Abstract
Objectives: To compare visual and anatomical outcomes between eyes treated with fluocinolone acetonide (FAc) 190 µg intravitreal implant for clinically significant chronic diabetic macular edema (DME) and fellow eyes not treated with FAc implant using data from the Iluvien Clinical Evidence study in the UK (ICE-UK) study.
Methods: In this retrospective cohort study, data on people attending hospital eye services and treated with the FAc implant between April 1, 2013 and April 15, 2015 were collected. Changes in visual acuity (VA), central foveal thickness (CFT) and intraocular pressure (IOP) were compared between study eyes (intervention) and fellow eyes.
Results: A total of 208 people were selected. Mean age was 68.1 years and 62% were male. Mean change in VA was −0.09 LogMAR units for study eyes and 0.04 LogMAR units for fellow eyes at 12 months post-implant (p < .001). Over the same period, ≥5 letter, ≥10 letter and ≥15 letter improvements in Early Treatment Diabetic Retinopathy Study (ETDRS) score were achieved by more FAc treated eyes than by fellow eyes (41% versus 23%, p < .001; 28% versus 11%, p < .001; and 18% versus 4%, p < .001 at 12 months, respectively). Differences in the mean change in CFT (−113 µm versus −13 µm, p < .001) and IOP (3.2 mmHg versus −0.2 mmHg, p < .001) were also observed between study and fellow eyes at 12 months.
Conclusion: Visual acuity improved in study eyes over the 12 months following FAc implant and worsened in fellow eyes. Over the same period, study eyes showed a larger improvement in central foveal thickness. Intraocular pressure worsened in study eyes only. Change in visual acuity, central foveal thickness and intraocular pressure between FAc implant and the end of the 12-month follow-up period differed significantly between study and fellow eyes.
Introduction
Diabetic retinopathy is amongst the most feared of the microvascular complications of type 1 and type 2 diabetes mellitus and was until very recently the most common cause of visual loss and blindness in working-aged peopleCitation1,Citation2. Up to 2010, it was estimated that 35% of people with diabetes worldwide had diabetic retinopathyCitation3. As diabetic retinopathy progresses, microvascular changes in the retina can lead to increased permeability, with leakage causing the accumulation of fluid in the macula. The global prevalence of diabetic macular edema (DME) in people with diabetes has been estimated as 7%Citation3 and the condition is the major cause of vision loss in the diabetic populationCitation4.
Several types of therapy are licensed for use in DME. The Early Treatment Diabetic Retinopathy Study (ETDRS) showed that focal photocoagulation can lead to a reduced loss of vision in people with clinically significant DMECitation5. Subsequently, data from several landmark trials have shown that anti-VEGF (vascular endothelial growth factor) therapies can improve vision in people with DMECitation6–9, and anti-VEGF therapy has become the treatment of choice for the management of DME. Nevertheless, anti-VEGF therapies are not effective in all peopleCitation10. Dong and colleagues suggest that several cytokines associated with inflammation and angiogenesis contribute to the pathogenesis of diabetic retinopathyCitation11. Therefore, a multifactorial approach to the management of diabetic retinopathy could be beneficialCitation11. Intravitreal corticosteroids have been shown to be effective in improving visual acuity in DMECitation12–14 and reduce not only the expression of the VEGF gene but also suppress other inflammatory mediators. Intravitreal steroids are therefore potentially useful second-line agents in the management of DME.
Fluocinolone acetonide (FAc) 190 µg intravitreal implant is licensed in 17 European countries for the treatment of visual impairment associated with chronic DME considered insufficiently responsive to available therapies. Two separate randomized clinical trials, Fluocinolone Acetonide in Diabetic Macular Edema (FAME) A and FAME B, have been conducted to study the clinical effectiveness of FAc intravitreal implant in DMECitation13,Citation15,Citation16. These studies recruited people with DME previously treated with retinal laser therapy, and the combined results of the two trials indicated that the FAc intravitreal implant provided visual benefit for up to 3 yearsCitation12,Citation13. Due to its long duration of action, the FAc intravitreal implant needs to be administered less frequently compared with anti-VEGF and other steroid therapies for DMECitation17. However, intravitreal corticosteroids have been shown to be associated with side effects including steroid-induced cataracts and raised intraocular pressure (IOP)Citation12–14. The UK National Institute of Health and Care Excellence (NICE) currently recommends that FAc intravitreal implant be used only in eyes with pseudophakic lenses where the DME has been insufficiently responsive to available therapiesCitation18.
