Abstract
Objective: This study assessed training time with the dulaglutide single-use pen (SUP) and the insulin degludec disposable prefilled pen (FlexTouch®) in self-injection–naïve patients with type 2 diabetes mellitus (T2DM) in Japan.
Methods: This multi-center, open-label, comparative, crossover study measured training time with the dulaglutide SUP vs FlexTouch®. Participants learned how to use both devices in a randomly assigned order. Healthcare providers (HCP) conducted the training. The primary end-point was the time required to train self-injection-naïve T2DM participants to self-inject correctly using each device. Secondary end-points included performance measures, such as success and error rates, patient perceptions related to ease-of-use, and factors associated with training time and performance.
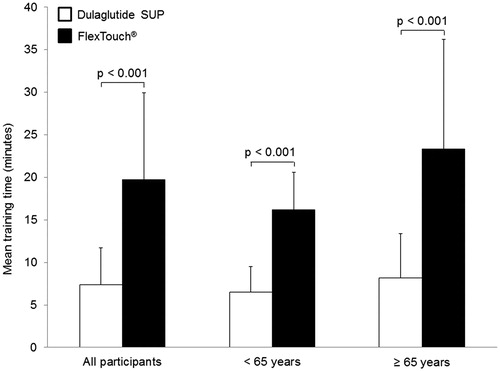
Results: Overall, 48 participants were randomized and completed the study. The mean training time to achieve correct administration was significantly shorter with the dulaglutide SUP vs FlexTouch® (7.4 min vs 19.7 min, p < .001). The proportions of participants who successfully completed the mock injection without error were similar for both devices. Ninety-two percent (44/48) of participants reported that the dulaglutide SUP was easier to use than FlexTouch®.
Conclusions: In this study, participants required a shorter training time to achieve correct administration with the dulaglutide SUP, and had a higher preference for the dulaglutide SUP, when compared to FlexTouch®. These data suggest that the dulaglutide SUP is easy-to-use, which may decrease the burden on HCPs to train diabetic patients how to administer injection therapy and reduce patient injection hurdles, such as needle fear.
Introduction
Diabetes mellitus is one of the most common chronic diseases, with an estimated global prevalence of 8.8% among people aged 20–79 years in 2017Citation1. In Japan, there were an estimated 7.2 million cases of diabetes in 2017, with a 7.7% prevalence among adults aged 20–79 yearsCitation1. Type 2 diabetes mellitus (T2DM) is the most common form of diabetes. The goal of managing T2DM is to maintain quality-of-life and life expectancy, which is similar to management goals of other diseases in non-diabetics. In order to achieve this goal, it is critical to maintain blood glucose levels within acceptable limits. Target glycemic control is not often achieved with lifestyle modifications and oral anti-T2DM medications. In such cases, Japan Diabetes Society (JDS) guidelines for the management of T2DM recommend intensifying treatment with injectable medications, such as insulin and glucagon like peptide-1 (GLP-1) receptor agonistsCitation2.
The dulaglutide (Trulicity) single-use pen (SUP) is a disposable device containing a pre-filled syringe that is designed for subcutaneous delivery of a 0.5 mL volume dose of the GLP-1 receptor agonist dulaglutideCitation3. The actual injection is user-initiated; however, the needle insertion, dose delivery, and needle retraction are automated via a spring-loaded mechanism after initiation. In a single-arm study in the US, the dulaglutide SUP was found to be a safe and effective device for use by patients with T2DM who were self-injection–naïveCitation4.
Several studies have compared diabetes injectable therapy devices, including those for insulin and GLP-1 receptor agonists, for patient ease-of-use, satisfaction, and preferenceCitation5–11. However, these studies did not investigate the dulaglutide SUP, or were single arm in design. Thus, the usability of the dulaglutide SUP compared to other devices has not yet been thoroughly explored. In addition, in phase 3 clinical trials of dulaglutide in Japan, prefilled syringes were used for the dulaglutide arm instead of the commercially available SUPCitation12,Citation13. Therefore, the usability of the dulaglutide SUP among Japanese patients with T2DM compared to other devices currently available as first-line injectables is unknown. Furthermore, there has been no comparison to date between the dulaglutide SUP and the insulin degludec (Tresiba) disposable prefilled pen (FlexTouch®), and, therefore, limited data is available on potential differences between auto-injector and semi-manual devices.
