Abstract
Objective: We evaluated compliance of recent narrative systematic reviews with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidance.
Research design and methods: We searched EMBASE and MEDLINE databases on 21-September-2017, for English-language records with a print publication date of 01-June-2017, for “systematic review” (in the title field) and terms relating to drug therapy (in the subject field). Case studies, conference reports, letters, surveys, errata, editorials, and meta-analyses were excluded. A manual screen excluded protocols and reports providing statistical analysis. Articles were scored for fulfilment of PRISMA checklist items for the objectives, methods (information sources, search, study selection), and results (study selection, study characteristics), and whether they reference PRISMA.
Results: Of the 99 abstracts identified, 46 (46.5%) were selected for analysis. Reasons for exclusion were: not drug related (n = 35), statistical analysis conducted (n = 10), protocol (n = 7), and not in English (n = 1). Twenty-seven (58.7%) publications did not fully adhere to our set of PRISMA items. More than 82% of publications appropriately reported on objectives, information sources, and study selection (methods) items, whereas 76.1% and 50.0% reported study selection (results) and search items, respectively. Publications citing PRISMA (n = 28; 60.8%) tended to report on more items with the exception of search criteria.
Conclusions: Just over half of these recent publications described as systematic reviews did not follow PRISMA criteria, despite referencing them. These findings suggest a need for improvement in performing systematic reviews and/or reporting how they were conducted. Such improvements may lead to greater confidence in the findings of systematic reviews.
Abstract
Objective: Forums to comment on and discuss publications online are emerging, but their nature and utility have not been evaluated. This analysis characterised commentary and discussion in PubMed Commons, a closed pilot forum which is accessible only to approved PubMed authors.
Research design and methods: Literature searches were conducted in PubMed for articles with authors from 15 pharmaceutical companies and 6 high-profile research institutions that also had comments on PubMed Commons. The prevalence of comments across affiliations was summarised, and comments were classified as discussion, curation (annotation of relevant information), criticism, or endorsement.
Results: The prevalence of comments was similar between articles with industry or research institution authors (). Curation was the most common category and was more common with industry authors. Discussion was more common with research institution authors. Some criticism comments to company-authored articles (4/17) noted the funding source as a potential bias.
Conclusions: Use of PubMed Commons is low, regardless of author affiliation, and the most common comments were curation.
Keywords:
Table. Prevalence and type of comments in PubMed Commons.
Abstract
Objective: Online activity extends the reach of important data, and monitoring where discussion and interest in congress or publication activity occurs can inform future publication plans. This exploratory study analysed activity surrounding three different drugs that released clinical trial results in congress presentations at EASD 2017 with simultaneous publication in high-impact journals.
Research design and methods: Online activity including data from Twitter (number of tweets), online news articles, and page views relating to all three drugs were collected over a period of three weeks starting one week prior to the conference/publication date. A quantitative comparison of engagement (based on interactions including replies, retweets, and likes) with the published/presented clinical trial was made according to whether the activity related to the congress presentation, the manuscript, or another source. The proportion of overall activity that was captured by some of the different commonly available methods and automated tools was also assessed.
Results: We identified a total of 136 news articles, and 723 tweets related to the three studies, with 1667 interactions. While a greater proportion of online activity consistently related to the manuscripts than the congress presentations (40% vs 16% overall), activity relating to the congress was associated with a greater level of engagement. The majority of activity was driven indirectly by news articles that related to the manuscript or congress presentations.
Conclusions: In this small sample, with a simultaneous presentation/publication, the publication drives online activity, but the presentation disproportionately drives online engagement. It would be interesting to examine any differences should the presentation and publication be separated in time.
Keywords:
Abstract
Objective: We conducted a systematic review to examine the relationship between professional medical writing support and the quality, ethics and timeliness of medical research publications.
Research design and methods: Using terms related to ‘medical writ*’ and ‘medical publication professional’, we searched MEDLINE and Embase (no date limits) and abstracts and posters from meetings of the International Society for Medical Publication Professionals (2014–2017). We also hand-searched the bibliographies of studies identified in the electronic searches. We screened the results to identify studies comparing the quality, ethics and timeliness of manuscripts written with professional medical writing support with those written without declared professional medical writing support.
Results: Our searches identified 90 potentially relevant studies, of which 81 were excluded during screening and full paper review. The remaining 9 studies, all observational, analysed 870 articles with professional medical writing support and 2519 without. Professional medical writing support was associated with increased adherence to reporting guidelines (in 3/4 studies in which this was assessed), higher quality of written English (1/1 study), publication in journals with higher impact factors (1/1 study), increased transparency in reporting of conflicts of interest (1/1 study) and lower likelihood of reporting non-pre-specified outcomes (1/1 study). Professional medical writing was associated with a reduced time from clinical trial completion to primary publication (1/1 study), while time from manuscript submission to acceptance was either reduced (1/2 studies) or increased (1/2 studies).
Conclusions: Observational studies suggest that medical writing support increases the quality and ethics of reporting of clinical trials and may improve the timeliness of publication.
