Abstract
Objective: To assess the relationship between copay amount and vaccination claim submission status for tetanus-diphtheria-acellular pertussis (Tdap) and herpes zoster (GSK study identifier: HO-14-14319).
Methods: Retrospective analyses were performed using vaccination administrative claims data in patients aged ≥65 years with ≥1 claim for Tdap or zoster vaccines between 2012 and 2014. To avoid confounding by other financial responsibility, analyses were conducted among patients in the copayment phase of insurance. The impact of patient copay amount on vaccination claim status (“canceled” vs. “paid”) was evaluated by logistic regression separately for Tdap and zoster, adjusting for patient and provider characteristics.
Results: A total of 81,027 (39.2% with canceled claims) and 346,417 patients (56.8% with canceled claims) were included in the Tdap and zoster analyses, respectively. Mean (standard deviation) copay for canceled vs. paid claims was $37.2 (18.4) vs. $31.1 (20.1) for Tdap and $64.9 (36.9) vs. $53.5 (38.8) for zoster. The adjusted odds ratios (ORs) for a canceled Tdap vaccine claim, compared with $0 copay, were 1.19 ($1–25 copay), 1.76 ($26–50 copay), 2.42 ($51–75 copay) and 2.40 ($76–100 copay), all p < .001. The adjusted ORs for a canceled zoster vaccine claim, compared with $0 copay, were 1.02 ($1–25), 1.39 ($26–50), 1.66 ($51–75), 2.07 ($76–100) and 2.71 (>$100), all p < .001 except for $1–25 (p = .172).
Conclusions: High patient copay is a barrier to Tdap and zoster vaccinations in Medicare Part D patients. Providing vaccines at low or no copay may improve vaccination rates in these adults.
GSK study identifier: HO-14-14319.
Introduction
In the US, adult vaccination has been actively endorsed as an effective measure for reducing morbidity and mortality associated with vaccine-preventable diseasesCitation1. The recommendations of the Advisory Committee on Immunization Practices (ACIP) include vaccinations against zoster for adults aged 60 years or older and against pertussis (with the tetanus, diphtheria, acellular pertussis vaccine, Tdap) for all adultsCitation2. However, coverage rates in adults remain low for most vaccines, even for those routinely recommendedCitation3.
As a result, the burden of herpes zoster and pertussis remains high in US adults. Previous research indicates that approximately 1 million episodes of zoster occur in the US every year, with one in three people developing zoster over the course of their lives; the risk of zoster is even higher for adults aged 60 years and olderCitation4,Citation5. The reported incidence of pertussis varies greatly by data source and study design (i.e. from 1 to 5 cases per 100,000 persons per year); however, according to ACIP, the true burden of pertussis is at least 100 times greater than reportedCitation6. Vaccination against both pertussis and zoster has been shown to be cost-effective for older adults from both a societal and payer perspectiveCitation6–10. ACIP also recommended Tdap vaccination in adults 65 years and older who are in close contact with children less than 12 months of ageCitation11 to reduce the burden of infant pertussis. Still, the 2015 report from the Centers for Disease Control and Prevention (CDC) shows little progress in adult vaccination coverage in the past 10 years, with moderate increases for Tdap and zoster vaccines in 2015 compared to 2014. In 2015, 16.5% of adults aged 65 years or older reported vaccination with a Tdap vaccine (14.0% in 2014), and 34.2% of adults aged 65 years or older reported vaccination against herpes zoster (31.0% in 2014)Citation3.
In the US, adults aged 65 or older are eligible to receive Medicare benefits (a federal health insurance program administered by the Center for Medicare & Medicaid Services [CMS] for adults aged 65 or older, or younger patients with certain conditions) covering inpatient hospital stays (Part A) and some medical outpatient services and products (Part B). Individuals can also purchase Medicare Part D prescription drug plans. According to the CMS guidelines, Medicare Part B fully covers the cost of vaccines for influenza, pneumococcal infections and hepatitis B (for moderate- and high-risk patients), or vaccines directly related to the treatment of injuries or direct exposure to a disease. However, most injectable vaccines are currently covered by Medicare Part DCitation12, including Boostrix (GSK) or Adacel (Sanofi Pasteur, Inc.) against pertussis and Zostavax (Merck Sharp & Dohme Corp.) against zoster. Medicare Part D prescription drug plans classify covered medications into different tiers or categories, which may differ between plans. Medicare Part D usually employs five tiers (1 to 5), with generic or preferred generic drugs being included in Tier 1, and specialty drugs included in Tier 5. Beneficiaries may incur a copayment to receive vaccines covered by Medicare Part D. The copay amount may vary based on the setting in which a patient gets vaccinated and the patient’s type and phase of insurance (deductible, copayment or coinsurance, “donut hole” or catastrophic coverage)Citation12. The copay for a vaccination may be greater than its commercial price as the negotiated price of vaccination for an insurance plan may routinely include a dispensing fee, vaccine administration fees and covered vaccine product amount.