The aim of the Iluvien Clinical Evidence study in the United Kingdom (ICE-UK) was to assess the real-world effectiveness of the FAc intravitreal implant for chronic DME in routine clinical practice. Changes in visual acuity, central foveal thickness (CFT) and IOP were compared between FAc treated and fellow eyes post implant. Whilst randomized trials of DME-related treatments provide a direct comparison between FAc treated and untreated subjects, here we compared study eyes (first eyes treated with FAc implant) and fellow eyes (the patient’s eye not treated with FAc implant, but which may have other standard care) in the same subject since this provides a natural experiment whereby the reference (control) eye is exposed to exactly the same physiological milieu. The low daily release of FAc from the intravitreal implant provides systemic FAc levels that are undetectable in the systemic circulationCitation19; therefore the effect of the FAc implant in the study eye on the disease in the fellow eye is low. However, unlike randomized controlled trials, the decision as to which eye to treat is likely to be based on a clinical decision. Nevertheless, the premise of the study was based on the assumption that there was homogeneity between study and fellow eyes.
Methods
Data source
This study used a retrospective cohort design. The data source has been described previouslyCitation20. Briefly, data were extracted from patient medical records for a representative cohort of patients registered at 13 participating UK hospitals. Data were pseudonymized and combined into a single dataset. Data included demographics, medical history, implant data, and data from multi-disciplinary and medication reviews at several time points within a designated period.
Ethical approval
The lead clinician and Caldicott Guardian at each center gave written approval for extraction of anonymized data. The study protocol was approved by the head of research governance at the lead clinical center. This study was conducted in accordance with the Declaration of Helsinki and the UK Data Protection Act.
Subjects
People with type 1 or type 2 diabetes treated with the FAc intravitreal implant for DME in at least one eye were included in the study. In order to allow for sufficient follow-up, it was required that the implant be inserted between April 1, 2013 and April 15, 2015 at a participating site as part of the patient’s routine care. The period of observation ended on 15 April 2016. A minimum history of 12 months prior to implant was also required, providing information that is not available in randomized controlled trials. People with a history of participating in any interventional study for DME or with insufficient follow-up were excluded. Reasons for insufficient follow-up included: non-attendance at the clinic and missing the last appointment post index. Study eyes were defined as the first eye to be treated with the FAc implant. Fellow eyes were defined as the eye that received no FAc implant; these could receive other DME treatments as deemed clinically necessary in routine practice. People whose fellow eye was treated with the FAc intravitreal implant within 12 months of the study eye’s first treatment were censored at the time of implant, with all remaining subjects followed for 12 months post implant. The index date was defined as the date of the first recorded FAc intravitreal implant into the study eye.
Outcomes
Changes in visual acuity, CFT and IOP were investigated at 3, 6 and 12 months post index date in study and fellow eyes. Visual acuity was measured using one of: Early Treatment of Diabetic Retinopathy Study (ETDRS) score, Snellen fraction or LogMAR scores. All Snellen fractions were converted to approximate ETDRS scores for analysis using the following formula derived by Gregori and colleagues: approximate ETDRS = 85 + 50 × log (Snellen fraction). All approximate ETDRS scores were rounded to the nearest letterCitation21. Snellen fractions were converted to LogMAR scores using the following formula: −1 × log (Snellen fraction)Citation21. These formulae were rearranged to convert between LogMAR and ETDRS. People who could only detect movement, detect light or count fingers were allocated to 2.3 on the LogMAR scale, the value attributed to people who can count fingers. As this was an observational study, there was no restriction on the optical coherence tomography (OCT) machine type used to measure retinal thickness.
Statistical analysis
Results were compared between study eyes and fellow eyes. Baseline characteristics were displayed for study and fellow eyes overall and by subgroup based on the difference in the mean change in visual acuity between study and fellow eyes at 12 months post implant (> and ≤−0.12 LogMAR units). Baseline characteristics were compared using the paired t-test or Wilcoxon signed rank test for continuous variables, depending on their distribution. Changes in visual acuity, CFT and IOP were compared between study and fellow eyes using the Wilcoxon signed rank test. Categorical variables were compared using McNemar’s test. Due to the observational nature of this study, people did not visit the clinic at set times prior to and following insertion of the FAc intravitreal implant. Therefore last observation carried forward prior to and after implant was implemented in order to impute missing valuesCitation22.
Mean change in the four study outcomes on each day following baseline and mean values on each day in the 12 months prior and post index date were calculated using a dataset where missing values had been imputed using linear interpolationCitation22. As linear interpolation was not suitable following the last observed value or prior to the first observed value, nearest observation carried forward and backward was used to impute the remaining missing values.
Generalized linear mixed multinomial modeling with a random intercept at subject level and a generalized logit link function was conducted. The dependent variable was change in visual acuity categorized as ≥5 letter improvement, between −4 and +4 letter change (i.e. stable visual acuity) and ≥5 letter worsening. The following variables were added as fixed effects: age, baseline visual acuity (LogMAR units), insulin treatment, sex, FAc implant, lens status, number of prior anti-VEGF injections, prior IOP-lowering therapy, number of prior laser therapies, number of prior steroid injections (triamcinolone or dexamethasone) and prior vitrectomy. All statistical analyses were carried out using IBM SPSS statistics version 20.
