Abstract
Backgrounds: Although evidence is mounting on the role of hyperuricemia in cardio-renal disease, continuing doubt remains as to whether hyperuricemia can be considered a major causal cardiovascular risk factor. In addition, available data suggest that treatment may be beneficial, even in the absence of overt gout, when hyperuricemia accompanies other clinical conditions, such as urate deposition, advanced chronic kidney disease, or cardiovascular risk factors.
Methods and Results: Analysis of the literature suggests there would be sufficient evidence warranting clinical trials to determine whether lowering uric acid levels would be clinically beneficial in the prevention or treatment of cardiovascular and renal diseases.
Conclusion: Under a practical profile, it becomes progressively more important to investigate the possibility of reducing serum uric acid levels in the general population below the level of 5.0 mg/dL.
Introduction
Several experimental and clinical studies reported that hyperuricemia may trigger hypertension, metabolic syndrome, vascular damage, and renal diseaseCitation1–4. Furthermore, a substantial proportion of epidemiological studies are compatible with the hypothesis that hyperuricemia may be an independent risk factor for cardiovascular disease, as well as for an increased cardiovascular mortalityCitation5. Although such evidence is mounting, continuing doubt remains as to whether hyperuricemia can actually be considered a causal major cardiovascular risk factor. In addition, available data suggest that treatment may be beneficial, even in the absence of overt gout, when hyperuricemia accompanies other clinical conditions, such as urate deposition, advanced chronic kidney disease (CKD), or cardiovascular risk factorsCitation6. However, conflicting results do not support the general treatment of asymptomatic hyperuricemia to reduce cardiovascular risk, or to prevent progression of renal disease. There would seem to be sufficient evidence to warrant clinical trials to determine whether lowering uric acid levels would be of clinical benefit in the prevention or treatment of cardiovascular and renal diseasesCitation6.
Xanthine oxidase
Although a causal relationship between uric acid level and cardiovascular conditions has not been fully clarified, the capacity of uric acid to negatively affect vascular function by pro-oxidant effects and by decreasing nitric oxide bioavailability and consequently inducing endothelial dysfunction suggests that some pathophysiologic mechanisms may be the basis of the association among hyperuricemia, hypertension, metabolic syndrome, and cardiovascular disease.
Xanthine oxidoreductase (XOR) is a critical source of both uric acid and reactive oxygen species, contributing to vascular inflammation and endothelial dysfunctionCitation7. It is a rate-limiting enzyme in purine metabolism and catalyzes the last two steps of purine catabolism (the oxidation of hypoxanthine to xanthine and the oxidation of xanthine to uric acid) by utilizing either NAD + or O2. Two reactive oxygen species (ROS): superoxide anion (O2–) and hydrogen peroxide (H2O2), are produced in these reactions. XOR can exist in two forms; xanthine dehydrogenase (XDH), mostly when NAD + is available as a substrate, and xanthine oxidase (XO), when O2 is availableCitation8. XDH is the main form present in the liver, but it can be converted to XO form by reversible sulfhydryl oxidation or by irreversible proteolytic modificationCitation8. XOR is also present in the intestines, mammary gland, cardiac and skeletal muscle, corneal epithelium, and endothelial cells of vascular vesselsCitation9–11.
Endothelial cell-associated XO inhibition should be an attractive strategy for the treatment of conditions associated with endothelial damage. Clinical studies are needed to confirm that a potent inhibitor, unaffected by enzyme redox state and interaction with XO, such as febuxostat, may be effective to reduce cardiovascular riskCitation12.
Clinical studies
Some studies that controlled for multiple risk factors suggested that uric acid could be an independent risk factor for both cardiovascular diseaseCitation13–17 and kidney diseaseCitation18–20. Other studies have noted that an elevated level of uric acid predicted the development of hypertensionCitation21–23, kidney diseaseCitation18–20, and diabetesCitation24,Citation25. In addition, there have been reports of cardiovascular and renal benefits from lowering uric acid levels in recent preliminary clinical trialsCitation13,Citation26–28. Notwithstanding, the Framingham Heart Study reported that uric acid was not a causal risk factor for cardiovascular events, because uric acid was not independent of hypertensionCitation29. Independence of cardiovascular and renal disease risk factors is very difficult to be discerned by epidemiologic studies because of the biologic interlinking among them. Although a causal relationship of uric acid with renal and cardiovascular disease has not been demonstrated, evidence toward the hypothesis that urate lowering therapy should be effective in cardiovascular risk management is still growing.