Abstract
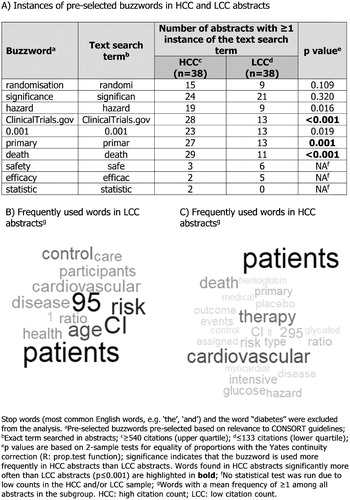
Objective: To investigate the relationship between “buzzwords” in New England Journal of Medicine (NEJM) article abstracts and citation counts (CCs).
Research design and methods: Abstracts mentioning “diabetes” from 2007–2016 NEJM articles were identified via PubMed and their CCs recorded from the NEJM website. High and low CC (HCC; LCC) cut-offs were the upper and lower quartiles of all CCs. 10 “buzzwords” were pre-selected (). The numbers of HCC and LCC abstracts with ≥1 mention of each buzzword were compared using the prop.test function in R. Words frequently used in HCC and LCC abstracts were determined.
Results: 153 abstracts, with 4440 unique words, met the inclusion criteria. “ClinicalTrials.gov”, “death” and “primary” were used significantly more in HCC abstracts (). “Outcome” and “placebo” were among the most common words used in abstracts of HCC (), but not LCC (), articles.
Conclusions: Buzzwords associated with good publication practice, including “ClinicalTrials.gov”, in manuscript abstracts may increase their citation likelihood.
Keywords:
* Oral Presentation
* Oral Presentation
Abstract
Objective: To assess the long-term impact and reach of a series of published articles via their social-media footprint.
Research design and methods: Social-media footprints were monitored for several Shire-sponsored articles published from July to December 2016. Data included the Altmetric Attention Score (a measure of social-media attention), journal impact factor, journal-provided downloads and ResearchGate reads. Publication metrics were updated and citation numbers were recorded in September 2017.
Results: For the 35 articles monitored, most social-media attention occurred within 4 weeks from online publication (mean Altmetric Attention Score: 14.5 at week 4; 18.2 at week 40–64). No correlation was observed between Altmetric Attention Score at week 4 and journal impact factor (n = 30), journal-provided downloads (n = 14), or ResearchGate reads (n = 35) at 10–17 months from publication. Of 29 articles with ≥1 tweet, 51.7% were promoted on a social-media platform by the publisher and the numbers of tweeters and tweets per article were greater for those articles with publisher tweets (7.9 and 9.9, respectively) than without (4.6 and 5.4, respectively). For the article with the highest Altmetric Attention Score of 2371, there were 67 individual tweeters (44 of whom were health organisations, healthcare professionals and scientists/researchers), with a potential reach of 225,233 followers. The articles with the five highest Altmetric Attention Scores 4 weeks after publication included the three most cited articles 10–17 months later.
Conclusions: These findings, based on a small sample of scientific publications, illustrate how social-media activity may be used to estimate publication reach. Preliminary data suggests social-media activity 4 weeks after publication may predict citation rates up to 17 months later.
Keywords:
Reference
- Novak SP, Håkansson A, Martinez-Raga J, Reimer J, Krotki K, Varughese S. Nonmedical use of prescription drugs in the European Union. BMC Psychiatry 2016;16:274
Abstract
Objective: Evidence of the value of professional medical writing support is increasing, and various guidelines on the role of professional medical writers now existCitation1. Our aim was to develop a unified set of concise, practical, evidence-based guidelines for responsible medical and scientific writing.
Research design and methods: A Writing Committee was established that consisted of four members from three leading professional organizations, AMWA, EMWA and ISMPP, representing more than 6500 medical communicators around the world. The Writing Committee agreed on the scope of, and the process and timelines for developing, the Joint Position Statement (JPS), which was reviewed by organizational representatives located in Europe, North America and Asia Pacific. A broader Joint Outreach Committee developed a multi-channel communications strategy.
Results: A concise, evidence-based position statement was drafted, approved and finalized within 4 months of the Writing Committee being formed. The resulting JPS, which is being translated into multiple languages, was launched at the European ISMPP meeting in January 2017, presented at an AMWA webinar and an EMWA conference, and published in a peer-reviewed journal with associated commentaryCitation2,Citation3. The JPS publication attracted 10,878 unique page views in the first month after launch, and over 23,000 impressions, 1000 engagements and 41 posts on social media in the first 9 months.
Conclusions: The JPS represents the first global standard for the work of professional medical writers. The JPS is intended to serve as a reference for professionals engaged in the generation of scientific/medical publications, and may also be used as a practical tool for communicating with other stakeholders.
References
- Hamilton CW, Gertel A, Jacobs A, Marchington J, Weaver S, Woolley K. Mythbusting Medical Writing: Goodbye, Ghosts! Hello, Help! Account Res 2016;23:178-94
- AMWA–EMWA–ISMPP Joint Position Statement on the Role of Professional Medical Writers. Medical Writing 2017;26:7-8
- Winchester C. Advancing the medical writing profession: the Joint Position Statement on the role of the professional medical writer. Medical Writing 2017;26:6
Abstract
Objective: To evaluate disclosure of clinical trials registered by pharmaceutical companies using an independent, semi-automated tool (TrialsTracker; https://trialstracker.ebmdatalab.net/#/).