Results
Data were collected on 311 people, of whom 208 people contributing 208 study eyes and 208 fellow eyes were eligible for inclusion in the study cohort. The number of study subjects excluded has been described previouslyCitation20.
Patient characteristics
Of the 208 people treated with the FAc intravitreal implant in the study eye, 128 (62%) were male. Mean age was 68.1 years (). A total of 176 (85%) people had type 2 diabetes. Median (IQR) duration of diabetes was 18.0 (11.0–27.0) years. For 137 (70%) people, vision was worse in the study eye.
Table 1. Baseline characteristics.
A total of 185 (89%) study eyes and 111 (53%) fellow eyes had a pseudophakic lens (p < .001). At the time of implant, median visual acuity was worse in the study eye than in the fellow eye (median 0.69, IQR 0.49–1.00 LogMAR units versus 0.40, 0.19–0.80 LogMAR units, p < .001). Mean (standard deviation, SD) CFT was 483 (189) µm for study eyes and 371 (176) µm for fellow eyes (p < .001). Study eyes had a history of receiving more anti-VEGF (p < .001), steroid (p < .001) and macular laser treatments (p = .028) when compared with fellow eyes. IOP at baseline was similar in study and fellow eyes (median 15 versus 15 mmHg, p = .236) and there was no significant difference in the number of study and fellow eyes that had been previously treated with IOP-lowering therapy (20% versus 16%, p = .143). The percentage of patients receiving the FAc implant in a pseudophakic eye who had a phakic lens in their fellow eye was 37% for those receiving the FAc implant in their worse-seeing eye and 31% in those receiving the FAc implant in the study eye with the same or better visual acuity. Baseline characteristics by subgroup defined by difference in mean change in visual acuity between study eye and fellow eye at 12 months are displayed in Supplementary Table 1.
Other intraocular interventions
The number of eyes treated with anti-VEGF post FAc implant in the study eye were: 5% of study eyes versus 18% of fellow eyes between 0 and 3 months, 9% of study eyes versus 20% of fellow eyes between 3 to 6 months and 18% of study eyes versus 24% of fellow eyes between 6 and 12 months (). The number of eyes treated with steroids (other than FAc) post FAc implant in the study eye were: 2% of study eyes versus 1% of fellow eyes between 0 and 3 months, 1% of study eyes versus 1% of fellow eyes between 3 and 6 months and 5% of study eyes versus 1% of fellow eyes between 6 to 12 months. The corresponding figures for macular laser therapy were 2% of study eyes versus 2% of fellow eyes, 3% of study eyes versus 2% of fellow eyes and 5% of study eyes versus 4% of fellow eyes at 0 to 3 months, 3 to 6 months and 6 to 12 months, respectively.
Table 2. Number of eyes prescribed therapies before and after treatment with fluocinolone intravitreal implant.
Twenty-three study eyes (11%) had a phakic (natural) lens at baseline. A pseudophakic lens status or a cataract operation was recorded in 15 eligible phakic eyes (68%) between 0 and 3 months post implant (including 11 cataract operations performed on day of FAc implant) and one eye (14%) between 3 and 6 months post implant. At the time of FAc implant in the study eye, 97 (47%) fellow eyes had a phakic lens. Ten, three and four eligible phakic eyes received a cataract operation or a change in lens status to pseudophakic at 0 to 3 months, 3 to 6 months and 6 to 12 months post implant.
Change in visual acuity
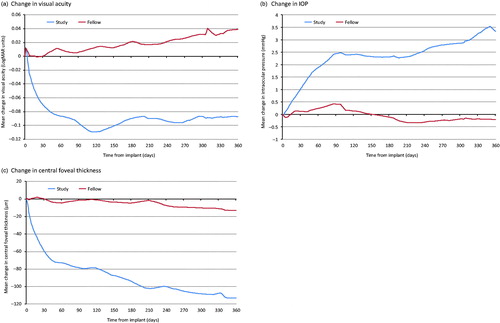
For the study eye, mean visual acuity was better in the 12 months following FAc implant than in the 12 months preceding implant (0.73 LogMAR units at −12 months, 0.76 LogMAR units immediately prior to implant and 0.67 LogMAR units at +12 months, ). Visual acuity in study eyes improved over the first 4 months of study follow-up (mean change −0.10 LogMAR units). Improvements in visual acuity decreased slightly between 4 and 12 months and mean change in visual acuity was −0.09 LogMAR units at 12 months post FAc implantation (). For the fellow eye, mean visual acuity improved prior to index date (0.54 LogMAR units 4 months prior to implant to 0.5 LogMAR units at FAc implant) but then gradually worsened over the 12-month follow-up period post implant (0.54 LogMAR units at 12 months). Change in visual acuity was significantly different in study eyes and fellow eyes at 3 months (mean −0.08 versus −0.02 LogMAR units, respectively, p = .008), 6 months (−0.11 versus 0.03 LogMAR units, respectively, p < .001) and 12 months post implant (−0.09 versus 0.04, respectively, p < .001, Supplementary Table 2).