Recent advances
Hyperuricemia was shown to be a strong independent risk factor for major cardiovascular events (MACE) in a study that collected baseline clinical data through a 1998/1999 longitudinal survey as part of the Valle dell’Irno Prevenzione Project, and evaluated MACE incidence after 10 yearsCitation30. A total of 1,175 patients (aged 25–74 years, 50% men) completed the study. At least one MACE was reported by 135 patients, whose mean serum uric acid (SUA) values were significantly higher compared with patients without events (6.0 ± 4.8 and 4.6 ± 4.0 mg/dl, respectively; p < .01). Patients with SUA values of at least 6 mg/dl (prevalence of 14.6%) had significantly lower levels of high-density lipoprotein (HDL) cholesterol and increased values of body mass index (BMI), blood pressure, cholesterol, triglycerides, white blood cells, complement component 3 (C3), and creatinine. Confounder-adjusted stepwise linear regression revealed SUA, age, creatinine, glucose, and systolic blood pressure as independent predictors of MACE. Diastolic blood pressure and creatinine were independently correlated with SUA in the entire population, diastolic blood pressure only in men, and BMI, creatinine, age, and C3 in womenCitation30.
In elderly women from the general population, SUA value ≥6.8 mg/dl was found to triple the risk of resistant hypertension (failure to maintain blood pressure values below 140/90 mmHg despite therapeutic interventions)Citation31. In a cohort of 580 elderly subjects aged ≥65 years, the prevalence of resistant hypertension was 5.7% in the cohort and was higher in women (8.3%) than in men (3.0%, p < .05). Independent of chronic kidney disease (odds ratio = 3.89, 95% confidence interval = 1.49–10.1), hyperuricemia predicted resistant hypertension in women (odds ratio = 3.11, 95% confidence intervals = 1.06–9.1, p = .03), but not in menCitation31.
Prasad et al.Citation32 investigated the relationship of SUA, inflammatory markers, and coronary endothelial dysfunction (CED), to study the link of SUA levels to early coronary atherosclerosis in post-menopausal women. In a prospective cohort study, SUA, high-sensitivity C-reactive protein levels, and neutrophil count were measured in 229 post-menopausal women who underwent diagnostic catheterization, without obstructive coronary artery disease, and undergoing coronary microvascular function testing, to measure coronary blood flow response to intracoronary acetylcholineCitation32. Hypertension was present in 48% of patients, type 2 diabetes mellitus in 5.6%, and hyperlipidemia in 61.8%. CED was diagnosed in 59% of post-menopausal women. Mean SUA level was 4.7 ± 1.3 mg/dL. Post-menopausal women with CED had significantly higher SUA compared with patients without CED (4.9 ± 1.3 vs 4.4 ± 1.3 mg/dL; p = .02). There was a significant correlation between SUA and percent change in coronary blood flow to acetylcholine (p = .009), and this correlation persisted in multivariable analysis. SUA levels were significantly associated with increased neutrophil count (p = .02) and high-sensitivity C-reactive protein levels (p = .006) among patients with CED, but not among those without CEDCitation32.
Vascular stiffness has been proposed as a valid, reproducible, and useful surrogate endpoint of kidney disease and cardiovascular risk, in studies investigating the link with hyperuricemiaCitation33. Observational evidence in large epidemiological cross-sectional studies suggests that there is an independent association between uric acid and arterial stiffness, and there seems to be sufficient evidence to warrant larger clinical trials to determine whether lowering uric acid concentrations would be useful for prevention or treatment of vascular stiffness and, subsequently, of cardiovascular and kidney diseasesCitation33.
Recently, the longitudinal relationship between SUA and pulse wave velocity, a measure of arterial stiffness, in a community-dwelling population, was studied in 446 women and 427 men participating in the BLSA (Baltimore Longitudinal Study of Aging), with 1,409 and 1,434 observations respectively, over an average period of 6 yearsCitation34. Higher SUA was associated with a greater increase in pulse wave velocity in men (β = 0.997; p = .012, higher vs lower SUA tertiles), but not women; this association was lost when men with SUA ≥6.2 mg/dL were not included, suggesting a threshold for SUA association with arterial stiffness, which is more frequently reached in menCitation34.