Prohibitive cost sharing (copayment) has been identified as one of the barriers to vaccine uptake among the elderlyCitation13. In 2011, CMS included a new tier in Medicare Part D (Tier 6), which would allow vaccines to be provided to patients without requiring copays. However, few health plans currently implement Tier 6 (i.e. a dedicated Vaccine Tier with zero-dollar cost sharing) for their membersCitation14.
The main objective of this study was to assess the relationship between patient copayment/coinsurance amount and vaccination claim submission status (“paid” or “canceled”) for Tdap and zoster vaccines among patients in the copay/coinsurance phase of their insurance. The “paid” or “canceled” status of the submitted claim was considered indicative of the patient receiving or not receiving the vaccine, as “canceled” indicates that the provider had examined a patient’s coverage level (and financial responsibility) in the system but did not submit the claim to be paid. The secondary objective extended this assessment to all patients, regardless of the phase of their insurance coverage.
Methods
Data source and study design
To conduct this study (GSK study identifier: HO-14-14319), we used 2012–2014 vaccine administration claims data from TransactRxCitation15, a leading Medicare Part D vaccine-specific physician billing service system in the US. The system allows enrolled physicians to determine the patient’s coverage, the physician’s reimbursement amount, and the patient’s copayment amount and total financial responsibility before the patient receives a vaccine. Thus, patients may obtain information about their coverage and financial responsibility for a vaccine before deciding whether to receive or decline it. In addition to vaccination claims (recorded as either “canceled” or “paid”), other relevant data was obtained from TransactRx: date of visit, location of the provider site, patient’s insurance plan, national provider identifier (NPI), type and brand of vaccine, together with financial variables related to the covered product amount, covered administration fee and patient’s total out-of-pocket expense (including deductible, copay, coinsurance, “donut hole”, and catastrophic coverage amounts). The patient’s copayment/coinsurance amount depends on the insurance plan and varies with the insurance coverage phase (as shown in ).
Table 1. Insurance coverage phases for Medicare Part DCitation1 beneficiaries.
Certain variables available from TransactRx were linked to other databases to obtain additional information. Specifically, the provider site zip code was linked to US Census data to obtain socioeconomic and demographic characteristics (e.g. estimated income based on the provider zip code, rural/urban classification), insurance plan identifiers were linked to CMS Landscape files to obtain the type of insurance plan, and provider NPI was linked to the NPI database to obtain provider classification and gender.
Two analytical samples were defined based on patients’ insurance coverage phase: the primary analytical sample included patients only in the copay or coinsurance phase of their insurance; the secondary analytical sample included all patients, regardless of insurance coverage phase. The main predictor in the study was copayment or coinsurance amount (if both were paid, then the total amount was considered).
Study population
Patients included in the study were Medicare Part D beneficiaries aged 65 years or older, with a vaccination claim submission status of either “canceled” or “paid” for Tdap or zoster vaccines occurring between 2012 and 2014. Data was screened and cleaned to ensure that patients with multiple claim submissions were accurately captured in the analytical dataset with a single “canceled” or “paid” claim submission status. Patients with multiple records from providers with different NPIs and records with negative cost values were excluded from the study.
Two study cohorts were established, based on the vaccine type: (i) patients with either a “paid” or “canceled” claim submission status for Tdap vaccination; and (ii) patients with either a “paid” or “canceled” status for herpes zoster vaccination.
Endpoints
The outcome under study was vaccination claim submission status for Tdap or zoster vaccinations, i.e. “paid” or “canceled”. A “paid” status indicates that the patient received the vaccine, while a “canceled” status refers to instances where the claim was submitted but the vaccine was not administered.