Figure 1. Mean visual acuity, central foveal thickness and intraocular pressure for study and fellow eyes. Missing values were imputed using linear interpolation. As linear interpolation was not suitable following the last observed value or prior to the first observed value, nearest observation carried forward and backward was used to impute the remaining missing values.
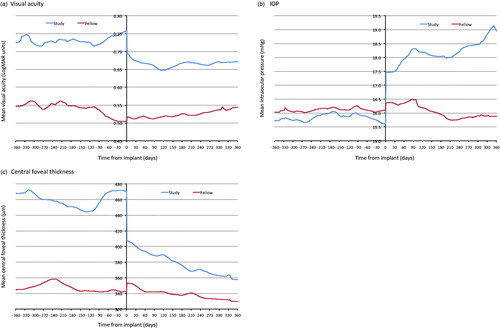
Figure 2. Mean change in visual acuity, central foveal thickness and IOP post index date for study and fellow eyes. Missing values were imputed using linear interpolation. Last observation carried forward was then used following the last recorded value within the 365 day follow-up period. Last observation carried forward was used in the year before index date in order to impute missing values at the time of implant in the study eye.
A ≥5 letter improvement in ETDRS score was achieved by more study eyes than fellow eyes at each follow-up time point (44% versus 22%, p < .001, at 3 months; 44% versus 30%, p = .002, at 6 months; 41% versus 23%, p < .001, at 12 months post implant, ). More study eyes also achieved a ≥10 letter improvement at 3 months (26% versus 12%, p < .001), 6 months (30% versus 10%, p < .001) and 12 months (28% versus 11%, p < .001) post index date. For a ≥15 letter improvement in ETDRS the corresponding figures for study eyes and fellow eyes were 14% versus 7%, p = .012, at 3 months; 16% versus 4%, p < .001, at 6 months; and 18% versus 4%, p < .001, at 12 months, respectively. At 6 and 12 months post FAc implant, fewer study eyes exhibited a worsening in visual acuity of ≥5, ≥10 and ≥15 letters compared with fellow eyes. However, this only achieved statistical significance for a ≥10 letter worsening in ETDRS letter score at 6 months (10% of study eyes versus 19% of fellow eyes, p = .014).
Figure 3. Percentage of study and fellow eyes achieving (a) ≥ 5, ≥10 and ≥15 letter improvement in ETDRS score and (b) ≥ 5, ≥10 and ≥15 letter worsening in ETDRS score. *Significant at the <.05 level. **Significant at the <.001 level.
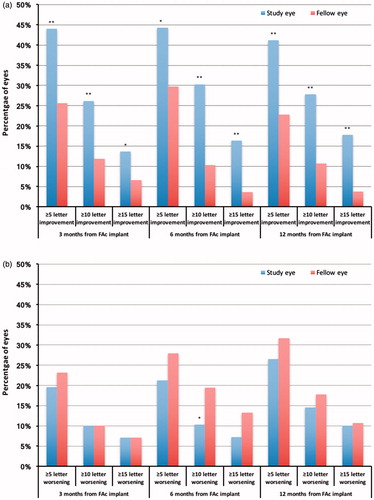
Results from the generalized linear mixed multinomial modeling showed that the study eyes were more likely to achieve a ≥5 letter gain in visual acuity, and this result was significant at 3 months (exponential of the coefficient 2.09, 95% CI 1.13–3.88, p = .019) post implant (). Study eyes were not significantly more or less likely to have a worsening of ≥5 letters in visual acuity at 3, 6 and 12 months post implant.
Table 3. Results from the generalized linear mixed model.
Change in central foveal thickness
In the study eye, CFT fluctuated from 468 µm at −12 months to 445 µm at −4 months before increasing to 471 µm immediately prior to implant (). Mean CFT then decreased gradually post implant from 407 µm immediately following implant to 358 µm at the end of the 12 month follow-up. For the fellow eye, there was little change in mean CFT in the 12 months before and after implant (CFT 345 µm, 343 µm and 330 µm at −12 months, index date and +12 months, respectively). A difference in the change in CFT was observed between study and fellow eyes between index date and 3 month follow-up (mean −87 µm versus −3 µm, respectively, p < .001), 6 month follow-up (−90 µm versus −3 µm, respectively, p < .001) and 12 month follow-up (−113 µm versus −13 µm, respectively, p < .001, Supplementary Table 2).