The relationship between SUA and measures of endothelial function was studied in a cohort of elderly community-dwellers, 424 males and 426 females aged 70 years, from the Prospective Study of the Vasculature in Uppsala Seniors (PIVUS), having complete data on SUA and endothelial function assessed by flow-mediated vasodilation and by intra-arterial infusion of acetylcholine (endothelium-dependent vasodilation, EDV) and sodium nitroprusside (endothelium-independent vasodilation, EIDV)Citation35. Especially in women, SUA was found to be associated with endothelial function not independently of a list of confounders including BMI and trunk fat mass, suggesting a role as a surrogate metabolic marker rather than an active player in endothelial function. SUA was inversely associated in both genders with log(EDV) (β ± SE males = –0.39 ± 0.17, p = 0.03; females = –0.57 ± 0.19, p = .003) and log(EIDV) (males = –0.23 ± 0.12, p = .05; females = –0.49 ± 0.15, p = .002). After adjustment for BMI, only the association between SUA and log(EIDV) in females persisted, although attenuated (–0.32 ± 0.16, p = .049), and it was no longer significant in the fully-adjusted multivariate model including waist/hip ratioCitation35.
A prospective analysis of baseline serum UA measurement and 15-year follow-up data from the Busselton Health Survey (n = 4,173), on the general population, stratified by existence or absence of baseline cardiovascular disease, found that hyperuricemia did associate with an increased risk of cardiovascular death only in participants with gout and existing cardiovascular diseaseCitation36. The authors concluded that, despite the considerable prevalence of hyperuricemia in 10.7% of the population, single or time averaged measures of UA were not independently predictive of incident cardiovascular disease or mortalityCitation36.
Some conflicting data could not support the use of urate lowering therapy in patients without gout, to prevent progression or risk of cardiovascular and kidney disease. A systematic review and meta-analysis of randomized clinical trials, published in 2015, found that urate lowering therapy was associated with reductions in serum uric acid, systolic, and diastolic blood pressure. On the contrary, data were not sufficient for evaluation of effects of urate-lowering therapy with end stage renal disease and cardiovascular diseaseCitation37.
Uric acid and metabolic syndrome
Based on the literature suggesting a role of hyperuricemia in cardiovascular risk, our study group evaluated whether uric acid level could be considered as a predictive factor of metabolic syndrome. SUA levels and metabolic parameters were evaluated in 139 subjects. It was found that SUA levels were significantly higher in subjects with than without metabolic syndrome (p < .0001), and rose gradually with the increasing number of metabolic syndrome components (p for trend <.0001)Citation38.
Our cohort was further analysed and other associations between SUA levels and several cardiometabolic parameters were found. Subjects were divided into low (<5 mg/dl) and high SUA group (≥5 mg/dl), choosing as a cut-off the precipitation concentration of urate. In the low SUA group, SUA levels directly correlated with: creatinine, body mass index, waist circumference, blood pressure, glucose and insulin levels, triglyceride and C-reactive protein levels. In the high SUA group, SUA levels directly correlated with: body weight, triglyceride, C-reactive protein, and inversely correlated with HDL-cholesterol concentrations ()Citation39.
Figure 1. Correlations between serum acid uric levels and cardiometabolic parameters. Reproduced with permissionCitation39.
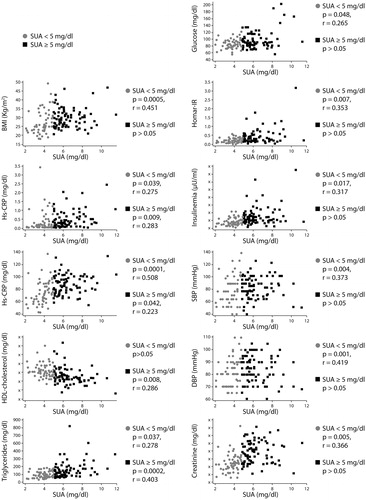
Results indicated that the progressive association between SUA and cardiometabolic risk factors rises quite below the precipitation concentration of uric acid, i.e. < 5 mg/dl. Thus, it is conceivable that the threshold value for SUA levels should be located lower than the currently suggested value in the healthy population, as previously recommended by Desideri et al.Citation40. Accordingly, the few associations we found in the high SUA levels population (SUA ≥5 mg/dl) could imply needing to redefine the range of normality for SUA, and strongly suggest oxidant generation promoted by uric acid already starts at low SUA concentration and does not further increase for higher SUA levelsCitation39.