In Japan, studies of insulin devices have reported that HCPs have a relatively short amount of time available to train patients to self-injectCitation5,Citation9. Reducing patient training time is essential, since HCPs in Japan have one of the highest numbers of patient visits per physician globallyCitation14. The aims of this study were to compare training time, ease-of-use, and performance measures of the dulaglutide SUP and FlexTouch® in self-injection-naïve participants with T2DM in Japan.
Methods
Study design
This was a multi-center, open-label, comparative, crossover study designed to measure training time with two self-injection devices, the dulaglutide SUP (Trulicity, Eli Lilly and Company, Kobe, Japan) and the insulin degludec FlexTouch® (Tresiba, Novo Nordisk, Tokyo, Japan) (). The study did not involve active ingredient administration. The active ingredients and brand names of both devices were concealed from study participants by either obscuring the name on the device and the instructions-for-use (IFU), or labelling the device and the IFU with a dummy name, for example “device X”, so that participants were not aware that one of the devices was for administration of insulin. Since weakened thumb strength can limit a patients’ ability to press the button on each device, thumb strength was measured between training periodsCitation15.
Figure 1. Study design and sequence. Questionnaire 1 = Diabetes Treatment with Self-injection questionnaire; Questionnaire 2 = Sociodemographic and Clinical Information questionnaire; Questionnaire 3 = Device Usability questionnaire. Abbreviations: FlexTouch®, insulin degludec disposable prefilled pen; SUP, single-use pen.
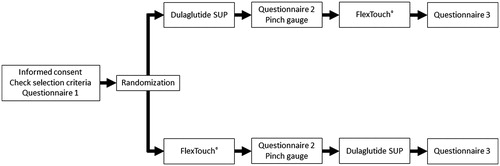
HCPs trained participants using the IFUs publicly available from each manufacturerCitation16,Citation17. Study participants were randomized in a 1:1 ratio, with half receiving training for the dulaglutide SUP first and the other half receiving training for FlexTouch® first. Training was performed at one visit as followsCitation5,Citation9:
Step 1. A designated trainer taught a participant each step of the injection procedure using the IFU for each device.
Step 2. The participant performed the mock injection into a rubber pad with support from the trainer.
Step 3. The participant performed the mock injection into a rubber pad without any support from the trainer.
Both devices used in this study and their IFUs are marketed and available in Japan. When developing IFUs for injectable devices, manufacturers work with Japanese diabetes experts (Professor Asakura T, Niigata University of Pharmacy and Applied Life Sciences) to ensure that the commercially available IFUs contain all information essential for patients to correctly administer the medicine. The device training procedures used in the study followed the current IFUs for both devices, which included all of the required steps to be taught to patients to achieve correct administration, as recommended by diabetes specialists.
Participants could read the IFUs at any time during the process described above. If a participant made a mistake during the mock injection procedure at step 3, the participant was asked to perform the procedure again until the procedure was mastered.
For this study, the training procedure was limited to self-administration though, in the real-world setting, HCPs also provide education on other topics, such as hypoglycemia management. For training with FlexTouch®, the dose was set at 10 IU (0.1 mL), reflecting the initial dose of insulin degludec used in a phase 3 clinical trialCitation18.
All participants gave written informed consent. This study was performed in accordance with the principles of the Declaration of Helsinki, the Ethical Guidelines for Medical and Health Research Involving Human Subjects in JapanCitation19, and with the approval of Institute Review Board Committees.
Participants
Study participants were recruited from two sites in Japan. Both sites were medical centers specializing in clinical trials: the P-One Clinic, Keikokai Medical Corporation in Tokyo (with a patient database) and the PS Clinic, Souseikai Medical Group in Fukuoka. The same number of participants was recruited at each site. Inclusion criteria included adults ≥20 years of age, diagnosed with T2DM, taking at least one oral anti-T2DM medication, able to read and understand Japanese, and willing and able to provide written informed consent. Exclusion criteria included cognitive impairment, hearing difficulty, visual impairment, severe psychopathology, gestational diabetes, type 1 diabetes mellitus, T2DM treated only with diet and exercise, prior treatment with regular self-injectable medication (e.g. insulin, epinephrine), or insulin pump for any medical condition including T2DM, and employment in a position with a direct role in treating patients with diabetes.
Trainers
In Japan, patients are usually trained to use devices by HCPs who are either a nurse or a pharmacist. Trainers in this study were, therefore, nurses or pharmacists, to reflect this practice. Two HCP trainers were recruited from each site. Each trainer provided training for 12 study participants who were screened in advance. The trainers were self-taught using the publicly available IFUs for each device. After self-learning, the lead investigator confirmed mastery of the training procedures by the trainers.
Data collection and measures
The primary end-point was time required to train injection-naïve T2DM patients to self-inject correctly using each device. This was measured as the time taken to complete the three steps listed aboveCitation5,Citation9, defined as the time between the trainer putting the IFU on the table and the time the participant put the device on the table at the end of the third step. Participants were also assessed by trainers using an injection procedural checklist, which lists the procedures described in the IFUs, to determine whether each procedure was completed with/without errorCitation20.
Before the first training period, study participants completed a Diabetes Treatment with Self-injection questionnaire. Between training periods, participants completed a Sociodemographic and Clinical Information questionnaire, and measured their thumb strength with a pinch gauge (Jamar Hydraulic Pinch Gauge, Patterson Medical)Citation21, which ensured study participants had enough time to minimize the learning effect. Following mastery of both devices, participants completed a Device Usability questionnaire developed by the study team and based on several previous studiesCitation22–26 ().
Statistical analyses
All subjects who provided consent, fulfilled the study entry criteria, and completed the training and questionnaires were included in the analyses. Descriptive statistics, including mean, standard deviation, and frequency, were used to summarize and characterize the sample in terms of sociodemographic and clinical characteristics. A 2-sided paired t-test with a 5% alpha error was used to determine if dulaglutide SUP training time was statistically different from that of FlexTouch®. Descriptive statistics, including means and percentages, were used to summarize the performance measure. Fisher’s exact test was used to determine whether there was a statistically significant difference in the percentage of participants who could complete all of the procedures (without any error) and perceptions between the two devices. Frequencies of procedural errors for each device were compared. A sub-group analysis by age (<65 years and ≥65 years) was used to evaluate its effect on training time.
Results
Participant characteristics and demographics
A total of 49 participants were randomized. One of the randomized participants was found to meet an exclusion criterion (was previously treated with regular self-injectable medication [e.g. insulin, epinephrine] or insulin pump for any medical condition including T2DM). This participant did not complete the study, and was excluded from the analysis. No imputation was applied for missing data. Baseline participant characteristics and demographics are shown in . Half (50.0%) of all participants were at least 65 years of age. The mean age was 63.5 years (range = 38–79 years) and 77.1% of the participants were male. The mean body mass index was 25.09 kg/m2 (standard deviation [SD] = 2.86). The majority of participants (55.6%) had baseline HbA1c levels of at least 7%. The proportions of participants who had one, two, or at least three concomitant oral diabetes medicines were 29.2%, 39.6%, and 31.3%, respectively. Seven participants (14.6%) reported they had comorbidities requiring treatment that affected their eyesight, such as glaucoma and cataract. Injectable medicines had not been recommended to any study participants in the prior 3 months.
Table 1. Baseline participant characteristics and demographics.
Training time
The mean training time to achieve correct administration with the dulaglutide SUP (7.4 min, SD = 4.3) was significantly shorter (p < .001) than with FlexTouch® (19.7 min, SD = 10.2) (). The sub-group analysis showed that mean training time was also significantly shorter for the dulaglutide SUP compared with FlexTouch® for participants aged <65 years (6.5 min vs 16.2 min, p < .001), as well as participants aged ≥65 years (8.2 min vs 23.3 min, p < .001) (). All trainers in this study were nurses.
Procedural errors
The proportions of participants who successfully completed the mock injection without a procedural error were similar for both devices (dulaglutide SUP, 54.2% vs FlexTouch®, 56.3%) (). The most common reported procedural errors involved disposing the cap as soon as it is pulled when preparing for injection for the dulaglutide SUP (16 of 48 participants) and putting the cap on the device after the injection for FlexTouch® (16 of 48 participants), both of which are not considered significant in achieving successful administration in practice.