Research design and methods: For the top 50 pharmaceutical companies (2014 global sales; EvaluatePharma, London, UK), registered interventional phase 2–4 clinical trials completed in 2006–2015 were identified in TrialsTracker, which calculates annual disclosure rates for sponsors of over 30 studies registered on ClinicalTrials.gov. The proportion of trials with results disclosed by April 2017 was analysed by company membership of the European Federation of Pharmaceutical Industries and Associations (EFPIA) and Pharmaceutical Research and Manufacturers of America (PhRMA).
Results: In total, 323 clinical trial sponsors were listed in TrialsTracker, of which 69 were pharmaceutical industry sponsors and 31 were ranked in the top 50 pharmaceutical companies. Of these, 25/31 were EFPIA/PhRMA members and 6/31 were non-members. The disclosure rate for each year from 2006 to 2015 was 42.9%, 54.4%, 81.0%, 86.1%, 84.6%, 87.2%, 89.3%, 82.1%, 84.1% and 73.4%; reporting of clinical trial results became mandatory in 2008. The disclosure rate (disclosed trials/eligible trials) between 2006 and 2015 was greater for all pharmaceutical industry sponsors (7037/9511 [74.0%]) than for non-industry sponsors (9074/19866 [45.7%]) (p < .01). For the top 50 companies, results were disclosed for 4761/6235 trials (76.4%) between 2006 and 2015, with similar disclosure rates for EFPIA/PhRMA members (4336/5697 [76.1%]) and non-members (425/538 [79.0%]).
Conclusions: According to TrialsTracker, the pharmaceutical industry has disclosed the results of three quarters of trials completed between 2006 and 2015. The disclosure rate for pharmaceutical industry sponsors is greater than for non-industry sponsors. Because TrialsTracker excludes sources other than ClinicalTrials.gov (e.g. company websites), this figure may be an underestimate.
Keywords:
Abstract
Objective: Building on a 2014 surveyCitation1 and the 2017 AMWA-EMWA-ISMPP Joint Position StatementCitation2, this survey aims to assess the value that corresponding authors (CAs) attribute to working with professional medical writers (PMWs).
Research design and methods: CAs were selected by a blind, unbiased and systematic four-step process: (1) Therapy area; (2) Journal; (3) Article selection (January 2013–April 2017); and (4) CA. CAs were invited by e-mail to complete a 10-question survey rating (5-point scale: “not-at-all” to “extremely”) both their general and specific (14 domains) experiences working with PMWs. Responses were collected anonymously and data summarised using descriptive statistics.
Results: In total, 298 CAs of randomised controlled trial manuscripts were identified; 75 were excluded (58 published >1 article, 14 invalid contacts, 2 rejections, 1 annual leave). Overall, 223 email invitations were sent and 32 CAs responded (50.0% [n = 16] European; 37.5% [n = 12] North American; 12.5% [n = 4] Asian). Of those CAs who responded, 56% (18/32) reported PMW support and 78.8% (14/18) considered this moderately to extremely useful. Management of timelines, collation of comments and conformity with guidelines were each ranked as valuable support by 55% of respondents (10/18). PMWs’ scientific expertise was considered moderately (5.6%; 1/18), slightly (50.0%; 9/18) or not-at-all (44.4%; 8/18) valuable. “Lack of need” was the most reported reason for not involving PMWs for the 44% of CAs (14/32) who had not worked with PMWs.
Conclusions: This small sample suggests that CAs appreciate the overall value of PMWs in publication development. CAs reported a more positive attitude towards PMWs’ publication assistance than scientific expertise.
Keywords:
References
- Marchington JM, Burd GP. Author attitudes to professional medical writing support. Curr Med Res Opin 2014;30:2103-2108
- Winchester C. AMWA-EMWA-ISMPP Joint Position Statements on the role of professional medical writers. Medical Writing 2017;26:7-8
† Encored poster at the 14th Annual Meeting of ISMPP
† Encored poster at the 14th Annual Meeting of ISMPP
Abstract
Objective: Journal publishers increasingly offer governmental and charitable research funders the option to pay for open access with a CC BY licence. Recommended by the Open Access Scholarly Publishers Association as the most liberal Creative Commons licence available, a CC BY licence allows sharing and adaption of published materials for commercial as well as non-commercial use. We set out to investigate whether pharmaceutical companies are offered the same terms.
Research design and methods: Journals with an Impact Factor (IF) ≥ 15 (accurate on 24 May 2017) were identified using Journal Selector (Sylogent, Newtown PA), and their open access policies were obtained from online information and by email contact between 29 June 2017 and 26 July 2017 (up to 3 attempts). Journals that only publish review articles were excluded from the analysis.
Results: Thirty-seven journals, from 18 publishers, had an IF ≥15, and relevant information was obtained for 36/37 journals. All 36 journals offered some form of open access within 12 months of first publication. Of these journals, 21 (58%) offered immediate open access with a CC BY licence. Only two of these journals had an open access policy that potentially allowed a CC BY licence to commercial funders/pharmaceutical companies.
Conclusions: The open access policies of most high impact medical journals restrict the dissemination of medical research funded by the pharmaceutical industry. To give the scientific community full access to read, reuse and adapt medical publications, publishers and academic journal editors should allow unrestricted and immediate open access with a CC BY licence to authors, independent of their funding source.