Conclusion
Although a causal relationship between uric acid and hypertension, metabolic syndrome, and cardiovascular disease has not been clarified, the capacity of uric acid to negatively affect vascular function by pro-oxidant effects and by decreasing nitric oxide bioavailability and, consequently, induce endothelial dysfunction may explain the association among hyperuricemia and endothelial dysfunction, also by a common mechanistic point of view. In this context, some data indicate that uric acid levels start to favour the onset of the metabolic syndrome since the range of normality, with a cut-off value of 5.0 mg/dL. Under a practical profile, it becomes progressively more important to investigate the possibility of reducing serum uric acid levels in the general population below the level of 5.0 mg/dL.
Transparency
Declaration of funding
This review was funded by Fondazione Menarini.
Declaration of financial/other relationships
CF declares sponsorship from and speakers bureau from Menarini. Peer reviewers on this manuscript have received an honorarium from CMRO for their review work, but have no other relevant financial relationships to disclose.
Acknowledgments
Editorial assistance for this supplement was provided by Content Ed Net funded by Fondazione Menarini.
References
- Tomiyama H, Higashi Y, Takase B, et al. Relationships among hyperuricemia, metabolic syndrome, and endothelial function. Am J Hypertens 2011;24:770-4
- Feig DI. The role of uric acid in the pathogenesis of hypertension in the young. J Clin Hypertens (Greenwich) 2012;14:346-52
- Lv Q, Meng XF, He FF, et al. High serum uric acid and increased risk of type 2 diabetes: a systemic review and meta-analysis of prospective cohort studies. PLoS One 2013;8:e56864
- Zoppini G, Targher G, Chonchol M, et al. Serum uric acid levels and incident chronic kidney disease in patients with type 2 diabetes and preserved kidney function. Diabetes Care 2012;35:99-104
- Stack AG, Hanley A, Casserly LF, et al. Independent and conjoint associations of gout and hyperuricaemia with total and cardiovascular mortality. QJM 2013;106:647-58
- Borghi C, Rosei EA, Bardin T, et al. Serum uric acid and the risk of cardiovascular and renal disease. J Hypertens 2015;33:1729-41
- Chen C, Lü JM, Yao Q, et al. Hyperuricemia-related diseases and xanthine oxidoreductase (XOR) inhibitors: an overview. Med Sci Monit 2016;22:2501-12
- Hille R, Nishino T. Flavoprotein structure and mechanism. 4. Xanthine oxidase and xanthine dehydrogenase. FASEB J 1995;9:995-1003
- Hellsten-Westing Y. Immunohistochemical localization of xanthine oxidase in human cardiac and skeletal muscle. Histochemistry 1993;100:215-22
- Cejková J, Ardan T, Filipec M, et al. Xanthine oxidoreductase and xanthine oxidase in human cornea. Histol Histopathol 2002;17:755-60
- Linder N, Rapola J, Raivio KO. Cellular expression of xanthine oxidoreductase protein in normal human tissues. Lab Investig 1999;79:967-74
- Malik UZ, Hundley NJ, Romero G, et al. Febuxostat inhibition of endothelial-bound XO: implications for targeting vascular ROS production. Free Radic Biol Med 2011;51:179-84
- Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on the blood pressure of adolescents with newly diagnosed essential hypertension. JAMA 2008;300:924-32
- Niskanen LK, Laaksonen DE, Nyyssönen K, et al. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: a prospective cohort study. Arch Intern Med 2004;164:1546-51
- Fang J, Alderman MH. Serum uric acid and cardiovascular mortality: the NHANES I epidemiologic follow-up study, 1971–1992. JAMA 2000;283:2404-10
- Alderman MH, Cohen H, Madhavan S, et al. Serum uric acid and cardiovascular events in successfully treated hypertensive patients. Hypertension 1999;34:144-50
- Niskanen L, Laaksonen DE, Lindström J, et al. Serum uric acid as a harbinger of metabolic outcome in subjects with impaired glucose tolerance: the Finnish diabetes prevention study. Diabetes Care 2006;29:709-11
- Iseki K, Ikemiya Y, Inoue T, et al. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis 2004;44:642-50
- Iseki K, Oshiro S, Tozawa M, et al. Significance of hyperuricemia on the early detection of renal failure in a cohort of screened subjects. Hypertens Res 2001;24:691-7
- Tomita M, Mizuno S, Yamanaka H, et al. Does hyperuricemia affect mortality? A prospective cohort study of Japanese male workers. J Epidemiol 2000;10:403-9
- Krishnan E, Kwoh CK, Schumacher HR, et al. Hyperuricemia and incidence of hypertension among men without metabolic syndrome. Hypertension 2007;49:298-303
- Jossa F, Farinaro E, Panico S, et al. Serum uric acid and hypertension: the Olivetti Heart Study. J Hum Hypertens 1994;8:677-81
- Forman JP, Choi H, Curhan GC. Plasma uric acid level and risk for incident hypertension among men. J Am Soc Nephrol 2007;18:287-92
- Nakanishi N, Okamoto M, Yoshida H, et al. Serum uric acid and risk for development of hypertension and impaired fasting glucose or Type II diabetes in Japanese male office workers. Eur J Epidemiol 2003;18:523-30
- Dehghan A, van Hoek M, Sijbrands EJ, et al. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes Care 2008;31:361-2
- Siu YP, Leung KT, Tong MK, et al. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis 2006;47:51-9
- Talaat KM, el-Sheikh AR. The effect of mild hyperuricemia on urinary transforming growth factor beta and the progression of chronic kidney disease. Am J Nephrol 2007;27:435-40
- Kanbay M, Ozkara A, Selcoki Y, et al. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearence, and proteinuria in patients with normal renal functions. Int Urol Nephrol 2007;39:1227-33
- Culleton BF, Larson MG, Kannel WB, et al. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med 1999;131:7-13
- Capuano V, Marchese F, Capuano R, et al. Hyperuricemia as an independent risk factor for major cardiovascular events: a 10-year cohort study from Southern Italy. J Cardiovasc Med (Hagerstown) 2017;18:159-64
- Mazza A, Lenti S, Schiavon L, et al. Asymptomatic hyperuricemia is a strong risk factor for resistant hypertension in elderly subjects from general population. Biomed Pharmacother 2017;86:590-4
- Prasad M, Matteson EL, Herrmann J, et al. Uric acid is associated with inflammation, coronary microvascular dysfunction, and adverse outcomes in postmenopausal women. Hypertension 2017;69:236-42
- Ramirez-Sandoval JC, Sanchez-Lozada LG, Magdalena Madero M, et al. Uric acid, vascular stiffness, and chronic kidney disease: is there a link? Blood Purif 2017;43:189-95
- Canepa M, Viazzi F, Strait JB, et al. Longitudinal association between serum uric acid and arterial stiffness: results from the Baltimore longitudinal study of aging. Hypertension 2017;69:228-35
- Ticinesi A, Lauretani F, Ceda GP, et al. Uric acid and endothelial function in elderly community-dwelling subjects. Exp Gerontol 2017;89:57-63
- Nossent J, Raymond W, Divitini M, et al. Asymptomatic hyperuricemia is not an independent risk factor for cardiovascular events or overall mortality in the general population of the Busselton Health Study. BMC Cardiovasc Disord 2016;16:256
- Kanji T, Gandhi M, Clase CM, et al. Urate lowering therapy to improve renal outcomes in patients with chronic kidney disease: systematic review and meta-analysis. BMC Nephrology 2015;16:58
- Ciarla S, Struglia M, Giorgini P, et al. Serum uric acid levels and metabolic syndrome. Arch Physiol Biochem 2014;120:119-22
- Ciarla S, Giorgini P, Struglia M, et al. Associations between low levels of serum uric acid and cardiometabolic parameters. Arch Physiol Biochem 2015;121:139-43
- Desideri G, Castaldo G, Lombardi A, et al. Is it time to revise the normal range of serum uric acid levels? Eur Rev Med Pharmacol Sci 2014;18:1295-306