Statistical methods
Statistical analyses to assess the relationship between vaccination claim status (i.e. “canceled” or “paid”) and the copay/coinsurance amount, the predictor of interest, were conducted separately for Tdap and zoster.
Baseline covariates, including patient, provider and insurance characteristics, were described using means, medians and standard deviations for continuous variables, and frequency distributions for categorical variables. Statistical comparisons between patients with vaccination claims with a submission status of “canceled” and “paid” were performed using the Wilcoxon–Mann–Whitney non-parametric test for continuous variables and chi-square tests for categorical variables. Statistical tests were evaluated using a significance level of α = .05.
Univariate logistic regression analyses were performed to examine the relationship between vaccine claim submission status and the patient’s copayment/coinsurance amount. For the primary objective, multivariate analyses were conducted, adjusting for age, gender, estimated income based on the provider zip code (which served as a proxy for patient zip code), geographic location, urban/rural classification, month of visit, insurance plan type, phase of insurance coverage, provider classification and provider gender.
A secondary analysis was performed to examine the association between total patient responsibility and claim submission status using multivariate logistic regression and adjusting for age, gender, estimated income based on the provider zip code, geographic location, urban/rural classification, month of visit, insurance plan type, phase of insurance coverage, provider classification and provider gender. Patient copay as an individual covariate was not included in this model, but was included as a component of the total patient responsibility amount. Including the two variables in the same model would present collinearity challenges, as over 80% of the sample was in the copay/coinsurance phase of insurance coverage.
All analyses were performed separately for the Tdap and zoster vaccines using SAS version 9.3.
Results
Study population identification
Of the initial 111,569 patients with records for Tdap and 456,145 patients with records for zoster in the dataset, after implementation of sample selection criteria and data cleaning, 99,146 were included in the final Tdap sample and 426,474 in the final zoster sample (secondary objective). The sample selection criteria are described in Supplementary Table 1. For the primary objective (only including patients in the copay phase), 81,027 (81.72% of the total sample) and 346,417 patients (81.23% of the total sample) were included in the Tdap and zoster cohorts, respectively.
Demographic, insurance plan and provider characteristics
Patient demographic and insurance characteristics for the Tdap and zoster cohorts are described in . In the Tdap cohort, 39.2% of patients had canceled claims. On average, patients with a canceled Tdap claim were 75.1 years vs. 74.4 years of age for patients with paid Tdap claims; 61% of patients in the Tdap cohort were women. A greater proportion of claims were from patients in urban areas rather than rural areas. In addition, for patients with “canceled” claim submission status the estimated income based on the provider zip code was of $50,667.7, compared to $54,456.9 among patients with “paid” status. The mean copay/coinsurance amount among patients with canceled Tdap claims was $37.2 (standard deviation [SD] $18.4) vs. $31.1 (SD $20.1) among patients with paid Tdap claims. Most patients with canceled Tdap claims had copay amounts between $26 and $50. The provider classifications for the majority of patients in the Tdap cohort were family and internal medicine (89.7%).
Table 2. Patient demographics, insurance characteristics and provider characteristics for patients in the coverage phase of their insurance.
In the zoster cohort, 56.8% of patients had canceled claims and 62% of patients were women. The average age was similar between the “canceled” and “paid” groups (74.8 vs. 74.2 years). Most vaccine claims came from patients from urban areas. In contrast with the Tdap cohort, patients with a “canceled” zoster claim submission status had higher estimated income based on the provider zip code ($65,261.7) than those with a “paid” claim submission status ($55,013.5). The average copay amount for zoster vaccination was found to be greater for patients with canceled claims ($64.9 [SD $36.9]) than for those with paid claims ($53.5 [SD $38.8]). Family and internal medicine (89.2%) were the most common provider types among patients in the zoster cohort.
Baseline characteristics were similar in the secondary analytical sample (i.e. patients in all phases of the insurance plan), as about 80% of the sample was included in the primary analysis for both vaccines. Patient demographics, insurance characteristics and provider characteristics for the secondary analytical sample are shown in Supplementary Table 2.
Univariate analyses
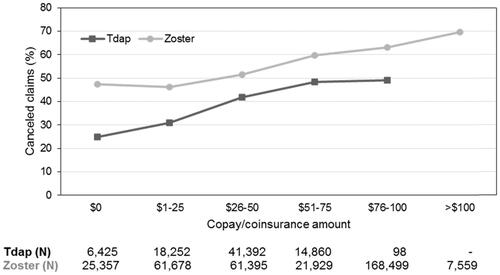
Among patients with $0 copay, 24.9% of Tdap patients and 47.4% of zoster patients had canceled claims. An increase in the number of canceled claims was observed with higher copay amounts for both primary sample cohorts, as shown in . Although the percentage of “canceled” claims for Tdap levels off from the $51–75 category to $76–100, this is likely due to the small sample size (N = 98) in the latter category rather than truly reflecting the behavior of patients with copays of $76–100. However, the proportion of canceled zoster claims decreases from the $0 category to the $1–25 category, but steadily increases in larger copay categories. Thus, for zoster, there is a decrease in the proportion of canceled claims for small copay amounts ($1–25) compared to $0, but this proportion increases as copay increases. A small proportion of zoster patients are in the extreme copay category of >$100.
Multivariate regression analyses
In order to address the primary objective, a multivariate logistic regression analysis was conducted using data on patients in the coverage phase of their insurance plan. Treating copay as a continuous variable and adjusting for patient and provider characteristics, the adjusted odds ratios (ORs) for a canceled claim associated with an increase in copay of $25 were 1.44 (95% confidence interval [CI]: 1.41, 1.47; p < .001) and 1.23 (95% CI: 1.22, 1.23; p < .001) for Tdap and zoster, respectively.
Adjusted associations between a categorical copay variable and the vaccination claim submission status are shown in . The ORs for a canceled vaccine claim using $0 copay as the reference category were: for Tdap, 1.19 ($1–25), 1.76 ($26–50), 2.42 ($51–75) and 2.40 ($76–100), all p < .001; for zoster, 1.02 ($1–25), 1.39 ($26–50), 1.66 ($51–75), 2.07 ($76–100) and 2.71 (>$100), all p < .001, except for the $1–25 category (p = .172).
Table 3. Covariate adjusted association between copay on vaccine claim status estimated using a logistic regression.
In both models, age, estimated income based on the provider zip code, urban vs. rural area, prior submitted claims, insurance plan characteristics and patient geographical location (reference: Northeast) were significantly associated with having a canceled vaccination claim. The adjusted ORs for Tdap showed that older patients and patients who had submitted prior claims were more likely to have a canceled vaccination claim, while those living in urban areas were more likely to have a paid vaccination claim compared with rural patients. In the zoster cohort, gender was also significantly associated with vaccination claim submission status.
To address the secondary objective, the association between patient total financial responsibility and vaccination claim submission status was estimated among patients in any phase of insurance. The results are shown in Supplementary Table 3. A $25 increase in total patient responsibility was associated with an increase in the odds of a canceled claim, with an OR of 1.41 (95% CI: 1.38, 1.44; p < .001) in the Tdap cohort and 1.23 (95% CI: 1.22, 1.23; p < .001) in the zoster cohort.
Discussion
This study assessed the relationship between copay amount and vaccination claim submission status for Tdap and zoster vaccines, and found that higher copay was associated with higher odds of having a canceled vaccination claim in elderly Medicare Part D patients. After adjusting for covariates related to patient and provider characteristics, the association remained significantly high for all categories, except the $1–25 copay amount in the zoster cohort.
presents a summary of the context, outcomes and impact of this study for healthcare providers. The results from this study support previous research which reported that higher patient copayment amounts were associated with lower vaccination ratesCitation16, and that reductions in copayment resulted in increased medication adherenceCitation17. However, the proportion of patients with canceled claims remained high among those with $0 copay/coinsurance (25% for Tdap and 47% for zoster), suggesting that factors other than copay amount may have also played an important role in determining whether patients received vaccinations. Thus, according to these findings, removing financial barriers may not be the only measure to change vaccination behaviors in a sizeable proportion of adults. Past research suggests that immunization rates in elderly Americans may be influenced by education level, race, ethnicity, geographic location, current health status, marital status and accessibility/availability of the care providerCitation18. Vaccine recommendations by providers are strong predictors of vaccination, and substantiate the provider’s role in helping overcome gaps in patient knowledge and appropriate disease risk assessment in vaccination decisionsCitation19. In this study, geographic location was found to be an important predictor, which may be correlated with patients’ beliefs and attitudes or with the extent of benefits provided by healthcare plans. This study did not account for racial and ethnic factors, but they have been shown to be of significance, with higher immunization rates observed among Whites for both Tdap and zoster vaccinesCitation1,Citation3. It is also interesting to note that being from an urban area is associated with decreased likelihood of having a canceled Tdap claim but greater likelihood of having a canceled zoster claim. This is an interesting finding that merits further research. Unfortunately, this cannot be explored further with the TransactRx database used in this study. Both unadjusted and adjusted associations between the copay variable and vaccination claim submission status were of greater magnitude for Tdap (adjusted OR for $25 increase in copay =1.44) than zoster (adjusted OR for $25 increase in copay =1.23). Further investigation is needed to fully understand how patients’ decisions regarding vaccination are made and to find reasons explaining the difference in magnitude of association between copay and vaccination claim status for the two vaccines.
Provider characteristics were also significantly associated with the odds of having a canceled vaccination claim. Healthcare providers play a crucial role in educating their patients about vaccines and achieving higher immunization rates in adultsCitation20–22. Patients’ or physicians’ attitude towards a vaccine and vaccination in general may also play an important role in a patient’s decision-making process, as there may be differences in a patient’s knowledge about the herpes zoster disease, the perception of low risk of contracting the disease and differences in the patients’ willingness to heed the advice of their physicians. For instance, the odds of having a canceled vaccination claim were higher in patients receiving care from a specialist as opposed to a family medicine physician: 1.86 for Tdap (95% CI: 1.61, 2.14; p < .001) and 1.11 for zoster (95% CI: 1.05, 1.18; p < .001). This finding is in agreement with previous studies showing that elderly patients with a primary care generalist had higher vaccination odds than those consulting a specialist as their usual physicianCitation18. Cost-related issues associated with the provider’s practice (e.g. lack of adequate reimbursement, costs of acquisition and storing of the vaccines, and failure to assess vaccination status during visits) have also been identified as barriers in adult vaccination in several national surveys assessing vaccination practices among general internists and family medicine physiciansCitation13,Citation23–25.
There are several potential limitations to this study. First, patients receiving vaccinations in pharmacies were not captured in the TransactRx database, and thus the results of the study might not be representative of all Medicare Part D beneficiaries. Second, similar to other administrative claims databases, the TransactRx vaccination claims database may be subject to errors and omissions, and proxies used for patient characteristics or socioeconomic status may be imperfect measures that result in residual confounding. In addition, only the status of the vaccination claim was recorded in the database. Thus, no inference about the impact of copay/coinsurance on a patient’s decision to get vaccinated can be made. However, it should be noted that the claim submission status (“paid” or “canceled”) was very likely to be recorded in TransactRx following the physician’s consultation with a patient about the disease and the vaccine. Therefore, the claim status recorded is expected to depend on financial variables to a greater extent compared with what may be observed in other administrative claims databases. Additionally, the timing of the inquiry about the cost of vaccination, e.g. at the start of an office visit, immediately after discussing with the physician, or following the appointment, is unknown in this study. This could have implications on the ultimate decision a patient makes about whether or not to receive the vaccination. Finally, estimated median income retrieved from US Census data based on the healthcare provider’s zip code was used as an estimate of income, assuming that patients, and particularly those who are elderly, are likely to live in close proximity to family practice or primary care physiciansCitation26. Indeed, the fact that increased distance between residents and healthcare providers results in decreased utilization of healthcare is well known, especially for subpopulations with limited access to transportation, such as the elderlyCitation27. In addition, according to the State Standards for Access to Care in Medicaid Managed Care published by the US Department of Health and Human Services, 32 states have established a maximum distance or time patients have to travel to see a primary care provider with most of these limits being around 10 miles or 30 minutesCitation28. A study conducted by the Washington State Office of Financial Management also indicated that current trips to routine care took an average of 17.5 minutes and an average distance of 8.6 milesCitation29. Thus, provider zip code can serve as a proxy for patient zip code. Regardless of distance traveled, however, the zip codes of patients and providers may differ, as patients may travel to different areas for physician services. Additionally, zip codes are of varying sizes with income heterogeneity that may not be accurately captured in the description of patient characteristics and may influence study findings. Thus, this is an important limitation of the approach used. Lastly, given that the Medicare population is the focus of this study, it may be likely that many patients live on fixed incomes and any effect of copay could have a greater impact on medical decision-making than the study demonstrated.
Conclusions
Patient copay/coinsurance is a substantial barrier to the utilization of zoster and Tdap vaccines in US Medicare Part D beneficiaries, both of which are covered under Medicare Part D. Reducing or eliminating patient cost sharing (i.e. providing vaccines at low or no copay) may help improve vaccination rates and reduce morbidity and mortality related to these vaccine-preventable diseases in adults. However, a quarter of patients with $0 copay for Tdap and almost half of all patients with $0 copay for zoster had “canceled” vaccination claim submission status, suggesting the need for further research on barriers to and other factors that may affect vaccination decisions. Ensuring that insurance plans implement Tier 6 or offer vaccines for little to no cost sharing would be a first step to making adult vaccines more accessible.
Transparency
Declaration of funding
GlaxoSmithKline Biologicals SA funded this study (GSK study identifier: HO-14-14319) and was involved in all stages of study conduct, including analysis of the data. GlaxoSmithKline Biologicals SA also took in charge all costs associated with the development and publication of this manuscript.
Author contributions: All authors participated in the study and the development of this manuscript: S.Y., M.D., P.L., M.S.D. and G.K. were involved in the conception or the design of the study; S.Y., M.D., R.H.B., P.L., M.S.D. and G.K. collected, analyzed and interpreted study data. All authors had full access to the data, revised the manuscript critically for important intellectual content and gave final approval before submission. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The work described was carried out in accordance to ICMJE recommendations for conduct, reporting, editing and publication of scholarly work in medical journals. The corresponding author had final responsibility to submit for publication.
Declaration of financial/other relationships
S.Y. and G.K. have disclosed that they were employed by the GSK group of companies at the time the study was conducted and during the development of the manuscript, and are currently employed by CSL Behring. S.Y. has disclosed that he holds shares in the GSK group of companies as part of his employee remuneration. G.K. has disclosed that she holds shares in the GSK group of companies and in CSL Behring as part of her employee remuneration. M.D., R.H.B., P.L. and M.S.D. have disclosed that their employer, Analysis Group, has received research funds from the GSK group of companies during the conduct of the study and outside the submitted work.
A peer reviewer on the manuscript has disclosed that they are a paid consultant for GSK, for work on studies related to the risk of cardiovascular disease and zoster, and for advisory committees related to the GSK candidate vaccine for zoster and for regulatory presentation related to the burden of zoster in the US. All other CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplementary_Table_1.docx
Download MS Word (33.8 KB)Acknowledgements
The authors would like to thank Grégory Leroux for editorial support and manuscript coordination (publications manager, Business & Decision Life Sciences on behalf of GSK); Véronique Gochet for editorial support (publications assistant, Business & Decision Life Sciences on behalf of GSK); and Vasile Coman and Petronela M. Petrar (medical writers, XPE Pharma and Science on behalf of GSK) for writing support.
Previous presentation: The data reported in this manuscript was partly presented at the 3rd Nexus Conference of the Academy of Managed Care Pharmacy (AMCP), Orlando, FL, USA, 26–29 October 2015.
Notes
Boostrix is a trademark of the GSK group of companies.
Adacel is a trademark of Sanofi Pasteur Inc.
Zostavax is a trademark of Merck Sharp & Dohme Corp.
References
- Williams WW, Lu PJ, O’Halloran A, et al. Noninfluenza vaccination coverage among adults – United States, 2012. Morb Mortal Wkly Rep 2014;63:95-102
- Bridges CB, Coyne-Beasley T; Advisory Committee on Immunization Practices (ACIP); ACIP Adult Immunization Work Group, Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices recommended immunization schedule for adults aged 19 years or older – United States, 2014. Morb Mortal Wkly Rep 2014;63:110-12
- Williams WW, Lu PJ, O’Halloran A, et al. Surveillance of vaccination coverage among adult populations – United States, 2015. Morb Mortal Wkly Rep Surveill Sum 2017;66:1-28
- Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb Mortal Wkly Rep Recomm Rep 2008;57:1-30
- Yawn BP, Saddier P, Wollan PC, et al. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc 2007;82:1341-9
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine in adults aged 65 years and older – Advisory Committee on Immunization Practices (ACIP), 2012. Morb Mortal Wkly Rep 2012;61:468-70
- Hales CM, Harpaz R, Ortega-Sanchez I, et al. Update on recommendations for use of herpes zoster vaccine. Morb Mortal Wkly Rep 2014;63:729-31
- McGarry LJ, Krishnarajah G, Hill G, et al. Cost-effectiveness of Tdap vaccination of adults aged ≥65 years in the prevention of pertussis in the US: a dynamic model of disease transmission. PLoS One 2014;9:e72723
- McGarry LJ, Krishnarajah G, Hill G, et al. Cost-effectiveness analysis of Tdap in the prevention of pertussis in the elderly. PLoS One 2013;8:e67260
- McLaughlin JM, McGinnis JJ, Tan L, et al. Estimated human and economic burden of four major adult vaccine-preventable diseases in the United States, 2013. J Prim Prev 2015;36:259-73
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. Morb Mortal Wkly Rep 2011;60:13-15
- Reimbursement for Vaccines and Vaccine Administration Under Medicare Part D. (MLN Matters. no: SE077272013). Baltimore (MD): Centers for Medicare & Medicaid Services, 2013
- Hurley LP, Lindley MC, Harpaz R, et al. Barriers to the use of herpes zoster vaccine. Ann Intern Med 2010;152:555-60
- Wouters AV, Manthe K, Stone DA. Few Medicare Part D Plans Offer Vaccines Without Cost-Sharing. Available at: https://www.manatt.com/Insights/Newsletters/Health-Update/HIPAA-and-Emerging-Technologies#Article5 [Last accessed 24 October 2017]
- TransactRx. Simplifying Vaccine Billing. POC Network Technologies Inc. 2014. Available at: http://www.transactrx.com/ [Last accessed 5 January 2015]
- Stoecker C, Stewart A, Lindley M. Cost of cost-sharing: the impact of Medicaid benefit design on vaccination coverage rates. Abstract presented at: APHA 142nd Annual Meeting & Expo, New Orleans, LA, USA, 15–19 November 2014
- Chernew ME, Shah MR, Wegh A, et al. Impact of decreasing copayments on medication adherence within a disease management environment. Health Aff (Millwood) 2008;27:103-12
- O’Malley AS, Forrest CB. Immunization disparities in older Americans: determinants and future research needs. Am J Prev Med 2006;31:150-8
- Schneeberg A, Bettinger JA, McNeil S, et al. Knowledge, attitudes, beliefs and behaviours of older adults about pneumococcal immunization, a Public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network (PCIRN) investigation. BMC Public Health 2014;14:442
- Armstrong K, Berlin M, Schwartz JS, et al. Barriers to influenza immunization in a low-income urban population. Am J Prev Med 2001;20:21-5
- Bridges CB, Hurley LP, Williams WW, et al. Meeting the challenges of immunizing adults. Am J Prev Med 2015;49(6Suppl4):S455-S464
- Winston CA, Wortley PM, Lees KA. Factors associated with vaccination of Medicare beneficiaries in five U.S. communities: results from the racial and ethnic adult disparities in immunization initiative survey, 2003. J Am Geriatr Soc 2006;54:303-10
- Medicare: Many Factors, Including Administrative Challenges, Affect Access to Part D Vaccinations. (No. GAO-12-61.) U.S. Government Accountability Office, 2011
- Hurley LP, Bridges CB, Harpaz R, et al. U.S. physicians’ perspective of adult vaccine delivery. Ann Intern Med 2014;160:161
- Hurley LP, Harpaz R, Daley MF, et al. National survey of primary care physicians regarding herpes zoster and the herpes zoster vaccine. J Infect Dis 2008;197(Suppl2):S216-S223
- Gozu A, Nidiry MA, Ratanawongsa N, et al. Patient factors associated with following a relocated primary care provider among older adults. Am J Manag Care 2009;15:195-200
- Nemet GF, Bailey AJ. Distance and healthcare utilization among the rural elderly. Soc Sci Med 2000;50:1197-208
- Murrin S. State Standards for Access to Care in Medicaid Managed Care. (No. OEI-02-11-00320.) Washington (DC): US Office of Inspector General, Department of Health and Human Services, 2014
- Yen W. How Long and How Far Do Adults Travel and Will Adults Travel for Primary Care? (Washington State Health Services Research Project, Research Brief no. 70.) Washington (WA): Washington State Office of Financial Management, 2013