Change in intraocular pressure
Mean IOP was similar and remained relatively stable in both the study and fellow eyes prior to implant (15.7 mmHg and 16.0 mmHg at −12 months and 15.6 mmHg and 16.1 mmHg at implant, respectively, ). Post FAc implant in the study eye, mean IOP increased to 18.8 mmHg at the end of the 12 month follow-up period. Conversely, mean IOP remained stable in the fellow eye (15.9 mmHg at +12 months, ). Change in IOP differed between the study eye and fellow eye at 3 months (mean 1.9 mmHg versus 0.55 mmHg, p = .004), 6 months (2.4 mmHg versus 0.4 mmHg, p < .001) and 12 months (3.2 mmHg versus −0.2 mmHg, p < .001) post index date (Supplementary Table 2).
The number of study versus fellow eyes with an IOP of ≤21 mmHg was 12 (8%) versus 11 (7%) at baseline (N = 156 pairs of eyes, p > .999), 26 (18%) versus 19 (13%) at 3 months (N = 147, p = .167), 31 (20%) versus 12 (8%) at 6 months (N = 154, p = .001) and 39 (25%) versus 11 (7%) at 12 months post FAc implant (N = 157, p < .001).
IOP-lowering medicine was initiated in more study eyes than fellow eyes between 0 and 3 months (3% versus 1%), 3 and 6 months (7% versus 1%) and 6 and 12 months (11% versus 4%) post index date.
Discussion
In the study cohort, visual acuity and CFT improved in the 12 months following the insertion of the FAc intravitreal implant when compared with the 12 months prior to implant. Conversely, there was a small but significant increase in IOP in the study eye in the 12 months post FAc implant. For the fellow eye, CFT and IOP remained relatively stable across the 12 month periods prior to and following FAc implant in the study eye. However, there was a small deterioration in visual acuity in the fellow eye post FAc implant. At the end of the 12 month follow-up period, statistically significant improvements in visual acuity and CFT were observed in study eyes treated with the FAc intravitreal implant when compared with the fellow eye. However, during the same period, a small statistically significant increase in IOP was observed in study eyes only. More fellow eyes were treated with anti-VEGF therapies in the 12 months post FAc implant when compared with study eyes. However, more study eyes than fellow eyes were treated with steroid therapy between 6 and 12 months post implant. The number of fellow eyes and study eyes receiving macular laser therapy post implant were similar. An important limitation of this study was the difference in visual acuity and CFT between study and fellow eyes prior to FAc implant. However, prior to implant, IOP was similar in study and fellow eyes, suggesting that the comparison between study and fellow eyes is reasonable for this outcome.
To date, the largest randomized controlled trials investigating the effectiveness of FAc have been the FAME studies, where people with DME previously treated with macular laser therapy were randomized to receive sham injection (n = 185), low-dose FAc implant (n = 375) and high dose FAc implant (n = 393)Citation12,Citation13. Campochiaro and colleagues reported that 28.7% of people randomized to 0.2 µg/day FAc implant achieved a ≥15 letter improvement in ETDRS score after 3 years compared with 18.9% of people randomized to sham injection (p = .018)Citation13. The 0.2 µg/day FAc group also received fewer off-protocol treatments for DME (15.2% compared with 33% for the sham group)Citation13. In this study, where the fellow eye acted as a control, we found that a ≥15 letter improvement was observed in 18% of study eyes and 4% of fellow eyes after 12 months follow-up (p < .001). Here, some of the study eyes and fellow eyes also received other DME treatments as part of their routine care. However, a higher number of fellow eyes received other treatments for DME post FAc implant. Unlike the people included in the FAME studies, whose DME had previously only been managed with macular laser therapy, 82% of study eyes and 47% of fellow eyes in this study had previously been treated with anti-VEGF injections, and 43% of study eyes and 19% of fellow eyes had been previously treated with steroids (triamcinolone or dexamethasone). Compared with the FAME study, a higher proportion of study eyes and fellow eyes had a pseudophakic lens at baseline (89% of study eyes and 53% of fellow eyes in this study versus 34.6% of those randomized to sham and 37.3% of those eyes randomized to the 0.2 µg/day FAc implant in the FAME study). In addition, people in this study were generally older and there was a higher proportion of people with type 1 diabetes. In a pre-specified subgroup analysis, Cunha-Vaz and colleagues reported that a higher percentage of people with chronic DME gained a 15-letter improvement in visual acuity when compared with those with non-chronic DMECitation23. Although the chronicity of DME was not collected as part of the ICE-UK study, we undertook a subgroup analysis to determine the baseline characteristics of those people who went on to respond better to FAc implant (difference in mean change in visual acuity between study eye and fellow eye at 12 months ≤−0.12 LogMAR units). We found that people who responded better were in general younger, had worse vision in their study and fellow eye at baseline and were more likely to have been previously treated with at least one steroid injection in the study eye.