Keywords:
Abstract
Objective: The International Society for Medical Publication Professionals (ISMPP) encourages its members to publish their own research. Nevertheless, Carey et al. (Citation2016) revealed a dawning low 2.4% publication rate of abstracts presented at American (US) ISMPP meetings (2009–2014). A more comprehensive analysis was now conducted to assess the publication rates of abstracts from all European (EU) and US ISMPP meetings.
Research design and methods: PubMed-indexed journals were searched for peer-reviewed full publications of original abstracts from EU (2011–2017) and US (2006–2017) ISMPP meetings using title key words, author names and affiliations. For each identified publication, author affiliations, time from abstract to full publication (T2P) and the journal impact factor (IF) were assessed. Regional differences and interrelations between T2P and IF were statistically analysed using 2-sided Student’s t-tests and Pearson correlation and regression analyses.
Results: To date, a total of 384 original abstracts were presented at ISMPP meetings (EU: 19%, US: 81%). The overall publication rate was 4.7% (EU: 5.4%, US: 4.5%), and the annual publication rates remained mainly below 10%. 62% of abstracts were (co-)authored by communication agencies without industry involvement whereas 33% of abstracts included industry authors. The latter had slightly higher publication rates (all: 5.6%, EU: 6.3%, US: 5.5%) than agency-authored abstracts (all: 4.2%, EU: 5.5%, US: 3.8%). T2P (mean ± SD: 2.0 ± 1.79 years) and IF (mean ± SD: 2.8 ± 1.11) were similar between EU and US (p > .05) and were found not to be correlated (p = .745).
Conclusions: The present analysis confirms previous concerns of low abstract publication rates. Possible hurdles such as low incentives to publish need to be overcome by ISMPP members to finally lead by example.
Reference
- Carey LC, Stretton S, Kenreigh CA, Wagner LT, Woolley KL. High nonpublication rate from publication professionals hinders evidence-based publication practices. PeerJ 2016;4:e2011
* Oral Presentation
* Oral Presentation
Abstract
Objective: There is a growing trend towards patient involvement in healthcare communications and a rise in use of new technologies and social media as a tool to share and discuss publicationsCitation1. This analysis aimed to assess the impact of freely-available publications on lowering barriers for health communication.
Research design and methods: Sylogent was used to filter journals from highest to lowest impact factor (IF) under the heading ‘Medicine, General and Internal’. The following filters were applied; Peer reviewed journal(s), Optional Open Access, Original article. Any journals without an IF or Altmetric scoring were eliminated from the analysis. The nine journals with the highest, median and lowest IFs were selected and from these; the 10 most recently published articles were analysed for Altmetric score, and open- or non-open access. Any articles which required a free or paid for registration were excluded from the analysis.
Results: Overall, 61 journals appeared in the initial search. Of the 90 articles retrieved, 35 were open access. The mean Altmetric score for the open access articles was 85.2 and for the non-open access was 13.9. The mean Altmetric scores for the highest, median and lowest IF journals were 69.5, 3.7 and 137.2, respectively, for open access articles, and 106.7, 0.6 and 9.0, respectively for the non-open access articles.
Conclusions: This research suggests that the open access articles gain more online attention and, therefore, potentially influence, compared with the non-open access articles. More work is needed to determine the correlation between Altmetric score and patient involvement, and the subsequent impact on research and medical communications.
Keywords:
Reference
- Fox CS, Barry K, Colbert J. Importance of social media alongside traditional medical publications. Circulation 2016;133:1978-83
Abstract
Objective: Expert guidelines are important resources to provide education, inform treatment decisions and improve patient care. We investigated whether these publications are freely accessible online or whether they have pay-per-view/subscriber-only access and are therefore restricted to clinicians/patients with the means to pay.
Research design and methods: We performed a systematic search of PubMed for articles published January 2014 to September 2017 using search terms ‘treatment’ and ‘guideline’, and other relevant synonyms, in English. Results were assessed to include only original guidelines, position/consensus statements, or other publications providing clinical practice recommendations. Ease of accessibility was determined by assessing whether the publication was free to access via PubMed, the journal website, Google or the website of any related society/group.
Results: We identified 3087 guidance publications, 1020 in 2014, 993 in 2015, 782 in 2016 and 292 in 2017 (as of 18 September 2017). Overall, 508 (16.5%) required a payment to access, with 2579 (83.5%) freely available. The greatest free access to guidelines was seen in 2014 (90.3%). However, consistently from 2015 to 2017 19.1–20.5% of articles required paid access. Twenty-eight broad therapy areas were represented in 2017, with no real pattern to ease of access observed in the individual areas. The top ten most represented areas were: oncology (50 articles, 78.0% free), obstetrics/gynaecology (34, 82.4% free), cardiology (29, 62.1% free), infectious disease (27, 77.8% free), gastroenterology (24, 95.8% free), rheumatology (17, 94.1% free), respiratory (12, 91.7% free), endocrinology (11, 72.7% free), genitourinary/sexual health (9, 100% free), immunology/allergy (7, 71.4% free).
Conclusions: Despite the importance of expert guidelines, about one fifth are not freely available to access.
Keywords:
† Encored poster at the 14th Annual Meeting of ISMPP
† Encored poster at the 14th Annual Meeting of ISMPP
Abstract
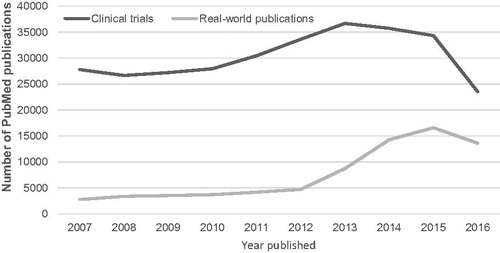
Objective: Medical research traditionally focused on clinical trials to obtain data. However, real-word data has become increasingly important as it can reflect clinical practice and populations outside of clinical trials. This analysis compared the publication history of clinical trials and real-world publications.
Research design and methods: PubMed was searched from 2007–2016 for all clinical trials and real-world publications. Clinical trials were identified by ‘Clinical trial’ publication type. Real-world publications were identified by ‘Observational study’ publication type or by a ‘Registry’ MeSH term.
Results: Number of clinical trials and real-world publications increased annually from 2008–2012 at a mean of 8.8% and 6.0% per year, respectively. From 2012–2015 the number of real-world publications greatly increased compared to a moderate decline in clinical trials. In 2016, both clinical trials and real-world publications experienced a large decrease in number ().
Conclusions: Real-world publications greatly increased in the last few years, symbolising their growing contribution to medical research. Further investigations are required to confirm and explain a decline in publications beyond 2016.
Keywords:
† Encored poster at the 14th Annual Meeting of ISMPP
† Encored poster at the 14th Annual Meeting of ISMPP
Abstract
Objective: To determine how medical publication professionals access and engage with publications-related news online.
Research design and methods: We analysed usage and user preferences of a freely-accessible, multichannel (website, email newsletter, LinkedIn, Twitter and Facebook) news service for medical publication professionals. Usage and demographic data were collected from website/LinkedIn metrics, and user feedback through a 13-question survey.
Results: At the time of writing (September 2017), the service had 1780 online subscribers and 3519 LinkedIn group members (44% Europe, 44% USA, 12% rest-of-world). Seventy-nine users completed the survey; the most frequent user-reported job roles were medical writing (41% of respondents), publications management (19%) and medical affairs (8%). Email newsletters were the preferred distribution channel (63% accessed these at least monthly), followed by the LinkedIn group (42%) and website (38%). Website page views revealed that the most-viewed topics were meeting reports, guidelines updates, and articles on plagiarism or predatory publishing. Survey respondents confirmed these observations, with 90%, 90% and 66% ‘interested’/‘very interested’ in publications news, guideline updates and meeting reports, respectively. Most respondents (62%) heard about a piece of news for the first time at least monthly via the service, rating quality and usefulness as ‘good’/‘excellent’ (89% and 87%, respectively). In spite of this, most respondents ‘never’/‘occasionally’ shared articles with colleagues (73%) or interacted with peers online (95%).
Conclusions: Based on this small survey, although medical publication professionals value online news services, this is not reinforced through online interaction and information sharing at the individual level. The professional community could improve the dissemination of news and information through more active engagement online, thereby improving education and advancing the field of medical publications.
Abstract
Objective: Patient centricity is increasingly important to the pharmaceutical industry. Today’s patients are more informed and want to be involved in the decision-making process. Some interesting initiatives have been reported, although much of the potential for patient engagement remains to be fulfilledCitation1,Citation2. We report a collaboration between patients, a registry scientific advisory board (SAB) and Alexion Pharmaceuticals.
Research design and methods: The atypical hemolytic uremic syndrome (aHUS) Registry is a phase IV trial (NCT01522183) with the objectives of understanding the natural history of aHUS, an ultra-rare disease, and evaluating the long-term safety and efficacy of eculizumab (approved for the treatment of aHUS). As a patient-centric organization, Alexion included a patient organization representative in the aHUS Registry SAB in 2014. The patient organization conducted a member survey to determine what research questions were of most importance to patients with aHUS in 2014 and 2016.
Results: Presentation of patient survey data to the SAB led to the re-prioritization and inclusion of new ideas in the analysis plan of the registry data. The questionnaire and outcomes were published with several of the SAB members, the patient representative and one patient as authorsCitation3. To maximize availability to patients, the article was provided as open-access, and has been actively promoted on social media by the patient organization, achieving an Altmetric score of 18.
Conclusions: This ongoing initiative demonstrates how a mutually beneficial relationship can be established between patients, their organisations and a pharmaceutical company. Inclusion of patient-proposed research ideas can enhance Registry protocol design and analysis plans.
Keywords:
References
- Sharma NS. Patient centric approach for clinical trials: Current trend and new opportunities. Perspect Clin Res 2015;6:134-8
- Javaid MK, Forestier-Zhang L, Watts L, et al. The RUDY study platform – a novel approach to patient driven research in rare musculoskeletal diseases. Orphanet J Rare Dis 2016;11:150
- Woodward L, Johnson S, Walle JV, et al. An innovative and collaborative partnership between patients with rare disease and industry-supported registries: the Global aHUS Registry. Orphanet J Rare Dis 2016;11:154
† Encored poster at the 14th Annual Meeting of ISMPP
Footnote‡‡ Winner, Most Reflective of Meeting Theme
† Encored poster at the 14th Annual Meeting of ISMPP
‡ Winner, Most Reflective of Meeting Theme
Abstract
Objective: The Open Researcher and Contributor ID identifier (ORCID iD) is a unique code for crediting researchers and a useful tool allowing organizations to track and report on their research. ORCID has been adopted across academia and some journals now require ORCID iDs from authors at submission. Pharmaceutical industry engagement with ORCID has been limited, but increasing numbers of employees are registering.
