Abstract
Objective: To compare the risk and cost of stroke/systemic embolism (SE) and major bleeding between each direct oral anticoagulant (DOAC) and warfarin among non-valvular atrial fibrillation (NVAF) patients.
Methods: Patients (≥65 years) initiating warfarin or DOACs (apixaban, rivaroxaban, and dabigatran) were selected from the Medicare database from 1 January 2013 to 31 December 2014. Patients initiating each DOAC were matched 1:1 to warfarin patients using propensity score matching to balance demographics and clinical characteristics. Cox proportional hazards models were used to estimate the risks of stroke/SE and major bleeding of each DOAC vs. warfarin. Two-part models were used to compare the stroke/SE- and major-bleeding-related medical costs between matched cohorts.
Results: Of the 186,132 eligible patients, 20,803 apixaban–warfarin pairs, 52,476 rivaroxaban–warfarin pairs, and 16,731 dabigatran–warfarin pairs were matched. Apixaban (hazard ratio [HR] = 0.40; 95% confidence interval [CI] 0.31, 0.53) and rivaroxaban (HR = 0.72; 95% CI 0.63, 0.83) were significantly associated with lower risk of stroke/SE compared to warfarin. Apixaban (HR = 0.51; 95% CI 0.44, 0.58) and dabigatran (HR = 0.79; 95% CI 0.69, 0.91) were significantly associated with lower risk of major bleeding; rivaroxaban (HR = 1.17; 95% CI 1.10, 1.26) was significantly associated with higher risk of major bleeding compared to warfarin. Compared to warfarin, apixaban ($63 vs. $131) and rivaroxaban ($93 vs. $139) had significantly lower stroke/SE-related medical costs; apixaban ($292 vs. $529) and dabigatran ($369 vs. $450) had significantly lower major bleeding-related medical costs.
Conclusions: Among the DOACs in the study, only apixaban is associated with a significantly lower risk of stroke/SE and major bleeding and lower related medical costs compared to warfarin.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia in the US population and is a significant risk factor for strokeCitation1. The prevalence of AF increases with age, and it is estimated that over 80% of US adults with atrial fibrillation are 65 years or older and approximately 37% are 80 years or olderCitation2.
The American College of Cardiology (ACC) and the American Heart Association (AHA) guidelines recommend oral anticoagulants (OACs) to patients with non-valvular AF (NVAF) and prior stroke, transient ischemic attack (TIA), or a CHA2DS2-VASc score ≥2Citation3.
Warfarin, a vitamin K antagonist (VKA), has been the most commonly used treatment for stroke prevention among NVAF patients since the 1950sCitation3. It was proven that dose-adjusted warfarin reduced stroke by more than 60% compared to controls and was more efficacious than antiplatelet therapyCitation4. Direct oral anticoagulants (DOACs; apixaban, rivaroxaban, dabigatran, edoxaban) have been approved by the US Food and Drug Administration (FDA) in recent years. While warfarin treatment requires a therapeutic range obtained by routine monitoring of the international normalized ratio (INR) through blood testing, DOACs provide a more convenient approach in reducing the risk of stroke/systemic embolism (SE) among NVAF patients with fewer drug and food interactionsCitation3.
Clinical trials have shown that DOACs are at least non-inferior to warfarin with respect to stroke and SE prevention and major bleeding riskCitation5–8. Compared to warfarin patients, apixaban patients had lower rates of stroke/SE and major bleedingCitation7; patients with rivaroxaban use had non-inferior rates of stroke/SE and similar rates of major bleedingCitation6; those prescribed 110 mg dabigatran (not approved in the US) had similar rates of stroke/SE and lower rates of major bleeding; those prescribed 150 mg dabigatran experienced lower rates of stroke/SE and similar rates of major bleedingCitation5; and patients with edoxaban use had non-inferior rates of stroke/SE and lower rates of major bleedingCitation8. Real-world comparisons between DOACs and warfarin exist, but little is known about the effectiveness and safety of apixaban because it was approved in December 2012, later than rivaroxaban and dabigatranCitation9–11.
AF carries a significant financial burden, costing the US healthcare system approximately $26 billion dollars annually, with AF-related hospitalizations accounting for the majority of these costs (52%)Citation12. In addition to understanding the clinical difference between DOACs and warfarin, it is important to evaluate the economic burden associated with these treatments.
Moreover, since elderly patients comprise the majority of NVAF patients, it is valuable to study the effectiveness and safety of OACs using Medicare data, which is the largest US database of patients older than 65 years. The aim of this study was to evaluate whether real-world study results in a large elderly population supplement clinical trial results. The current analysis is one of the largest real-world studies comparing the risk of stroke/SE and major bleeding and associated costs among OAC treatment-naïve NVAF Medicare beneficiaries who were prescribed apixaban, rivaroxaban, or dabigatran compared with warfarin in US clinical practice settings. Using the US Medicare data, this study provides new evidence of the effectiveness and safety comparisons of DOACs compared to warfarin among the elderly population, and adds comprehensive real-world cost data to limited literature.
Methods
Data source and patient selection
A real-world observational study was conducted using the US Center of Medicare and Medicaid Services (CMS) data from 1 January 2012 through 31 December 2014. Medicare is the federal health insurance program for those aged ≥65 years, certain younger people with disabilities, and people with end-stage renal disease in the United States, with an estimated 38 million fee-for-service beneficiariesCitation13. The database contains medical and pharmacy claims from 100% national Medicare data, which includes hospital inpatient, outpatient, Medicare carrier, Part D, skilled nursing facility, home health agency, and durable medical equipment claims. The medical claims are coded using International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM), Current Procedural Terminology, or Healthcare Common Procedure Coding System codes. Pharmacy claims include the drug dispensed using the National Drug Code coding system.
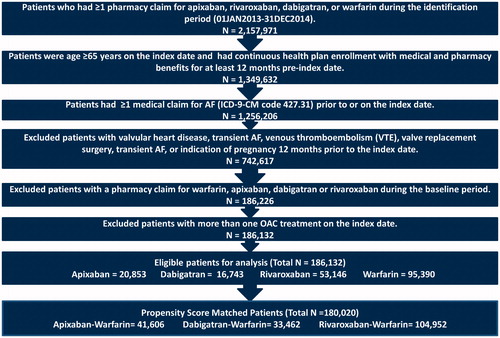
Elderly patients (age ≥65 years) with ≥1 pharmacy claim for warfarin, apixaban, rivaroxaban, or dabigatran from 1 January 2013 through 31 December 2014 were selected. Edoxaban was approved by the FDA in 2015; therefore, it was not included in the study. The date of the first OAC prescription fill was designated as the index date. Patients were required to have continuous medical (Part A and Part B) and pharmacy (Part D) enrolment for 12 months prior to the index date (baseline period). Patients were required to have ≥1 medical claim for AF (ICD-9-CM: 427.31) during the baseline periodCitation14. Patients with evidence of valvular heart disease, heart valve replacement or surgery, venous thromboembolism (VTE), transient AF (pericarditis, hyperthyroidism, thyrotoxicity), or a pharmacy claim for an OAC during the baseline period, more than one OAC claim on the index date, or indication of pregnancy during the study period, were excluded (Supplemental Table 1).
Patients were followed from the index date to the OAC prescription discontinuation date, switch to an OAC other than the index drug, death, interruption in continuous enrolment, or end of the study period (31 December 2014), whichever occurred the earliest. Discontinuation was defined as no evidence of index OAC prescription for 30 days from the last day of supply of the last filled prescription. The date of discontinuation was defined as the last day of days’ supply of last filled prescription. Switching was defined as a prescription for an OAC other than the index OAC prescription within 30 days before or after the discontinuation dateCitation15. The date of switching was defined as the prescription date of the OAC other than the index OAC.
Outcome measures
Primary outcome measures were stroke/SE and major bleeding defined by the primary discharge diagnosis in the inpatient setting. Stroke/SE included hemorrhagic stroke, ischemic stroke, and SE. Major bleeding included bleeding at key sites including – but not limited to – intracranial, gastrointestinal, liver, splenic, and ocular hemorrhage. The major bleeding code list was based on a validated administrative claim based algorithm and the International Society on Thrombosis and Haemostasis’ definition of major bleeding (Supplemental Table 2)Citation16,Citation17. The effectiveness and safety outcomes were measured independently; patients were censored at stroke/SE and/or major bleeding events for the respective analysis.
Stroke/SE-related and major bleeding-related medical costs were defined as the first stroke/SE or major bleeding hospitalization costs plus all additional costs after the hospitalization related to stroke/SE and major bleeding events during the follow-up. Stroke/SE- and major-bleeding-related medical costs were evaluated in all clinical settings. The medical costs included hospitalization, outpatient, and emergency room visit costs. The total paid amount was examined, which includes patient and payer paid amounts. Costs were calculated per patient per month (PPPM) and adjusted to 2014 US dollars using the medical care component of the consumer price index (CPI)Citation18.
Baseline variables
Patient demographics, clinical characteristics (clinical risk scores, comorbidities, and co-medications), and baseline hospitalizations were measured during the baseline period. Stroke and major bleeding risks were assessed using the CHADS2, CHA2DS2-VASc, and HAS-BLED scores, respectivelyCitation19,Citation20. The CHA2DS2-VASc stroke risk score was calculated as the summed total of the points determined for each diagnosis or characteristic, and was based on the CHADS2 score (congestive heart failure, hypertension, aged >75 years, diabetes, prior stroke or transient ischemic attack, or thromboembolism) plus vascular disease, aged 65–74 years, and sexCitation20. The HAS-BLED bleeding risk score was based on evidence of hypertension, abnormal kidney or liver function, stroke, bleeding, age >65 years, and drugs/alcohol abuse or dependenceCitation19. INR values were not available; therefore, they were not included in the calculation.
Statistical methods
All study variables, including baseline and outcome measures, were analyzed descriptively and stratified by cohort. Pearson’s chi-square tests were used to evaluate significant differences for dichotomous variables; Student’s t-tests were used for continuous variables. One-to-one propensity score matching (PSM) was used to balance demographics and clinical characteristics between each DOAC vs. warfarin (apixaban vs. warfarin, rivaroxaban vs. warfarin, and dabigatran vs. warfarin). Age, sex, US geographic region, Deyo–Charlson comorbidity index (CCI) score, CHA2DS2-VASc score, HAS-BLED score, stroke and bleeding history, comorbidities, baseline medication use, and inpatient admissions were used to calculate propensity scores for each patient using logistic regressionCitation21. The nearest neighbor method with a caliper of 0.01 was used to match patients. Mean standardized differences were used to assess the balance of baseline patient characteristics; values ≥10% were used as the thresholdCitation22.
Incidence rates of stroke/SE and major bleeding in PSM matched cohorts were calculated as the number of stroke/SE and major bleeding events, respectively, per 100 person-years. Cox proportional hazards models were used to compare the risk of stroke/SE and major bleeding in each of the three matched cohorts. Geographic region was included in the models as an independent variable due to imbalance after matching. Two-part models with bootstrapping were used to analyze the stroke/SE- and major-bleeding-related medical costs PPPM. There were a large number of zeroes in the stroke- and major-bleeding-related medical costs; therefore, two-part models were implemented, in which the first part was a logistic regression for the occurrence of the event (stroke or major bleeding) and the second part was a generalized linear model (GLM) regression of cost, conditional on the eventCitation23. Gamma distribution with log link was used for the GLM regression.
Sensitivity analyses
To ensure the robustness of the results in the main analysis, three sensitivity analyses were conducted. First, the cohorts were stratified by dosage (reduced and standard) to determine whether the treatment effects were modified by dosage. For the DOAC cohorts, standard-dose (apixaban 5 mg, rivaroxaban 20 mg, and dabigatran 150 mg) and reduced-dose (apixaban 2.5 mg, rivaroxaban 15 mg, and dabigatran 75 mg) cohorts were created based on the index dosage. Each warfarin patient was assigned to one of the two subgroups according to the dose of the matched DOAC patient. The balance of baseline characteristics was tested in each subgroup, and when imbalance was detected (standardized difference >10%), the variable was included in the multivariate model. The statistical significance of the interaction between treatment and dose was evaluated. Second, only patients with at least 30 days of follow-up were evaluated to exclude patients who had too short a follow-up to develop any stroke/SE or major bleeding events. The balance of the baseline characteristics was checked after excluding those patients with <30 days, and unbalanced variables were included in the multivariate model. Third, patients were censored at 6 months. Apixaban patients had a shorter follow-up time due to the drug’s recent market entry; therefore, the third sensitivity analysis helped create a more balanced follow-up period among the treatment groups.
Results
Baseline characteristics
Of 186,132 patients who were eligible for the analysis before PSM, 95,390 (51.2%) were prescribed warfarin, 20,853 (11.2%) were prescribed apixaban, 53,146 (28.6%) were prescribed rivaroxaban, and 16,743 (9.0%) were prescribed dabigatran. Patients initiating warfarin were older and had significantly higher baseline mean CHA2DS2-VASc and CCI scores followed by apixaban, rivaroxaban, and dabigatran (Supplemental Table 3). After 1:1 PSM, there were 20,803 apixaban–warfarin matched pairs, 52,476 rivaroxaban–warfarin matched pairs, and 16,731 dabigatran–warfarin matched pairs ().
Figure 1. Patient selection flow chart. Abbreviations. AF, atrial fibrillation; OAC, oral anticoagulant; ICD-9-CM; International Classification of Disease, 9th Revision, Clinical Modification; VTE, venous thromboembolism.
The descriptive baseline characteristics are shown in . After PSM, comparisons of baseline characteristics between matched cohorts did not show significant differences, with the exception of geographic regions; significant differences (standardized difference ≥10%) were found for the North Central and South regions. Apixaban–warfarin matched patients had a mean age of 78 years and mean CCI score of 2.8 and 2.9 for apixaban and warfarin patients, respectively. Rivaroxaban–warfarin matched patients had a mean age of 78 years and mean CCI score of 2.7. Dabigatran–warfarin matched patients had a mean age of 77 years and mean CCI score of 2.5 and 2.6 for dabigatran and warfarin matched patients, respectively. With regards to dosing, 72.0%, 64.3%, and 79.7% of matched patients were prescribed standard dose apixaban, rivaroxaban, and dabigatran, respectively.
Table 1. Baseline characteristics and follow-up time in propensity score matched cohorts.
Clinical outcomes
The follow-up time for each matched cohort is shown in . The incidence rates of stroke/SE and major bleeding are shown in and .
Figure 2. Hazard ratio of stroke/SE for propensity score matched patients. Abbreviations. HR, hazard ratio; CI, confidence interval; SE: systemic embolism.
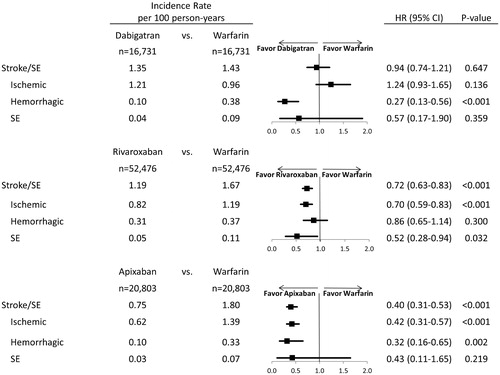
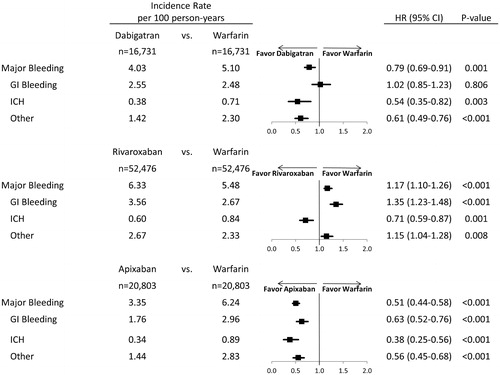
Figure 3. Hazard ratio of major bleeding for propensity score matched patients. Abbreviations. HR, hazard ratio; CI, confidence interval; GI, Gastrointestinal; ICH, Intracranial hemorrhage.
Apixaban (hazard ratio [HR]: 0.40; 95% confidence interval [CI]: 0.31, 0.53; p < .001) and rivaroxaban (HR: 0.72; 95% CI: 0.63, 0.83; p < .001) were associated with a significantly lower risk of stroke/SE compared to warfarin. Dabigatran (HR: 0.94; 95% CI: 0.74–1.21; p = .647) was associated with a similar risk of stroke/SE compared to warfarin (). The incidence rates and hazard ratios for ischemic stroke, hemorrhagic stroke, and SE are shown in .
Apixaban (HR: 0.51; 95% CI: 0.44, 0.58; p < .001) and dabigatran (HR: 0.79; 95% CI: 0.69, 0.91; p = .001) were associated with a significantly lower risk of major bleeding compared to warfarin. Rivaroxaban (HR: 1.17; 95% CI: 1.10, 1.26; p < .001) was associated with a significantly higher risk of major bleeding compared to warfarin (). The incidence rates and hazard ratios for gastrointestinal bleeding, intracranial hemorrhage, and other major bleeding are shown in .
Economic outcomes
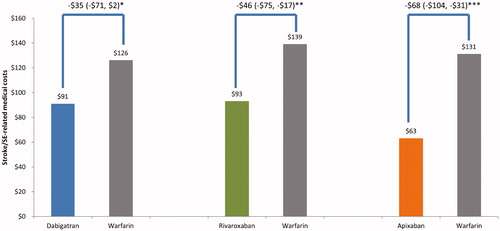
Compared to warfarin, apixaban ($63 vs. $131; difference: −$68; 95% CI: −$104, -$31; p < .001) and rivaroxaban ($93 vs. $139; difference: −$46; 95% CI: −$75, −$17; p = .002) had significantly lower PPPM stroke/SE-related medical costs; dabigatran ($91 vs. $126; difference: −$35; 95% CI: −$71, $2; p = .064) had similar stroke/SE-related medical costs ().
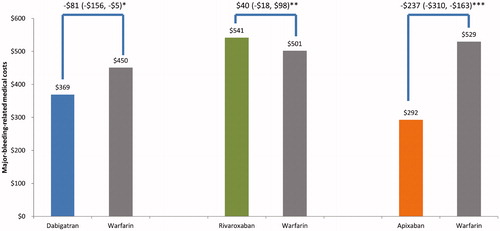
Figure 4. Stroke-related medical costs PPPM for propensity score matched patients. *p = .064; **p = .002; ***p < .001. Abbreviations. PPPM, per patient per month; CI, confidence interval; SE, systemic embolism.
Compared to warfarin, apixaban ($292 vs. $529; difference: −$237; 95% CI: −$310, −$163; p < .001) and dabigatran ($369 vs. $450; difference: −$81; 95% CI: −$156, −$5; p = .036) had significantly lower major-bleeding-related medical costs. Rivaroxaban ($541 vs. $501; difference: $40; 95% CI: −$18, $98; p = .177) had similar major-bleeding-related medical costs compared to warfarin ().
Sensitivity analyses
The results of the sensitivity analyses were consistent with those of the main analysis. In the dosing analysis, a significant interaction was found for stroke/SE between apixaban treatment and dosage (p = .046). Although both standard (HR: 0.34; 95% CI: 0.24, 0.47) and reduced dose (HR: 0.60; 95% CI: 0.38, 0.96) apixaban were associated with significantly lower risk of stroke/SE compared to warfarin, there was a difference in the magnitude caused by dosage. There was no significant interaction for major bleeding between apixaban treatment and dosage (p = .407). There were no significant interactions for rivaroxaban and dabigatran and dosage for major bleeding and stroke/SE ().
Table 2. Dose sensitivity analysis for propensity score matched patients.
In the sensitivity analyses for ≥30 days of follow-up and censoring at 6 months, the results were consistent with those of the main analysis. In both sensitivity analyses, apixaban and rivaroxaban patients had significantly lower risks of stroke/SE and dabigatran patients had similar risk of stroke/SE compared to warfarin patients. In the two sensitivity analyses, apixaban and dabigatran also demonstrated significantly lower risk of major bleeding and rivaroxaban demonstrated significantly higher risk of major bleeding compared to warfarin ().
Table 3. Hazard ratio of stroke/SE and major bleeding for propensity score matched patients in the sensitivity analyses.
Discussion
This is the largest US real-world evaluation of the effectiveness, safety, and associated costs of DOACs compared to warfarin to date in the elderly NVAF population using PSM. This real-world study of Medicare patients with NVAF demonstrated that, when compared to warfarin, apixaban was associated with significantly lower risk of stroke/SE and major bleeding; rivaroxaban was associated with significantly lower risk of stroke/SE but higher risk of major bleeding; and dabigatran was associated with similar risk of stroke/SE and significantly lower risk of major bleeding. The results also demonstrated that patients who initiated apixaban had lower stroke/SE- and major-bleeding-related medical costs compared to warfarin; patients who initiated rivaroxaban had significantly lower stroke/SE-related medical costs compared to warfarin; and patients who initiated dabigatran had significantly lower major-bleeding-related costs.
The effectiveness and safety results of apixaban versus warfarin observed in the study were generally consistent with those of the ARTISTOTLE clinical trial. In the ARISTOTLE trial, apixaban was superior to warfarin in reducing the risk of stroke/SE (HR: 0.79; 95% CI: 0.66, 0.95; p < .001 for non-inferiority; p = .011 for superiority) with fewer major bleeding events (HR: 0.69; 95% CI: 0.60, 0.80; p < .001)Citation7 In the ROCKET-AF clinical trial, patients with rivaroxaban use had non-inferior risk of stroke/SE (HR: 0.79; 95% CI: 0.66, 0.96; p < .001 for non-inferiority) and similar risk of major bleeding (HR: 1.04; 95% CI: 0.90, 1.20; p = .58) compared to warfarinCitation6. These results were different from those in our study where we found rivaroxaban was associated with significantly lower risk of stroke/SE and higher risk of major bleeding. In the RE-LY trial, 110 mg dabigatran (not approved in the US) was associated with similar risk of stroke/SE (HR: 0.91; 95% CI: 0.74, 1.11; p < .001 for non-inferiority) and lower risk of major bleeding (HR: 0.80; 95% CI: 0.69, 0.93; p = .003); 150 mg dabigatran was associated with significantly lower risk of stroke/SE (HR: 0.66; 95% CI: 0.53, 0.82; p < .001 for superiority) and similar risk of major bleeding (HR: 0.93; 95% CI: 0.81, 1.07; p = .31) compared to warfarinCitation5. The results from this clinical trial are different from our real-world study where we found dabigatran (150 mg and 75 mg) was associated with similar risk of stroke/SE and significantly lower risk of major bleeding.
Several real-world studies have compared the effectiveness and safety of rivaroxaban and dabigatran to warfarin with varying results. In the Graham et al. study using Medicare data, dabigatran patients had a significantly lower risk of ischemic stroke (HR: 0.80; 95% CI: 0.67, 0.96) and similar risk of major bleeding (HR: 0.97; 95% CI: 0.88, 1.07) compared to warfarinCitation24. Likewise, in the Lauffenburger et al. study using Truven MarketScan data, dabigatran was associated with a lower risk of ischemic stroke/SE (HR: 0.86; 95% CI: 0.79, 0.93) and similar risk of major bleeding (HR: 0.94; 95% CI: 0.87, 1.01)Citation25. Similarly, the Villines et al. study using US Department of Defense claims data demonstrated that patients with dabigatran use had lower risk of stroke (HR: 0.73; 95% CI: 0.55, 0.97) and similar risk of major bleeding (HR: 0.87; 95% CI: 0.74, 1.03) compared to warfarinCitation26. However, the Lip et al.Citation11 and the Yao et al.Citation9 studies showed contrary results, and these results are consistent with those of our analysis. In the Lip et al. major bleeding study using MarketScan data, patients initiating dabigatran (HR: 0.69; 95% CI: 0.50, 0.96) had significantly lower risk of major bleeding compared to warfarinCitation11. The Yao et al. study using OptumLabs data found that dabigatran (HR: 0.79; 95% CI: 0.67, 0.94) had significantly lower risk of major bleeding and similar risk of stroke/SE (HR: 0.98; 95% CI: 0.76, 1.26) compared to warfarin, which is consistent with our studyCitation9. The differences in databases and patient populations between our study and others may explain the inconsistencies in results.
Laliberté et al. compared rivaroxaban to warfarin using Symphony Health Solutions’ Patient Transactional data and found that patients prescribed rivaroxaban had similar bleeding and composite stroke and SE outcomes to those prescribed warfarinCitation27. Likewise, the Yao et al. study using OptumLabs data found that patients with rivaroxaban use had similar risk of major bleeding (HR: 1.04; 95% CI: 0.90, 1.20) and stroke/SE (HR: 0.93; 95% CI: 0.72, 1.19) compared to those with warfarin useCitation9. In the comparison of the types of major bleeding, the Yao study and our study were similar, in that rivaroxaban patients had significantly lower risk of intracranial hemorrhage but higher risk of gastrointestinal bleeding compared to warfarin patients. The Lip et al. major bleeding study using MarketScan data also found that patients initiating rivaroxaban treatment (HR: 0.98; 95% CI: 0.83, 1.17) had similar risk of major bleeding compared to those initiating warfarinCitation11. Contrary to those studies, our study found that rivaroxaban was associated with a significantly higher risk of major bleeding and a lower risk of stroke/SE in the elderly population. The inconsistency may be due to the difference in patient characteristics (e.g. older mean age, higher HAS-BLED score [higher hypertension and renal disease]), risks of different types of stroke and major bleeding in the elderly population, study design, and sample size (the sample size was larger in our study).
Several real-world data studies have consistently demonstrated that apixaban was associated with lower risk of stroke/SE and major bleeding compared to warfarin. In the Lip et al. major bleeding study, patients initiating apixaban (HR: 0.53; 95% CI: 0.39, 0.71) had significantly lower risk of major bleeding compared to those initiating warfarinCitation11. The Yao et al. OptumLabs data study found that apixaban users had significantly lower risk of major bleeding (HR: 0.45; 95% CI: 0.34, 0.59) and stroke/SE (HR: 0.67; 95% CI: 0.46, 0.98) compared to warfarin usersCitation9. Similarly, in a recently published study pooling four claims datasets, apixaban initiators were associated with a significantly lower risk of stroke/SE (HR: 0.67; 95% CI: 0.59, 0.76) and major bleeding (HR: 0.60; 95% CI: 0.54, 0.65) compared with warfarin initiatorsCitation28. Among the comparisons of DOACs to warfarin, only apixaban was consistently associated with significantly lower risks for both stroke/SE and major bleeding.
Limited real-world studies have compared medical costs between DOACs and warfarin. Laliberté et al. using Premier Perspective Comparative Hospital data demonstrated that rivaroxaban had significantly lower hospitalization cost compared to warfarin ($11,993 vs. $13,255; difference: -$1284; p < .001) per patient during the index hospitalization from 2010 to 2012Citation29. Another study by Laliberté et al. also found significantly lower all-cause and AF-related hospitalization costs for rivaroxaban compared to warfarin using Humana data from 2011 to 2012Citation30. However, Laliberté et al. did not report costs associated with clinical outcomes: stroke/SE and major bleeding.
One study comparing the costs related to DOACs versus warfarin using the Medco US health plans data and corresponding relative risks from the clinical trials of DOACs demonstrated that apixaban (difference: -$1245) and dabigatran (-$555) patients were associated with significantly lower stroke plus major bleeding excluding intracranial hemorrhage (MBEIH)-related medical costs, while rivaroxaban patients (difference: $144) had significantly higher costs compared to warfarinCitation31. Another analysis using the same patient population and methods showed that apixaban was associated with a $493 reduction in stroke-related medical costs and a $752 reduction in MBEIH-related medical costs compared to warfarinCitation32. The current study adds to the body of evidence regarding medical costs related to major bleeding and stroke/SE among OAC patients with NVAF.
There are several limitations to our study. Due to the nature of observational cohort studies, no causal relations could be inferred and only associations were assessed. Although cohorts were PSM matched, potential residual confounders exist, such as over-the-counter aspirin use and warfarin dose adjustment, which are not available in the dataset. Claims data lacks laboratory results and accuracy in medical information. For example, in this study, AF was identified first, and patients with ICD-9-CM codes for valvular heart disease were excluded. Diagnoses were identified using ICD-9-CM codes, which is different from the clinical trials. As a result, this study may not be directly comparable to clinical trials. Additionally, the presence of a claim for a filled prescription does not indicate whether the medication was consumed or taken as prescribed. This study included only treatment naïve OAC patients at baseline, which may have impacted the generalization of the results. OAC drug prescription(s) or other comorbid conditions were not evaluated prior to the 12-month baseline period. Moreover, the follow-up period was not uniform for the four study cohorts, which may have introduced bias into the results. However, our findings from sensitivity analyses were consistent with primary analysis, indicating that these results are less likely to be affected by different follow-up periods. Compared with clinical trials, the follow-up period for each cohort in this study was also shorter, which may impact our results. Finally, although understanding the US Medicare population is important in managing NVAF, findings from this elderly population may not be generalized to other populations.
Conclusions
This analysis is one of the first and largest real-world studies examining the risk of stroke/SE, major bleeding, and associated costs among US elderly NVAF patients using PSM.
This real-world study in the elderly Medicare population found that, when compared to warfarin, apixaban was associated with significantly lower risks of stroke/SE and major bleeding; dabigatran was associated with similar risk of stroke/SE and significantly lower risk of major bleeding; and rivaroxaban was associated with significantly lower risk of stroke/SE, but higher risk of major bleeding.
Cost analyses demonstrated that patients who were prescribed apixaban had both lower stroke/SE- and major-bleeding-related medical costs; dabigatran had similar stroke/SE-related costs and significantly lower major-bleeding-related costs, and rivaroxaban had significantly lower stroke/SE-related medical costs and similar major bleeding costs when compared to warfarin. The clinical results of this study, which supplement what has been reported in clinical trial data, along with the economic results may help healthcare providers with the selection of appropriate treatment for patients with NVAF.
Transparency
Declaration of funding
This work was funded by Pfizer Inc. and Bristol-Myers Squibb.
Declaration of financial/other relationships
A.A. has disclosed that he is an employee of University of California, Irvine, and was a paid consultant to Bristol-Myers Squibb in connection with this study and development of this manuscript. A.K., Q.Z. and O.B. have disclosed that they are employees of STATinMED Research, a paid consultant to Pfizer and Bristol-Myers Squibb in connection with this study and the development of this manuscript. J.T., O.D., J.M. and X.L. have disclosed that they are employees of Pfizer Inc., with ownership of stocks in Pfizer Inc. H.L., L.R. and L.V. have disclosed that they are employees of Bristol-Myers Squibb Company. L.R. and L.V. have disclosed that they have ownership of stocks in Bristol-Myers Squibb Company.
CMRO peer reviewers on this manuscript have received an honorarium from CMRO for their review work, but have no relevant financial or other relationships to disclose.
Supplemental material
Download MS Word (24.1 KB)Acknowledgements
Michael Moriarty provided medical writing and editorial support with STATinMED Research which is a paid consultant to Bristol-Myers Squibb and Pfizer. Statistical programming for this study was provided by Yingchu Zhao and Yiyun Lin of STATinMED Research which is a paid consultant to Bristol-Myers Squibb and Pfizer.
References
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983-8
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA 2001;285:2370-5
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation 2014;130:e199-267
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857-67
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-91
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093-104
- Yao X, Abraham NS, Sangaralingham LR, et al. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J Am Heart Assoc 2016;5:e003725
- Abraham NS, Singh S, Alexander GC, et al. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ 2015;350:h1857
- Lip GY, Keshishian A, Kamble S, et al. Real-world comparison of major bleeding risk among non-valvular atrial fibrillation patients initiated on apixaban, dabigatran, rivaroxaban, or warfarin. Thromb Haemost 2016;116:975-86
- Le Heuzey JY, Paziaud O, Piot O, et al. Cost of care distribution in atrial fibrillation patients: the COCAF study. Am Heart J 2004;147:121-6
- CMS/Office of Enterprise Data & Analytics (OEDA). Medicare enrollment dashboard: hospital/medical enrollment, December 2016. Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Dashboard/Medicare-Enrollment/Enrollment%20Dashboard.html [Last accessed 6 March 2017]
- Jensen PN, Johnson K, Floyd J, et al. Identifying atrial fibrillation from electronic medical data: a systematic review. Pharmacoepidemiol Drug Saf 2012;21:141-7
- Teutsch C, Huisman MV, Lip GY, et al. Persistence with dabigatran therapy for stroke prevention in patients with non-valvular atrial fibrillation: the Gloria-AF Registry. Blood 2016;128:2616
- Thigpen JL, Dillon C, Forster KB, et al. Validity of international classification of disease codes to identify ischaemic stroke and intracranial hemorrhage among individuals with associated diagnosis of atrial fibrillation. Circ Cardiovasc Qual Outcomes 2015;8:8-14
- Cunningham A, Stein CM, Chung CP, et al. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf 2011;20;560-6
- Crawford M, Church J, Akin B. CPI Detailed Report Data for December 2014. Bureau of Labor Statistics. 2014. Available at: https://www.bls.gov/cpi/cpid1412.pdf [Last accessed 5 April 2017]
- Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093-100
- Lip GYH, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263-72
- Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 2014;33:1242-58
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083-107
- Lachenbruch PA. Comparisons of two-part models with competitors. Stat Med 2001;20:1215-34
- Graham DJ, Reichman ME, Wernecke M, et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for non-valvular atrial fibrillation. Circulation 2015;4:e001798
- Lauffenburger JC, Farley JF, Gehi AK, et al. Effectiveness and safety of dabigatran and warfarin in real-world US patients with non-valvular atrial fibrillation: a retrospective cohort study. J Am Heart Assoc 2015;4:e001798
- Villines TC, Schnee J, Fraeman K, et al. A comparison of the safety and effectiveness of dabigatran and warfarin in non-valvular atrial fibrillation patients in a large healthcare system. Thromb Haemost 2015:114:1290-8
- Laliberté F, Cloutier M, Nelson WW, et al. Real-world comparative effectiveness and safety of rivaroxaban and warfarin in nonvalvular atrial fibrillation patients. Curr Med Res Opin 2014;30:1317-25
- Li X, Deitelzweig S, Keshishian A, et al. Effectiveness and safety of apixaban versus warfarin in non-valvular atrial fibrillation patients in “real-world” clinical practice. Thromb Haemost 2017;117:1072-1082
- Laliberté F, Pilon D, Raut MK, et al. Is rivaroxaban associated with lower inpatient costs compared to warfarin among patients with non-valvular atrial fibrillation? Curr Med Res Opin 2014;30:1521-8
- Laliberté F, Cloutier M, Crivera C, et al. Effect of rivaroxaban versus warfarin on health care costs among nonvalvular atrial fibrillation patients: observations from rivaroxaban users and matched warfarin users. Adv Ther 2015;32:216-27
- Amin A, Stokes M, Makenbaeva D, et al. Estimated medical cost reductions associated with use of novel oral anticoagulants vs. warfarin in a real-world non-valvular atrial fibrillation patient population. J Med Econ 2014;17:771-81
- Amin A, Stokes M, Wu N, et al. Estimated medical cost reductions associated with apixaban in real-world patients with non-valvular atrial fibrillation. J Med Econ 2013;16:1193-202