In an electronic medical record based study across 14 UK clinical sites mean best recorded visual acuity improved from 51.9 letters at baseline (n = 311), to 55.5 at 12 months (n = 160) and 57.2 at 24 months (n = 53)Citation24. In a retrospective study carried out by Elaraoud and colleagues, an improvement in visual acuity and CFT was observed in 15 out of 22 eyes treated with FAc intravitreal implantCitation25. The subjects included were similar to those included in the ICE-UK study in that all included eyes had a pseudophakic lens and the majority had been previously treated with multiple anti-VEGF and laser therapiesCitation25.
Unlike an RCT where people are randomized to FAc or sham injection, this study had a retrospective observational design whereby the untreated eye acted as a natural control that had been exposed to the same patient and disease factors. However, in 70% of people, the FAc intravitreal implant was inserted into the worse-seeing eye. At baseline, CFT was greater in the study eye when compared with the fellow eye. Baseline vision is likely to dictate the improvement or worsening of vision observed in an eye and the difference in visual acuity at baseline between study and fellow eyes. In addition, more fellow eyes received anti-VEGF treatments in the 12 months post FAc implant. These limitations need to be taken into account when interpreting the comparisons between study and fellow eye.
Five cataract operations were observed in study eyes over the follow-up period. However, the majority of people had a pseudophakic lens in the study eye at the time of implant (89%). In the FAME study, in those with no history of receiving a cataract operation, cataract surgery was reported in 75% of the 0.2 µg/day FAc group and 23% of the sham group after 24 months of follow-up and was the most commonly reported adverse eventCitation12. At 36 months, these figures were 82% and 51%, respectivelyCitation13. The percentage of eyes with a phakic lens at baseline was higher in the FAME study when compared with ICE-UK (11% versus 65%)Citation12. More study eyes initiated IOP-lowering medication post index date. IOP-lowering surgery was carried out in one study eye and two fellow eyes within 12 months of implant. However, following insertion of the FAc intravitreal implant, there was a small but statistically significant increase in IOP in study eyes when compared with fellow eyes. Furthermore, a statistically significant higher percentage of study eyes had an IOP of ≥21 mmHg when compared with fellow eyes. In the FAME study, all included individuals had no prior history of glaucomaCitation12. Laser trabeculoplasty and incisional IOP-lowering surgery were reported to have been carried out in 1.3% and 4.8% of the 0.2 µg/day FAc implant group and 0% and 0.5% of the sham group, respectivelyCitation13.
Strengths and limitations
The general study limitations have largely been discussed previouslyCitation20. The comparison of study and fellow eyes had statistical limitations. The decision to treat one eye with FAc instead of or before the fellow eye is based on clinical judgment and is not a random event. The study eye often had poorer visual acuity and CFT at baseline compared with the fellow eye. This could have led to confounding by severity. Conversely, worse characteristics at baseline could have potentially led to improved outcomes and therefore favored the study eye. The FAc implant is licensed for the treatment of DME considered insufficiently responsive to available therapies. Therefore, when the FAc implant was inserted into the worse-seeing eye, the better-seeing eye could still have been responsive to other DME treatments. However, little improvement was observed in the fellow eye over the 12 months before and after index date, and only 18%, 20% and 24% of fellow eyes received anti-VEGF therapy at 0 to 3 months, 3 to 6 months and 6 to 12 months post index date. The UK NICE guidelines recommend that the FAc intravitreal implant be prescribed in eyes with a pseudophakic lensCitation18 and this may have also influenced the decision as to which eye to treat with FAc. For 37% of patients who received an FAc implant in their worse-seeing eye, the fellow eye was phakic at the time of implant. For those patients whose visual acuity was the same or better in the study eye, 31% had a phakic lens in their fellow eye. Collection parameters included DME type for each eye, but a status of no DME could not be recorded. Unilateral cases of DME were reported separately for three study subjects. The level of misclassification of the unilateral and bilateral DME status is unknown, although 26% patients had no history of receiving any treatment for DME (laser, intravitreal steroids or anti-VEGF therapy) in the fellow eye prior to index date (compared with 3% of study eyes).
Several types of OCT machine were used to measure retinal thickness across the 13 participating ophthalmology centers, and this has been discussed in detail elsewhereCitation26. Retinal thickness measurements have been shown to vary depending on machine type, possibly due to variation in the retinal segmentation algorithms used by different OCT machinesCitation27. However, the same OCT machine type was used to measure retinal thickness in the left and right eye during the same visit.
Due to the low level of systemic absorption, the effect of the FAc implant on the fellow eye is thought to be low. However, bevacizumab has been reported to significantly reduce the level of VEGF in the blood plasma for up to 28 days post injection in people with DME and age-related macular degenerationCitation28,Citation29. Furthermore, studies have shown that anti-VEGF therapy administered unilaterally can have beneficial effects in the contralateral eye of people with bilateral DMECitation30, age-related diabetic macular degenerationCitation31, proliferative diabetic retinopathyCitation32, or uveitis-related cystoid macular edemaCitation33. The potential crossover effect of anti-VEGF therapy on the contralateral (study or fellow) eye is a limitation of the study design.
Conclusion
At the end of the 12-month follow-up period, a statistically significant improvement in visual acuity and CFT and a small but significant increase in intraocular pressure were observed in study eyes treated with the FAc intravitreal implant when compared with the fellow eye. Differences in baseline visual acuity and CFT between study and fellow eyes were a limitation of this study. However, considering the study and fellow eyes independently, trends in visual acuity and CFT over the 12 month periods before and after FAc implant highlight the benefit of the FAc intravitreal implant in the often poorer study eye. Over the same period, little or no improvement in these outcomes was observed in fellow eyes.
Transparency
Declaration of funding
This study was supported by Alimera Sciences, the manufacturer of Iluvien 190 µg intravitreal implant, who designed the study and commented on the manuscript.
Author contributions
C.J.C. contributed to the study design. S.E.H. and E.B. analyzed the data. S.E.H. wrote the initial draft of the manuscript. All co-authors interpreted the data and C.J.C., S.E.H. and D.R.O. commented on the manuscript.
Declaration of financial/other relationships
S.E.H. and E.B. have disclosed that they are employed by and C.J.C. has disclosed that he is a director of Pharmatelligence, a research consultancy receiving funding from Alimera Sciences for the submitted work and from other healthcare related organizations. D.R.O. has disclosed that he has received sponsorship from Sanofi to attend the American Diabetes Association Meeting, San Diego, June 2017.
CMRO peer reviewers on this manuscript have received an honorarium from CMRO for their review work, but have no relevant financial or other relationships to disclose.
Supplemental data
Download MS Word (28.3 KB)Acknowledgements
The authors thank Annette Beiderbeck of Alimera Sciences for designing the study and commenting on the manuscript. We acknowledge the contributions of the staff at the following ICE-UK study centres: New Cross Hospital, Wolverhampton; Maidstone Hospital, Maidstone; Bristol Eye Hospital, Bristol; Royal Victoria Hospital, Belfast; The James Cook University Hospital, Middlesborough; University Hospital, Norfolk; Sunderland Eye Infirmary, Sunderland; Royal Hallamshire Hospital, Sheffield; Royal Surrey County Hospital, Guildford; Queen Elizabeth Hospital, Birmingham; Moorfields Eye Hospital, London; Sandwell General Hospital, West Bromwich; and Royal Free Hospital, London. Particularly, the authors thank Prof. Yit Yang who was instrumental in project development, selection of clinically relevant endpoints, development of the protocol and study design. We also thank Prof. Yit Yang for his comments on the draft manuscript. The authors thank SVMPharma for collating the data, Dafydd Williams for initial data preparation and analysis and Sara Jenkins-Jones for her editorial work.
Previous presentation: These results have been presented at The Association for Research in Vision and Ophthalmology (ARVO) 2017 meeting 17th EURETINA Congress 2017 and the Royal College of Ophthalmology (RCOphth) Congress 2017.