Open Pharma, which brings together industry, publishers and other healthcare stakeholders to improve the model of medical publishing, recognized ORCID as an important innovation. Being an Open Pharma participant, GSK Vaccines initiated a pilot study to assess the feasibility of implementing ORCID and to encourage internal authors to register for ORCID iDs.
Research design and methods: Internal authors were educated about ORCID and Open Pharma via a newsletter and user guide. They were asked to confirm registration with ORCID through Datavision™ (from 14/08/2017 onwards). Unsolicited feedback was recorded.
Results: On 13/12/2017, 328 of 531 internal authors (62%) had registered with ORCID. Datavision™ enabled ORCID iD collection and extraction of a report of those who had registered. Unsolicited comments (n = 24) mostly related to technical issues and were adequately resolved. A few individuals enquired about easier ways to link their ORCID iD with their previous publications.
Conclusions: The Open Pharma initiative facilitated adoption of ORCID by GSK Vaccines. Internal authors are engaged and no significant hurdles have been identified in this ongoing pilot study. Continued efforts may encourage adoption of ORCID across the pharmaceutical industry. Moving forward, external authors will be encouraged to use ORCID iDs when working with GSK Vaccines.
Keywords:
* Oral Presentation
Footnote†† Encored poster at the 14th Annual Meeting of ISMPP
* Oral Presentation
† Encored poster at the 14th Annual Meeting of ISMPP
Abstract
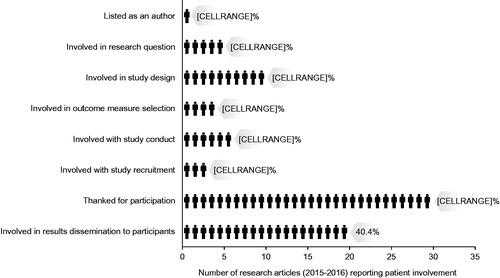
Objective: In 2014, The BMJ introduced a mandatory “Patient Involvement” statement in the Methods section of research articles. We investigated the extent of patient involvement described in clinical trial research publications in The BMJ. Our primary objective was to quantify patient authorship.
Research design and methods: We searched PubMed (journal: The BMJ; publication type: clinical trial; dates: 2015/01/01-2016/12/31) and electronically exported all retrieved articles. Non-research articles were removed. Two authors categorized patient involvement () based on the verbatim “Patient Involvement” and Acknowledgements sections in each publication. Results were cross-checked.
Results: Of the 62 articles retrieved, 10 were non-research articles. Reported patient involvement was generally low (). Involvement was lowest for authorship (1.9%; 1/52) and highest for thanking patients for their participation (57.7%; 30/52).
Conclusions: Despite The BMJ’s requirement, reported patient involvement in clinical trial publications remains low. Patient authorship is being encouraged, but remains rare. Advocacy efforts for meaningful patient involvement during research, including publication planning and preparation, must continue.
Keywords:
Abstract
Objective: Patient engagement in rare disease research is vital to ensure that relevant clinical questions and patient-centred health outcomes are addressed in future studies. It could therefore be expected that more attention is given to the accessibility of target journals when submitting rare disease articles compared to those describing common conditions. As 75% of rare diseases affect childrenCitation1, we aimed to investigate the accessibility of paediatric rare disease publications.
Research design and methods: A pragmatic literature review was conducted in September 2017 using EMBASE and Ovid MEDLINE® to identify paediatric rare disease articles published in the English language in 2017. Abstracts were screened by a single reviewer.
Results: A total of 889 abstracts were identified, 181 of which met the eligibility criteria. Of these, 4% (7/181) were published in rare disease-specific journals (71% [5/7] of articles published in these journals were published in the Orphanet Journal of Rare Diseases), 72% (130/181) in journals specialising in a common disease therapy area (excluding rare diseases and paediatrics), and 24% (44/181) in general medical journals which did not specify therapy area. 54.5% (24/44) of the journals in the final category exclusively published paediatric manuscripts. 24% (43/181) of articles were published in open access journals whilst 1% (2/181) were published in journals requiring lay summaries.
Conclusions: Improved accessibility to orphan disease literature is essential for the rare disease community, as only a small proportion of rare disease articles are published in open access journals, with fewer still providing accompanying lay summaries. Further analyses into individual metrics of these journals and their correlation with visibility are recommended.
Keywords:
Reference
- Great Ormond Street Hospital. Scale of Rare Diseases. Available at: http://www.gosh.org/what-we-do/research/zayed-centre-research-rare-disease-children/rare-diseases/scale-rare-diseases. [Last accessed 2 October 2017]
* Oral Presentation
* Oral Presentation
Abstract
Objective: To analyse reporting of the Delphi method to achieve consensus on diagnosis and management guidelines in rare diseases.
Research design and methods: A pragmatic literature review was conducted in September 2017 by searching EMBASE and MEDLINE® databases. Publications were screened by a single reviewer to include English language articles, in which the Delphi method was used for the development of guidelines in rare diseases. Reporting of the Delphi method was critiqued against the AGREE Reporting Checklist.
Results: Searches identified 26 results published between 2009–2017, 12 of which fulfilled the inclusion criteria. 11 unique Delphi studies were identified, which investigated 7 Orphanet-classified rare diseases and 2 author-defined rare conditions; all studies reported consensus results. The majority (8/11 [73%]) used a two-stage modified Delphi method, while the remainder used a classic three-stage process. Literature searches guided the development of statements for Delphi panel review in the majority of studies, but only 2/11 (18%) conducted systematic literature reviews and merely 6/11 (55%) of studies reported the number of statements assessed. Furthermore, 7/11 (64%) did not report collecting panellist feedback to inform subsequent Delphi stages, 5/11 (45%) of studies did not describe the rating scales used, and 2/11 (18%) omitted reporting the level of consensus reached.
Conclusions: There is a need for improved reporting of Delphi methods, with the majority of identified studies lacking AGREE-recommended detail. Since data from randomised controlled trials are scarce in rare diseases, well-designed Delphi studies are a valuable tool, and as such, publication should be considered and carried out in line with existing and newly developed recommendations on best practice.
Keywords:
* Oral Presentation
* Oral Presentation
Abstract
Objective: We assessed whether peer-reviewed publications and patient-targeted publication summaries are of relevance and interest to patients and caregivers.
Research design and methods: 100 patients and 50 caregivers of patients with atopic dermatitis/eczema were recruited from a national US database. Participants completed an online survey.
Results: The majority of respondents had college (35%) or graduate school (33%) education. A marked proportion of respondents (45%) were familiar with peer-reviewed publications, and 37% actively searched for them. Publications were accessed via general internet search (60%); scientific journals (49%); libraries (19%); patient organization websites (13%); or PubMed (12%). Most patients accessed freely available articles (90%). Publications helped patients be involved in the decision-making regarding their disease management (51%) and treatment options (45%). Although 53% of patients were relatively confident of their ability to understand publications, they pointed out publications contained too much jargon (51%), and conclusions and relevance to patients were unclear (40%). Respondents indicated that patient summaries were/would be useful to help patients interpret key findings of peer-reviewed publications (82%). Respondents were likely to discuss patient summaries with their healthcare providers (90%) and would find them helpful when discussing treatment options (81%). More than half the patients (54%) said they spent a lot of time looking for relevant information. Our sample is skewed towards more educated patients. Therefore, data on different education levels and therapy areas should be collected separately.
Conclusions: The information in peer-reviewed publications is of interest to patients and can help them participate in treatment and disease-management decisions. Our research highlights the need to improve the accessibility, clarity, and relevance of this information to patients.
Keywords:
Abstract
Objective: Timing of publications is an important consideration in publication plans. Our objective was to identify key challenges and make recommendations for developing multiple related scientific manuscripts intended for simultaneous publication in the same journal.
Research design and methods: We evaluated manuscripts developed for simultaneous publication; experiences and challenges related to author management, development timelines, and journal feedback were documented.
Results: We assessed 2 pairs of manuscripts reporting primary clinical trial findings (total 4 manuscripts). Each pair of manuscripts shared a specific disease setting (oncology/haematology). One manuscript in each pair presented results for a different but related drug. Each pair of manuscripts was submitted simultaneously to the same journal; both pairs were rejected following peer review. Upon resubmission to second choice journals, 1 manuscript was ultimately accepted; 3 remain under consideration. No target journals provided guidance on simultaneous submissions, even after enquiry. During manuscript development, management of timelines and author expectations was challenging, as submission was dependent on simultaneous manuscript finalisation and approval. Upon submission to first choice journals, one editor stated that dual submissions are not usually accepted. The same peer reviewers appeared to evaluate both manuscripts in each submission, leading to requests to report data uniformly between manuscripts. Additional findings will be presented for another 6 manuscripts under development for simultaneous submission.
Conclusions: Simultaneous submissions may increase the impact of published research, but journals do not typically provide guidance on their suitability. We recommend enquiring with journals during development, and informing authors of delays to related manuscripts. The potential increased impact of simultaneous publication should be weighed against the risk of manuscript delays.
Keywords:
† Encored poster at the 14th Annual Meeting of ISMPP
Footnote§§ Winner, Best Poster
† Encored poster at the 14th Annual Meeting of ISMPP
§ Winner, Best Poster
Abstract
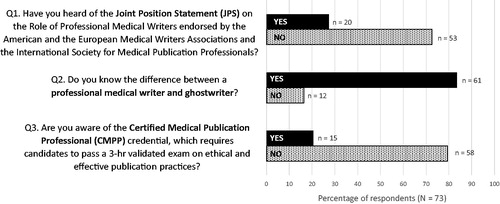
Objective: The Peer Review Congress (PRC), hosted by JAMA and The BMJ every 4 years, attracts world-leading journal editors and publication researchers. These influencers can affect how publication professionals are perceived. The PRC provided a rare opportunity to assess awareness of initiatives to differentiate and advance our profession.
Research design and methods: With PRC’s permission, we conducted face-to-face surveys of poster presenters (Chicago; September 11-12, 2017). A standardized data collection tool was used to obtain presenter consent, ask 3 binary-response questions (), and document demographic information.
Results: Of 84 presenters, 80 were available (response rate 95%) and 80 consented; duplicates were removed (73 unique responders; 29 from USA [40%], 42 from academia [58%]). Awareness was high for the difference between professional medical writers and ghostwriters, but low for the JPS and CMPP credential ().
Conclusions: Awareness of important publication professional initiatives is low among researchers in the publication ecosystem. These empirical results should help justify, guide, and advance advocacy efforts.
† Encored poster at the 14th Annual Meeting of ISMPP
† Encored poster at the 14th Annual Meeting of ISMPP
Abstract
Objective: Systematic literature reviews (SLRs) are a key component in the evidence-based assessment of healthcare interventions. Based on the utilization of a pre-defined structured methodology designed to identify all available research prior to the development of the study hypothesis, SLRs have become valuable resource in the critical impact analysis of therapeutic interventions across multiple research studies. Existing as standalone evidence, SLRs address broader clinical questions than single empirical studies, enabling SLRs to be prioritized above other research designs in the ‘hierarchy of evidence,’ due to the potential to provide practical evidence-based conclusions.
Research design and methods: Authors conducted an evidence-based literature search of all National Library of Medicine databases to determine if study investigators are conducting an SLR when designing the protocol for phase 2/3 clinical trials.
Results: A total of 16,856 publications utilizing “systematic literature review” in the title, MeSH heading, abstract body, or manuscript body were identified. No time limit was applied. Of these publications, 7995 utilized SLR in the title or abstract body, with 370 discussing systematic evidence and clinical trial design. Only 16 publications reported the results of an SLR that was conducted as part of the design of an interventional clinical trial (oncology: n = 3; emergency medicine, cardiovascular disease, dermatology: n = 2 each; endocrinology, gastroenterology, hematology, internal medicine, mental health, nephrology, rheumatology: n = 1 each), with 7 focusing on a therapeutic intervention.
Conclusions: Despite the well-documented importance of SLRs in evidence-based medicine and clinical practice decision-making, there is limited evidence to support the hypothesis that SLRs are being utilized when designing clinical trial protocols. A greater awareness of the potential role of SLRs in the design of clinical trial protocols is warranted.
Abstract
Objective: Manuscript submission can be costly and time-consuming, particularly when a new target journal is required. We used data from previous agency manuscript submissions and explored whether author h-index can help refine journal choice.
Research design and methods: This exploratory study examined original research articles produced with agency support that presented clinical trial data within the haematology field. These were identified using internal agency records. Impact factor (IF) data were collected from http://www.scijournal.org; h-index data were obtained from the Scopus database.
Results: A total of 33 articles were identified, published by 17 different journals from 2008 to 2017. Of these, 11 were published by the first-choice journal; 11, 6, and 5 manuscripts had 1, 2, and 3 prior submissions, respectively. Manuscripts with a low median author h-index (range 2–10) were more likely to have had more submissions. Few papers had a first author with a low h-index; this did not influence acceptance, and were on the first or second submission. The relationship between median author h-index and IF varied with number of submissions, with a positive relationship observed in manuscripts with ≥2 submissions compared with those with ≤1 prior submission.
Conclusions: Our data suggest that the relationship between author h-index and IF increases with number of prior submissions This could be due to the standard practice to initially target prestigious journals with high impact factor and visibility, whereas subsequent submissions may involve more agency input. Our data indicate that median author h-index is a more useful metric in predicting acceptance and choosing target journals compared with first or last author h-index.
Keywords:
Abstract
Objective: Analysing reasons for the low rate of publications in English from Spanish-speaking countries
Method: We conducted a Pubmed search with the criteria “Affiliation (Spanish speaking country)” and “Language (English)” and the filter “Last 5 years”. We selected 400 persons from that search who were corresponding authors. The selection matched the proportion of country of origin that we obtained from the Pubmed search. We sent a survey to them between September 1st and September 15th 2017. We received 22 answers (more than 5% response when the average for this kind of surveys is around 1%)
Results: The Pubmed search showed that 60% of Spanish-speaking physicians who have published in English during the last 5 years are from Spain. The rest (40%) are from Latin America
All responders to the survey were physicians with more than 10 years’ professional experience, more than 36 years old and had published more than 10 articles in the last 5 years.
The main obstacles that they encounter when they try to publish and article in English are: language (64%, n = 14), delay in receiving answers from the journals (64%, n = 14) and difficulties in addressing referee comments (45%, n = 10).
Finally, we asked about medical writing support in these countries. 77% of responders knew of companies or freelance medical writers in their countries but only 55% percent had used this kind of service. They think that these services could be helpful but there is not enough supply in their regions and prices are too high.
Conclusions: Physicians from Spanish-speaking countries needs help from professional medical writers to increase their publications in English but current supply and price are important constraints.
Notes
* Oral Presentation
† Encored poster at the 14th Annual Meeting of ISMPP
* Oral Presentation
† Encored poster at the 14th Annual Meeting of ISMPP
† Encored poster at the 14th Annual Meeting of ISMPP
† Encored poster at the 14th Annual Meeting of ISMPP
‡ Winner, Most Reflective of Meeting Theme
* Oral Presentation
† Encored poster at the 14th Annual Meeting of ISMPP
* Oral Presentation
* Oral Presentation
† Encored poster at the 14th Annual Meeting of ISMPP
§ Winner, Best Poster
† Encored poster at the 14th Annual Meeting of ISMPP