Table 2. Procedural errors.
Perception of device usability
The results of questions regarding usability of the devices are shown in . For all questions, the proportion of participants strongly agreeing or agreeing was numerically higher for the dulaglutide SUP compared to FlexTouch®. The greatest difference in usability response was for the statement, “I don’t feel it takes much time to prepare the injection”, with 100% of participants strongly agreeing or agreeing for the dulaglutide SUP, compared with 56.3% for FlexTouch®. The mean score for willingness to use each device was higher for the dulaglutide SUP (5.8) than FlexTouch® (3.4), based on a 0–10 visual analog scale (a higher score indicates a greater willingness). Of the 48 participants, 44 (92%) reported that the dulaglutide SUP was more user-friendly than FlexTouch®.
Table 3. Device usability.
Injection hurdle among injection-naïve patients
Participants’ willingness to take injectable medicines before training was 0.6 (mean) on a 0–10 visual analog scale (a higher score indicates a greater willingness) (). This score significantly improved to 1.6 (mean) after training with both devices (p = .007) ().
Discussion
Our study demonstrated that the training time required to achieve correct administration was significantly shorter with the dulaglutide SUP compared with FlexTouch® (means of 7.4 min and 19.7 min, respectively) among Japanese participants, regardless of age category (<65 years vs ≥65 years). The success rate for finishing the mock injection without error was similar for the two devices. The most commonly reported procedural error for the dulaglutide SUP was immediate cap disposal before injection, which is not considered clinically relevant to successful administration. Over 90% of participants reported that the dulaglutide SUP was more user-friendly than FlexTouch®. According to an estimation by the Japanese governmentCitation27, 66.1% of patients with T2DM (∼1.4 million of 2.2 million) are older than 65 years. Half of the participants in this study were aged 65 years or older and, hence, these findings are generalizable to injection-naïve patients with T2DM in Japan. The training time for FlexTouch® in our study is consistent with that in a previous study, which reported a training time of 18.3 min (SD = 4.3) in self-injection-naïve patients using FlexPen, the previous version of FlexTouch®Citation5. In the previous study, FlexPen had the shortest training time, when compared with four other insulin pen devicesCitation5. Therefore, superiority of dulaglutide SUP compared to FlexTouch®, the modified version of the insulin pen device previously shown to have the shortest training time of several devicesCitation5, may imply that the dulaglutide SUP has an advantage when compared with all other insulin pen devices currently available in Japan.
Despite advances in insulins and injection devices, many physicians are reported not to initiate injection therapies in a timely mannerCitation28. In the Diabetes Attitudes, Wishes and Needs (DAWN) JAPAN study, Ishii et al.Citation29 explored barriers to insulin initiation for physicians in Japan. A total of 52%, 20%, and 16% of non-JDS-affiliated physicians, JDS-affiliated physicians, and JDS-certified specialists, respectively, agreed that it is time-consuming to explain injection methods and use of injection devices; furthermore, 56%, 23%, and 17%, respectively, agreed that it is difficult to provide guidance and education on insulin injection to patients. These data suggest that difficulty in training patients for self-injection is one of the barriers to initiating insulin therapy. This barrier is greater for non-specialists, but is noteworthy even among specialists. Removing barriers to first-line injectable T2DM therapy is, therefore, vital in achieving optimal treatment. A typical self-injection procedure of insulin consists of the following five steps: (1) attach the needle, (2) check the air flow, (3) select the dose, (4) inject, and (5) dispose of the device. The dulaglutide SUP is a single-use, disposable, and ready-to-use device that does not require the first three steps. A patient only has to remove the cap, hold the device on their skin, and unlock the device to prepare the self-injection. The simpler procedure may explain why training time was significantly shorter with the dulaglutide SUP compared with FlexTouch® in this study, regardless of age category (<65 years vs ≥65 years). This is consistent with the pivotal study of dulaglutide, in which 92% of site trainers found it easy to train patients to use the dulaglutide SUPCitation30. When insulin therapy is initiated, patients receive training on how to use the relevant device, as well as additional training on subjects such as how to manage hypoglycemia. GLP-1 receptor agonists also require hypoglycemia management training, albeit this training is simpler compared to that of insulin therapy, because the risk of hypoglycemia is relatively low with GLP-1 receptor agonistsCitation31. In this study, the additional trainings above were not included; consequently, in the real-world setting, the difference in training time is likely to be even more pronounced.
The simpler procedure, which was measured in this study as shorter training time, associated with the dulaglutide SUP may explain why the dulaglutide SUP was preferred over FlexTouch® by more than 90% of the participants in this study. This is consistent with a previous study that showed both younger and older patients preferred the dulaglutide SUP over SoloSTAR, highlighting patient preference for a simple single-use deviceCitation32. Several studies have shown that FlexTouch® is superior to other insulin pen devices (i.e. SoloSTAR, MirioPen, and InnoLet) regarding overall preference, ease-of-use, performing injections, and confidenceCitation24,Citation26,Citation33. This study demonstrated that the dulaglutide SUP is superior to FlexTouch® for each of these measures, as well as for time to prepare an injection and fear of the needle. In a randomized, double-blind study, 95% of patients reported that the dulaglutide SUP was “easy” or “very easy” to use at 28 weeks, and more than 90% of patients were “mostly willing” or “definitely willing” to continue using either device at 28 weeksCitation34. Collectively, these data indicate that multiple attributes contribute to patient satisfaction with the dulaglutide SUP.
Injection-naïve Japanese participants with T2DM were reluctant to use injectable medications at baseline, which is consistent with previous researchCitation22. In this study, a higher willingness to use injectable medications was observed in the post-study period compared to the pre-study period. Injection-naïve patients are likely to have concerns about using injection therapy due to fears of pain and/or device complexityCitation4. Therefore, the higher willingness to use injectable medicines in the post-study period indicates that a greater understanding of the dulaglutide SUP device may decrease injection hurdles for patients.
Despite the relatively simpler procedure for the dulaglutide SUP compared with FlexTouch®, the rate of participants who successfully administered treatment without error was similar for both devices, albeit numerically lower for the dulaglutide SUP. This may be because the trainings for both devices were sufficiently comprehensible for the participants and that participants were successfully trained by the trainers. The fewer number of steps in procedures did not necessarily lead to a lower error rate. Errors related to disposal of the needle and/or device were the most common procedural errors for both devices. Within the procedures that might affect administration, the most common error with the dulaglutide SUP was related to locking/unlocking the device, while the most common error for FlexTouch® was related to keeping the device pressed against the skin for a sufficient time. With FlexTouch®, procedural errors that might lead to incorrect dosing were observed, whereas the dulaglutide SUP automates most procedures associated with correct dose administration (e.g. “Keep the needle under the skin for a full count (6 s)”). Thus, HCPs may be more confident that the dulaglutide SUP can achieve glucose control because it automates the procedures that ensure correct dose administration. In this study, the trainings were standardized based on the IFUs, whereas in the real-world setting training may be less formal. Therefore, there may be more procedural errors in the real-world setting. Further study is required to understand the relationship between the simplicity of the administration procedures and the corresponding error rate.
Although drug names, active ingredients, and label information were removed from the devices and IFUs, one limitation of our study was that the devices were not masked and therefore were potentially identifiable, hence possibly introducing bias if participants had prior knowledge of GLP-1 receptor agonists and insulins. However, any bias would have been limited, because only one participant had actively searched for information about subcutaneous self-injected T2DM medicine in the 3 months prior to the study. The study did not involve drug administration, and was not designed to investigate the efficacy of active ingredients, but to assign mock tasks to study participants. The study investigated only a SUP compared to a multi-use FlexTouch® pen with the facility for variable dosing. Thus, preference for a particular device may not necessarily equate with its content being superior to the content within the comparator device. Since each training process may vary by hospital, variability of actual training time in the real world would exist. Finally, the relatively small sample size may have precluded the ability to demonstrate associations between device type and participant demographics.
Conclusions
Our study demonstrates that the dulaglutide SUP is associated with a shorter dose administration training time and is more preferable than FlexTouch® among Japanese participants. The results of this study provide a greater understanding of the usability of the dulaglutide SUP in Japan, and suggest that it is less burdensome for HCPs to train patients to correctly use the dulaglutide SUP compared to other devices and easier for patients to learn self-injection with this device.
Transparency
Declaration of funding
This work was funded by Eli Lilly Japan, K.K.
Declaration of financial/other relationships
T.A. received lecture fees from Novo Nordisk Pharma Ltd. and Eli Lilly Japan, K.K., and received a manuscript fee from FUJIFILM RI Pharma Co., Ltd. T.A., Z.C., and S.S. are full-time employees of Eli Lilly Japan, K.K. Peer reviewers on this manuscript have received an honorarium from CMRO for their review work, but have no other relevant financial relationships to disclose.
Acknowledgments
We thank the participants, the study sites, and the study personnel who participated in this clinical trial. Medical writing support was provided by Andrew Sakko, and editorial support was provided by Noelle Gasco, of Syneos Health and funded by Eli Lilly Japan, K.K.
References
- International Diabetes Federation [Internet]. IDF Diabetes Atlas, 8th edn. IDF; 2017. Brussels, Belgium. Available online at: www.diabetesatlas.org accessed December 1, 2017
- Japan Diabetes Society [Internet]. Treatment guide for diabetes, 2014–2015. 2015. Tokyo, Japan. Available online at: www.jds.or.jp/modules/en/index.php?content_id = 1 access December 1, 2017
- Trulicity Ateos Eli Lilly Japan K.K. Ver 4. Revised March 2017. Kobe, Japan. Available online at: www.info.pmda.go.jp/downfiles/ph/PDF/530471_2499416G1029_1_10.pdf. Japanese accessed December 1, 2017
- Matfin G, Van Brunt K, Zimmermann AG, et al. Safe and effective use of the once weekly dulaglutide single-dose pen in injection-naïve patients with type 2 diabetes. J Diabetes Sci Technol 2015;9:1071-9
- Asakura T. The association between injection device and injection training time of insulin initial self-injection training. Prog Med 2003;23:3381-5. Japanese
- Asakura T, Seino H, Jensen KH. Patient acceptance and issues of education of two durable insulin pen devices. Diabetes Technol Ther 2008;10:299-304
- Clark PE, Valentine V, Bodie JN, et al. Ease of use and patient preference injection simulation study comparing two prefilled insulin pens. Curr Med Res Opin 2010;26:1745-53
- Ignaut DA, Schwartz SL, Sarwat S, et al. Comparative device assessments: Humalog KwikPen compared with vial and syringe and FlexPen. Diabetes Educ 2009;35:789-98
- Yamada KC, Aoki K. Evaluation of usability of insulin self-injection devices. Jpn J Ergonom 2006;42:112-18. Japanese
- Lorenzi GSB, Osther J, Boardman M. Application of adult-learning principles to patient instructions: a usability study for an exenatide once-weekly injection device. Clin Diabetes 2010;28:157-62
- Stauder U, Enginee D, Elton H, et al. Comparative assessment of lixisenatide, exenatide, and liraglutide pen devices: a pilot user-based study. J Diabetes Sci Technol 2014;8:123-31
- Araki E, Inagaki N, Tanizawa Y, et al. Efficacy and safety of once-weekly dulaglutide in combination with sulphonylurea and/or biguanide compared with once-daily insulin glargine in Japanese patients with type 2 diabetes: a randomized, open-label, phase III, non-inferiority study. Diabetes Obes Metab 2015;17:994-1002
- Miyagawa J, Odawara M, Takamura T, et al. Once-weekly glucagon-like peptide-1 receptor agonist dulaglutide is non-inferior to once-daily liraglutide and superior to placebo in Japanese patients with type 2 diabetes: a 26-week randomized phase III study. Diabetes Obes Metab 2015;17:974-83
- Xie J, Suzuki S, Cos X, et al. Cross-national variation in physicians’ characteristics and their goals for patients taking insulin: evidence from the MOSAIc study [abstract 1538-P]. Poster presented at the 74th American Diabetes Association; Jun 13–17, 2014; San Francisco, CA.
- Muto T, Asakura T, Toraishi K, et al. Comparison of new portable prefilled insulin pen with conventional insulin pens. Jpn J Pharm Health Care Sci 2006;32:1065-70. Japanese
- Eli Lilly Japan K. K. Instructions for Use, Trulicity® [Internet]. 2017. Kobe, Japan. Available online at: https://www.diabetes.co.jp/hcp/tlc/shared/pdf/material/material01_170626.pdf. Japanese accessed December 1, 2017
- Novo Nordisk. Instructions for Use, Tresiba® FlexTouch® [Internet]. 2016. Tokyo, Japan. Available online at: https://www.novonordiskpro.jp/content/dam/nnpro/japan/ja/products/FlexTouch®/Docs/FlexTouch®_Instruction-Manual_Pt_2016.pdf. Japanese accessed December 1, 2017
- Onishi Y, Iwamoto Y, Yoo SJ, et al. Insulin degludec compared with insulin glargine in insulin-naïve patients with type 2 diabetes: a 26-week, randomized, controlled, Pan-Asian, treat-to-target trial. J Diabetes Investig 2013;4:605-12
- Ministry of Education, Culture, Sports, Science and Technology, and Ministry of Health, Labour and Welfare [Internet]. Ethical guidelines for medical and health research involving human subjects. 2014. Tokyo, Japan. Available online at: http://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkanboukouseikagakuka/0000069410.pdf. Japanese accessed December 1, 2017
- Andre AD, Wang C, Berry B, et al. Comparative usability study of two once-weekly GLP-1RA pen injector products: Tanzeum™ and Bydureon® [abstract 1138-P]. Poster presented at the 75th American Diabetes Association; Jun 5–9, 2015; Boston, MA
- Smaby N, Johanson ME, Baker B, et al. Identification of key pinch forces required to complete functional tasks. J Rehabil Res Dev 2004;41:215-24
- Gelhorn HL, Bacci ED, Poon JL, et al. Evaluating preferences for profiles of glucagon-like peptide-1 receptor agonists among injection-naive type 2 diabetes patients in Japan. Patient Prefer Adherence 2016;10:1337-48
- Polinski JM, Curtis BH, Seeger JD, et al. Rationale and design of the multinational observational study assessing insulin use: the MOSAIc study. BMC Endocr Disord 2012;12:20
- Asakura T, Hyllestad-Winge J, Hoshino T. Usability and performance evaluation on prefilled insulin devices: FlexTouch®, SoloSTAR® and MirioPen® among diabetic patients and health care professionals in Japan. Jpn J Pharm Diabetes 2014;3:147-56. Japanese
- Bailey T, Thurman J, Niemeyer M, et al. Usability and preference evaluation of a prefilled insulin pen with a novel injection mechanism by people with diabetes and healthcare professionals. Curr Med Res Opin 2011;27:2043-52
- Oyer D, Narendran P, Qvist M, et al. Ease of use and preference of a new versus widely available prefilled insulin pen assessed by people with diabetes, physicians and nurses. Expert Opin Drug Deliv 2011;8:1259-69
- The Ministry of Health and Welfare [Internet]. Patient survey; 2014. Tokyo, Japan. Available online at: www.e-stat.go.jp/SG1/estat/List.do?lid = 000001141596. Japanese accessed December 1, 2017
- Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med 2001;135:825-34
- Ishii H, Iwamoto Y, Tajima N. An exploration of barriers to insulin initiation for physicians in Japan: findings from the Diabetes Attitudes, Wishes And Needs (DAWN) JAPAN study. PLoS One 2012;7:e36361
- Van Brunt K, Ignaut, DA, Zimmermann AG, et al. Patient experience with the single-use pen for injection of once weekly dulaglutide in injection-naïve patients with type 2 diabetes [abstract PDB125]. Poster presented at the International Society for Pharmacoeconomics 17th Annual European Congress; November 8–12, 2014; Amsterdam, The Netherlands
- Ahrén B. Avoiding hypoglycemia: a key to success for glucose-lowering therapy in type 2 diabetes. Vasc Health Risk Manag 2013;9:155-63
- Norrbacka K, Poon J, Gelhorn H, et al. Evaluating patients' preferences for dulaglutide versus insulin glargine medication profiles in T2D patient subgroups [abstract P-1210]. Poster presented at the International Diabetes Federation Congress; Dec 4–8, 2017; Abu Dhabi, United Arab Emirates
- