorter for patients treated with apixaban in comparison to those treated with rivaroxaban (6.3 vs. 7.4 months, p < .001).
Apixaban vs. dabigatran
Relative to dabigatran, patients treated with apixaban also had a higher mean age (78.0 vs. 76.8 years, p < .001), CCI score (3.0 vs. 2.7, p < .001), CHADS2 score (2.7 vs. 2.6, p < .001), CHA2DS2-VASc score (4.6 vs. 4.3, p < .001), and HAS-BLED score (3.1 vs. 2.9, p < .001). The mean duration of follow-up was shorter for patients treated with apixaban in comparison to those treated with dabigatran (6.3 vs. 7.6 months, p < .001).
Apixaban vs. warfarin
Unlike the other OAC comparisons, NVAF patients treated with apixaban vs. those treated with warfarin had a lower mean age (78.0 vs. 78.2 years, p = .03), CCI score (3.0 vs. 3.4, p < .001), CHADS2 score (2.7 vs. 2.9, p < .001), CHA2DS2-VASc score (4.6 vs. 4.7, p < .001), and HAS-BLED score (3.1 vs. 3.2, p < .001). The proportions of patients with stroke (11.8% vs. 15.8%, p < .001) and bleeding (18.9% vs. 24.0%, p < .001) diagnoses during the baseline period were lower among those treated with apixaban vs. warfarin. As in other OAC comparisons, the mean duration of follow-up was shorter for patients treated with apixaban than those treated with warfarin (6.3 vs. 8.3 months, p < .001).
Propensity score matched study cohorts
shows the baseline demographics and clinical characteristics after matching of study cohorts of patients treated with apixaban and other OACs.
Table 2. Baseline demographics and clinical characteristics of study cohorts treated with apixaban vs. other oral anticoagulants after matching.
Apixaban vs. rivaroxaban
After PSM, 6810 NVAF patients were in each cohort. Mean ages (77.1 vs. 77.0 years, p = .83), CCI scores (2.8 vs. 2.7, p = .97), and stroke and bleeding risks, according to CHADS2 scores (2.6 vs. 2.6, p = .97), CHA2DS2-VASc scores (4.4 vs. 4.4, p = .86), and HAS-BLED scores (2.9 vs. 2.9, p = .90), were not statistically different. The proportions of patients with stroke (10.0% vs. 9.8%, p = .69) and bleeding (16.8% vs. 16.6%, p = .81) diagnoses during the baseline period were also similar for NVAF patients treated with apixaban vs. rivaroxaban, as were the mean durations of follow-up (6.5 vs. 6.4 months, p = .54).
Apixaban vs. dabigatran
After PSM, 2327 NVAF patients were in each cohort. Mean ages (77.3 vs. 76.9 years, p = .11), CCI scores (2.6 vs. 2.6, p = .86), and stroke and bleeding risks, according to CHADS2 scores (2.6 vs. 2.6, p = .61), CHA2DS2-VASc scores (4.3 vs. 4.3, p = .58), and HAS-BLED scores (2.9 vs. 2.9, p = .77), were not statistically different. The proportions of patients with stroke (9.9% vs. 10.2%, p = .73) and bleeding (16.4% vs. 16.2%, p = .84) diagnoses during the baseline period were also similar for NVAF patients treated with apixaban vs. dabigatran, as were the mean durations of follow-up (7.1 vs. 7.0 months, p = .71).
Apixaban vs. warfarin
After PSM, 7107 NVAF patients were in each cohort. Mean ages (78.2 vs. 78.1 years, p = .54), CCI scores (3.0 vs. 3.0, p = .97), and stroke and bleeding risks, according to CHADS2 scores (2.7 vs. 2.7, p = .37), CHA2DS2-VASc scores (4.6 vs. 4.6, p = .66), and HAS-BLED scores (3.0 vs. 3.1, p = .22), were not statistically different. The proportions of patients with stroke (11.9% vs. 11.7%, p = .84) and bleeding (18.8% vs. 19.0%, p = .81) diagnoses during the baseline period were also similar for NVAF patients treated with apixaban vs. warfarin, as were the mean durations of follow-up (6.7 vs. 6.6 months, p = .66).
Post propensity score matching: unadjusted stroke/systemic embolism and major bleeding event rates of matched cohorts, apixaban vs. rivaroxaban
Among the matched populations, the unadjusted annual event rates of any S/SE (2.4% vs. 3.3%), ischemic stroke (1.7% vs. 2.6%), and hemorrhagic stroke (0.6% vs. 0.7%) during the follow-up were numerically lower for NVAF patients treated with apixaban in comparison to those treated with rivaroxaban. The rate of SE was 0.2% among apixaban treated patients and 0.1% among rivaroxaban treated patients. Additionally, event rates of any MB (4.7% vs. 9.5%), GI MB (2.5% vs. 5.9%), and other MB (1.8% vs. 3.6%) during the follow-up were numerically lower for NVAF patients treated with apixaban in comparison to those treated with rivaroxaban. The rate of intracranial hemorrhage was 0.7% among both apixaban and rivaroxaban treated patients. Illustrated with Kaplan Meier curves, times to any S/SE and any MB among matched cohorts treated with apixaban vs. rivaroxaban are shown in Supplementary Figures 1 and 2, respectively.
Cox regression results: apixaban vs. rivaroxaban
Cox regression results showed that among matched study cohorts the risks for any S/SE (hazard ratio [HR]: 0.72, p = .003), ischemic stroke (HR: 0.67, p = .012), any MB (HR: 0.49, p < .001), GI MB (HR: 0.43, p < .001), and other MB (HR: 0.51, p < .001) were significantly lower during the follow-up for patients treated with apixaban vs. rivaroxaban (). There were no significant differences in the risks for hemorrhagic stroke, systemic embolism, or intracranial hemorrhage between the two groups ().
Table 3. Cox regression results among matched cohorts: comparison of risks of stroke/systemic embolism and major bleeding during the follow-up associated with apixaban treatment vs. rivaroxaban, dabigatran, and warfarin.
Post propensity score matching: unadjusted stroke/systemic embolism and major bleeding event rates of matched cohorts, apixaban vs. dabigatran
Among the matched populations, the unadjusted annual event rates of any S/SE (2.6% vs. 3.3%), ischemic stroke (2.0% vs. 2.5%), and SE (0.2% vs. 0.3%) during the follow-up were numerically lower for NVAF patients treated with apixaban in comparison to those treated with dabigatran. The rate of hemorrhagic stroke was 0.7% among apixaban treated patients and 0.6% among dabigatran treated patients. Additionally, the event rates of any MB (4.7% vs. 5.8%), GI MB (2.6% vs. 3.8%), and other MB (1.7% vs. 2.0%) during the follow-up were numerically lower for NVAF patients treated with apixaban in comparison to those treated with dabigatran. The rate of intracranial hemorrhage was 0.7% among both apixaban and dabigatran treated patients. Illustrated with Kaplan Meier curves, times to any S/SE and any MB among matched cohorts treated with apixaban vs. dabigatran are shown in Supplementary Figures 3 and 4, respectively.
Cox regression results: apixaban vs. dabigatran
Cox regression showed that the risks for any S/SE (HR: 0.78, p = .27) and any MB (HR: 0.82, p = .23) among NVAF patients treated with apixaban vs. dabigatran trended to be lower, but did not reach statistical significance (). There were no significant differences in the risks for any of the subcategories of S/SE and MB ().
Post propensity score matching: unadjusted stroke/systemic embolism and major bleeding event rates of matched cohorts: apixaban vs. warfarin
Among the matched populations, the unadjusted annual event rates of any S/SE (2.8% vs. 4.2%), ischemic stroke (2.1% vs. 3.3%), and hemorrhagic stroke (0.6% vs. 0.9%) during the follow-up were numerically lower for NVAF patients treated with apixaban in comparison to those treated with warfarin. The rate of SE was 0.2% among apixaban treated patients and 0.1% among warfarin treated patients. Additionally, event rates of any MB (5.1% vs. 9.4%), intracranial hemorrhage (0.7% vs. 0.9%), GI MB (2.7% vs. 5.1%), and other MB (2.1% vs. 4.3%) during the follow-up were numerically lower for NVAF patients treated with apixaban in comparison to those treated with warfarin. Illustrated with Kaplan Meier curves, times to any S/SE and any MB among matched cohorts treated with apixaban vs. warfarin are shown in Supplementary Figures 5 and 6, respectively.
Cox regression results: apixaban vs. warfarin
Cox regression results showed that among matched study cohorts the risks for any S/SE (HR: 0.65, p < .001), ischemic stroke (HR: 0.63, p < .001), any MB (HR: 0.53, p < .001), GI MB (HR: 0.53, p < .001), and other MB (HR: 0.48, p < .001) were significantly lower during the follow-up for patients treated with apixaban vs. warfarin (). There were no significant differences in the risks for hemorrhagic stroke, systemic embolism, or intracranial hemorrhage between the two groups ().
Discussion
Elderly NVAF patients are at increased risk for stroke and bleedingCitation2,Citation18. Information on the stroke and bleeding risk in this unique patient population will help better manage these patients. This retrospective cohort study compared the risk of any S/SE and any MB between apixaban and other OACs among NVAF patients ≥65 years of age newly initiated on anticoagulant treatments in routine clinical practice. The findings from this study indicate that treatment with apixaban was associated with significantly lower risk of S/SE and MB than treatment with rivaroxaban and warfarin. The risks of S/SE and MB among elderly NVAF patients treated with apixaban vs. dabigatran trended to be lower, but did not reach statistical significance.
Elderly NVAF patients initiating different DOACs in routine clinical practice demonstrated several differences in patient characteristics. Patients treated with apixaban in comparison to those treated with rivaroxaban or dabigatran were older, more likely to have comorbidities, and have higher stroke and bleeding risks. These differences in patient characteristics are similar to those observed in an earlier evaluation of NVAF patients identified from the Premier Hospital and Cerner Health Facts databases who received apixaban, rivaroxaban, or dabigatranCitation16. Additionally, our results are consistent with those of the study conducted by Lip and colleagues of NVAF patients identified from the MarketScan Commercial and Medicare Supplemental databasesCitation14. In this latter study patients treated with apixaban vs. rivaroxaban and dabigatran were also older, more frequently diagnosed with comorbidities, and had higher stroke and bleeding risks according to CHADS2, CHA2DS2-VASc, and HAS-BLED scoresCitation14. Our study also shows some differences in patient characteristics between patients treated with warfarin vs. apixaban, with warfarin patients being older, more likely to have comorbidities, and having higher stroke and bleeding risks than apixaban patients. Consistently, Lip and colleagues reported that NVAF patients treated with warfarin were older and sicker than patients treated with other OACsCitation14. The underlying reasons for the different patient characteristics associated with different OAC use are not completely clear and warrant further investigations.
After balancing patient characteristics with PSM, the Cox regression analyses indicated that apixaban treatment in comparison to treatment with warfarin was associated with a lower risk for both stroke (35% lower S/SE risk) and MB (47% lower MB risk). These findings are consistent with that of the ARISTOTLE trial, in which apixaban demonstrated superiority vs. warfarin for both S/SE and MBCitation7. These findings are also consistent with a recent retrospective cohort study conducted by Yao and colleaguesCitation13. They reported that treatment with apixaban vs. warfarin was associated with a 33% lower risk for S/SE and 55% lower risk for MB among NVAF patients (median age: 73 years)Citation13.
Our study showed that in the matched cohorts, apixaban treatment in comparison to treatment with rivaroxaban was associated with a 28% lower risk for S/SE (any type) and a 51% lower risk for MB (any type). Furthermore, the risks for ischemic stroke, GI MB, and other MB were also significantly lower for patients treated with apixaban vs. rivaroxaban. When compared to dabigatran, treatment with apixaban was associated with numerically lower risks of S/SE and MB, but the differences did not reach statistical significance. Although our findings are consistent with that of randomized clinical trialsCitation8,Citation9, the event rates are not exactly the same, which is likely attributed to the differences in patient populations, with those in real-world settings being more diverse than those included in clinical trials. Thus, the results of the current study complement those of the clinical trials with those of real-world findings. While there are no head-to-head trials to compare different DOACs, an indirect comparison based on clinical trials that compared the outcomes of patients treated with each of the different DOACs vs. warfarin suggested that treatment with apixaban would be associated with a significantly lower risk of MB vs. treatment with rivaroxabanCitation22. Unlike the clinical trials, the current study focused on elderly NVAF patients and patients in routine clinical practice may be different from those participating in controlled clinical trials. Findings on MB from this study are generally consistent with the findings of Lip et al., who also compared the safety of DOACs in routine clinical practice after PSMCitation15. Lip et al. reported a nearly two-fold lower risk of MB (HR: 1.82; 95% CI: 1.36–2.43) for NVAF patients who received apixaban in comparison to those who received rivaroxaban (mean ages of cohorts: 68 years)Citation15. The risk of MB was also lower for patients who received apixaban vs. those who received dabigatran, but the difference was not statistically significant (mean ages of cohorts: 67 years)Citation15. The results on MB from this study are also similar to the findings of an early evaluation of MB risk among NVAF patients treated with different DOACs, which found the use of rivaroxaban compared to apixaban was associated with significantly greater risk of bleeding-related hospital readmissions across two different database analysesCitation16. Consistently, a recent study by Noseworthy et al. using the Optum database indicated that the MB risks were significantly lower for patients treated with apixaban vs. rivaroxaban (HR: 0.39, p < .001)Citation17. This study also found a significantly lower risk of MB for apixaban vs. dabigatran (HR: 0.50, p < .001)Citation17. Unlike the current study, Noseworthy and colleagues did not find a significant difference in the risk of S/SE among NVAF patients treated with apixaban vs. rivaroxaban (HR: 1.05, p = .85). While their study also used PSM to control for differences in patient characteristics among different DOAC cohorts, it included patients of all ages and did not exclude patients who had prior treatment with warfarinCitation17. In contrast, the current study focused on elderly NVAF patients newly initiating on OAC treatment.
It is important to understand treatment-related clinical outcomes among NVAF patients treated with different OACs in routine clinical practice to discern the most appropriate treatment strategies and reduce the disease burden. The results of our study provide further evidence about the risk of S/SE and MB associated with different OACs among NVAF patients, especially those ≥65 years of age. Future additional studies with larger sample sizes and longer time frames may be needed to further confirm the results of this real-world comparative study.
Study limitations
Retrospective, observational analyses using claims databases have certain inherent limitations, including that no causal relationship between treatment and outcomes can be established. Second, although a claim for a filled prescription may be documented, this does not indicate that the medication was consumed or that it was taken as prescribed. Also, medications filled over the counter or provided as samples by the physician are not captured in the claims data. Lab values are not reliably captured in the Humana database and therefore how often warfarin was within the therapeutic range could not be reliably assessed. Also, drug adherence to OACs was not evaluated and could impact outcomes. Edoxaban was not included in this analysis as its FDA approval was not long before the end of the study period and future studies are warranted to also include this more recently approved DOAC in the comparison of the effectiveness and safety of OACs in the real-world setting. Furthermore, NVAF patients may receive different dosages of DOACs in the real-world setting. In this study NVAF patients receiving any dosage of DOACs were included and future additional studies may be needed to examine whether our findings are consistent among those receiving only standard dosages or lower dosages of DOACs. Since a diagnosis code may be incorrectly coded or included as rule-out criteria rather than actual disease, the presence of a diagnosis code on a medical claim may not be positive indication of a particular disease. Additionally, mortality data is not reliably captured in the data source and was not evaluated as an outcome in this study. Although PSM was used to control for multiple confounders, there is potential for residual bias in this study. PSM does not take into account other confounding factors that were not included in the covariate list of the PSM process, such as drug adherence. However, many relevant real-world factors that may potentially impact the study outcomes were included in the PSM process already. Also, in the PSM process patients who did not have good matching were removed from the study cohorts and thus the findings may not be generalizable to the entire real-world population of elderly NVAF patients. Despite these potential limitations, the PSM process generated well balanced patient cohorts and provided a reliable tool for comparison of patient outcomes. Lastly, the Humana Research Database comprises claims of persons primarily residing in the Southern and Midwestern regions of the US and the results of this study may not be generalizable to the entire US elderly population.
Conclusions
In a real-world study of elderly NVAF patients in the US, after controlling for differences in patient characteristics, treatment with apixaban was associated with significantly lower risks of S/SE and MB than treatment with rivaroxaban and warfarin, and trended towards better outcomes compared with treatment with dabigatran. Further evaluations with longer time frames and/or based on other data sources may be needed to validate the findings of this study.
Transparency
Declaration of funding
This work was supported by Pfizer and Bristol-Myers Squibb.
Author contributions: S.D., X.L., K.G., J.T., J.M., T.C., and J.L. were responsible for study design. All authors contributed to data analysis and interpretation. M.L.-S., B.M., and J.L. drafted the manuscript with critical contributions from other authors. All authors approved the final manuscript for publication.
Declaration of financial/other relationships
S.D. has disclosed that he is a consultant for Pfizer and Bristol-Myers Squibb. X.L., J.T. and J.M. have disclosed that they are employees of Pfizer and own stock in the company. K.G. and T.C. have disclosed that they are employees of Bristol-Myers Squibb and own stock in the company. M.L.-S., B.M. and J.L. have disclosed that they are employees of Novosys Health, which has received research funds from Pfizer and Bristol-Myers Squibb in connection with conducting this study and development of this manuscript.
CMRO peer reviewers on this manuscript have received an honorarium from CMRO for their review work, but have no relevant financial or other relationships to disclose.
Supplementary figures
Download MS Power Point (118.6 KB)References
- Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994;154:1449-57
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370-5
- Ng KH, Hart RG, Eikelboom JW. Anticoagulation in patients aged ≥75 years with atrial fibrillation: role of novel oral anticoagulants. Cardiol Ther 2013;2:135-49
- Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol 2013;112:1142-17
- Pitt A, Ruff CT, Giugliano RP. Non-vitamin K antagonist oral anticoagulants in atrial fibrillation. Hematol Oncol Clin North Am 2016;30:1019-34
- Go AS, Hylek EM, Borowsky LH, et al. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med 1999;131:927-34
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. New Engl J Med 2011;365:981-92
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. New Engl J Med 2011;365:883-991
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. New Engl J Med 2009;361:1139-51
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955-62
- Graham DJ, Reichman ME, Wernecke M, et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation 2015;131;157-64
- Laliberte F, Cloutier M, Nelson WW, et al. Real-world comparative effectiveness and safety of rivaroxaban and warfarin in nonvalvular atrial fibrillation patients. Curr Med Res Opin 2014;30:1317-25
- Yao X, Abraham NS, Sangaralingham LR, et al. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J Am Heart Assoc 2016;5:e003725
- Lip GY, Pan X, Kamble S, et al. Major bleeding risk among non-valvular atrial fibrillation patients initiated on apixaban, dabigatran, rivaroxaban or warfarin: a “real-world” observational study in the United States. Int J Clin Pract 2016;70:752-63
- Lip GYH, Keshishian A, Kamble S, et al. Real-world comparison of major bleeding risk among non-valvular atrial fibrillation patients initiated on apixaban, dabigatran, rivaroxaban, or warfarin. A propensity score matched analysis. Thromb Haemost 2016;116:975-86
- Deitelzweig S, Bruno A, Trocio J, et al. An early evaluation of bleeding-related hospital readmissions among hospitalized patients with nonvalvular atrial fibrillation treated with direct oral anticoagulants. Curr Med Res Opin 2016;32:573-82
- Noseworthy PA, Yao X, Abraham NS, et al. Direct comparison of dabigatran, rivaroxaban, and apixaban for effectiveness and safety in nonvalvular atrial fibrillation. Chest 2016;150:1302-12
- Lip GY, Clementy N, Pericart L, et al. Stroke and major bleeding risk in elderly patients aged ≥75 years with atrial fibrillation: the Loire Valley atrial fibrillation project. Stroke 2015;46:143-50
- Jensen PN, Johnson K, Floyd J, et al. Identifying atrial fibrillation from electronic medical data: a systematic review. Pharmacoepidemiol Drug Saf 2012;21:141-7
- Thigpen JL, Dillon C, Forster KB, et al. Validity of international classification of disease codes to identify ischemic stroke and intracranial hemorrhage among individuals with associated diagnosis of atrial fibrillation. Circ Cardiovasc Qual Outcomes 2015;8:8-14
- Cunningham A, Stein CM, Chung CP, et al. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf 2011;20:560-6
- Lip GY, Larsen TB, Skjoth F, et al. Indirect comparisons of new oral anticoagulant drugs for efficacy and safety when used for stroke prevention in atrial fibrillation. J Am Coll Cardiol 2012;60:738-46