Dear Editor,
We read with great interest the article by Handelsman and colleagues, reporting that a once-weekly dipeptidyl peptidase-4 (DPP-4) inhibitor omarigliptin was non-inferior to a once-daily sulfonylurea glimepiride as add-on therapy to metformin in type 2 diabetic patientsCitation1. If glucose-lowering effect was similar, patient satisfaction would be a key determinant in choosing a once-weekly agent over a once-daily one in clinical practice. However, the impact of introducing a once-weekly DPP-4 inhibitor on treatment satisfaction remains to be assessed. It is possible that patients taking metformin will be less satisfied with the introduction of a once-weekly DPP-4 inhibitor, because it needs to be taken differently from metformin, which is taken every day. Indeed, our clinical data suggested this concern about treatment satisfaction, as described below.
We collected data on treatment satisfaction in 160 type 2 Japanese diabetic patients (aged 65 ± 11 years, 62% male, with hemoglobin A1c 6.4 ± 0.6%) who were treated with daily medications including a DPP-4 inhibitor and had the daily DPP-4 inhibitor switched to trelagliptin, another once-weekly DPP-4 inhibitor available in Japan. All patients were treated in Shiraiwa Medical Clinic, where treatment satisfaction was routinely checked at the change of the medical regimens. Treatment satisfaction was evaluated with a questionnaire for oral medication satisfaction, yielding a satisfaction score ranged from 0 (no satisfaction) to 60 (full satisfaction)Citation2. We analyzed the change in treatment satisfaction after the switch by using a linear mixed model with adjustment for age, sex, hemoglobin A1c levels, and insulin use. The study was in accordance with the Declaration of Helsinki, and was approved by the ethics committees of Shiraiwa Medical Clinic and Osaka University Hospital.
Consequently, as shown in , treatment satisfaction was significantly worsened in patients taking two medications (i.e. a DPP-4 inhibitor plus one agent) (p = .001 vs. at baseline), but not in the others, both those with monotherapy and those with more than two medications (all p > .1 vs. at baseline). The change in treatment satisfaction in patients with bipharmacy was significantly different from that in those with monopharmacy (p = .002 vs. bipharmacy) and those using more than two medications (p = .024 vs. bipharmacy). These findings indicate some concern about an unfavorable impact of the combined use of once-weekly DPP-4 inhibitor with one agent on treatment satisfaction.
Figure 1. Change in treatment satisfaction after switching a daily DPP-4 inhibitor to a once-weekly one. Dotted lines represent 95% confidence intervals. An asterisk denotes p < .05.
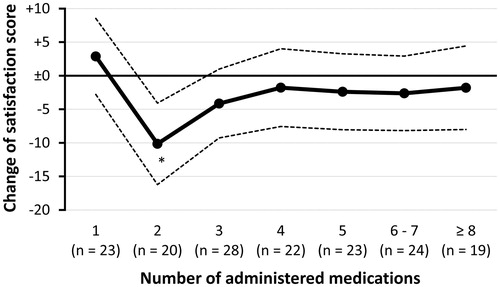
It is noteworthy that bipharmacy was distinguished not only from monotherapy, but also from polypharmacy with more than two medications. Although the true reasons remained unknown, the distinction might come from the relative impact of the once-weekly administration in patients’ overall medication regimens. In patients with two medications, the switch meant that the medications should be taken differently from each other, which might be confusing and troublesome rather than convenient. On the other hand, in patients with polypharmacy, in whom a once-weekly medication accounted for only a small portion of their medication regimens, the troublesomeness of taking one once-weekly medication might be less marked.
Treatment satisfaction considerably affects adherence and long-term disease control. For an effective use of a once-weekly agent in clinical practice, treatment satisfaction should be simultaneously assessed in clinical trials investigating its glucose-lowering effect.
Transparency
Declaration of funding
The current study was self-funded.
Author contributions: M.T. researched data and wrote the letter. T.S. researched data. N.K. and T.A.M. contributed to discussion. I.S. reviewed/edited the letter. All authors have approved the final letter and agreed to be accountable for all aspects of the work.
Declaration of financial/other relationships
M.T. has disclosed that he has received honoraria from Takeda Pharmaceutical and MSD and research funding from MSD. T.S. has disclosed that he has received research funding from Takeda Pharmaceutical and honoraria from Takeda Pharmaceutical and MSD. N.K. has disclosed that he has received honoraria and research funding from Takeda Pharmaceutical and MSD. T.A.M. has disclosed that he has received honoraria from MSD and research funding from Takeda Pharmaceutical and MSD. I.S. has disclosed that he has received honoraria and research funding from Takeda Pharmaceutical and MSD.
References
- Handelsman Y, Lauring B, Gantz I, et al. A randomized, double-blind, non-inferiority trial evaluating the efficacy and safety of omarigliptin, a once-weekly DPP-4 inhibitor, or glimepiride in patients with type 2 diabetes inadequately controlled on metformin monotherapy. Curr Med Res Opin 2017: published online 26 May 2017, doi: 10.1080/03007995.2017.1335638
- Takahara M, Shiraiwa T, Ogawa N, et al. Patient perspectives on combination therapy of a once-weekly oral medication plus daily medication for lifestyle-related chronic diseases. Intern Med 2017;56:615-20