References
- Stevens GA, White RA, Flaxman SR, et al. Global prevalence of vision impairment and blindness: magnitude and temporal trends, 1990–2010. Ophthalmology 2013;120:2377-84
- Liew G, Michaelides M, Bunce C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16–64 years), 1999–2000 with 2009–2010. BMJ Open 2014;4:e004015
- Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012;35:556-64
- Ferris FL, Patz A. Macular edema. A complication of diabetic retinopathy. Surv Ophthalmol 1984;28:452-61
- Narayan KMV, Boyle JP, Geiss LS, et al. Impact of recent increase in incidence on future diabetes burden: U.S., 2005–2050. Diabetes Care 2006;29:2114-16
- Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. Two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology 2016;123:1351-9
- Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology 2013;120:2013-22
- Elman MJ, Ayala A, Bressler NM, et al. Intravitreal ranibizumab for diabetic macular edema with prompt vs. deferred laser treatment: 5-year randomized trial results. Ophthalmology 2015;122:375-81
- The Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 2015;372:1193-203
- Bressler SB, Qin H, Beck RW, et al. Factors associated with changes in visual acuity and OCT thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Arch Ophthalmol 2012;130:1153-61
- Dong N, Xu B, Wang BCL. Study of 27 aqueous humor cytokines in patients with type 2 diabetes with or without retinopathy. Mol Vis 2013;19:1734-46
- Campochiaro PA, Brown DM, Pearson A, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology 2011;118:626-35
- Campochiaro PA, Brown DM, Pearson A, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology 2012;119:2125-32
- Haller JA, Bandello F, Belfort R, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology 2010;117:1134-46
- Electronic Medicines Compendium. SPC Iluvien 190 micrograms intravitreal implant in applicator. 2015. Available at: https://www.medicines.org.uk/emc/medicine/27636 [Last accessed October 20, 2016]
- Medicines and Healthcare products Regulatory Agency. Public Assessment Report. Mutual Recognition Procedure. Iluvien 190 micrograms Intravitreal Implant in Applicator (Fluocinolone acetonide). 2015. Available at: http://www.mhra.gov.uk/home/groups/par/documents/websiteresources/con171936.pdf [Last accessed October 20, 2016]
- Datapharm. eMC Dictionary of Medicines and Devices browser. Available at: http://dmd.medicines.org.uk/DesktopDefault.aspx?tabid = 1 [Last accessed January 6, 2017]
- National Institute for Health and Care Excellence. Fluocinolone acetonide intravitreal implant for treating chronic diabetic macular oedema after an inadequate response to prior therapy. 2013. Available at: www.nice.org.uk/guidance/ta301 [Last accessed October 24, 2014]
- Jaffe GJ, Martin D, Callanan D, et al. Fluocinolone acetonide implant (Retisert) for noninfectious posterior uveitis. Thirty-four-week results of a multicenter randomized clinical study. Ophthalmology 2006;113:1020-7
- Holden SE, Currie CJ, Owens DR. Evaluation of the clinical effectiveness in routine practice of fluocinolone acetonide 190 µg intravitreal implant in people with diabetic macular edema. Curr Med Res Opin 2017;33(Suppl):5--17
- Gregori NZ, Feuer W, Rosenfeld PJ. Novel method for analyzing Snellen visual acuity measurements. Retina 2010;30:1046-50
- Haukoos JS, Newgard CD. Advanced statistics: missing data in clinical research – part 1: an introduction and conceptual framework. Acad Emerg Med 2007;14:662-8
- Cunha-Vaz J, Ashton P, Iezzi R, et al. Sustained delivery fluocinolone acetonide vitreous implants: long-term benefit in patients with chronic diabetic macular edema. Ophthalmology 2014;121:1892-903
- Bailey C, Chakravarthy U, Lotery A, et al. Real-world experience with 0.2 μg/day fluocinolone acetonide intravitreal implant (ILUVIEN) in the United Kingdom. Eye 2017; (epub ahead of print)
- Elaraoud I, Andreatta W, Kidess A, et al. Use of flucinolone acetonide for patients with diabetic macular oedema: patient selection criteria and early outcomes in real world setting. BMC Ophthalmol 2016;16:3
- Currie CJ, Holden SE, Owens DR. Patterns of retinal thickness prior to and following treatment with fluocinolone acetonide 190 µg intravitreal implant for diabetic macular oedema. Curr Med Res Opin 2017;33(Suppl):33--43
- Wolf-Schnurrbusch UEK, Ceklic L, Brinkmann CK, et al. Macular thickness measurements in healthy eyes using six different optical coherence tomography instruments. Invest Ophthalmol Vis Sci 2009;50:3432-7
- Zehetner C, Kirchmair R, Huber S, et al. Plasma levels of vascular endothelial growth factor before and after intravitreal injection of bevacizumab, ranibizumab and pegaptanib in patients with age-related macular degeneration, and in patients with diabetic macular oedema. Br J Ophthalmol 2013;97:454-9
- Carneiro ÂM, Costa R, Falcão MS, et al. Vascular endothelial growth factor plasma levels before and after treatment of neovascular age-related macular degeneration with bevacizumab or ranibizumab. Acta Ophthalmol 2012;90:e25-30
- Bakbak B, Ozturk BT, Gonul S, Gedik S. The effect of intravitreal bevacizumab and ranibizumab on macular edema of the contralateral eye: a comparative study of two anti-VEGFs. Oman J Ophthalmol 2016;9:44-8
- Michalska-Małecka K, Kabiesz A, Kimsa MW, et al. Effects of intravitreal ranibizumab on the untreated eye and systemic gene expression profile in age-related macular degeneration. Cin Interv 2016;11:357-65
- Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology 2006;113:1695-705
- Acharya NR, Sttitvarakul W, Qian Y, et al. Bilateral effect of unilateral ranibizumab in patients with uveitis-related macular edema. Retina 2011;31:1871-6
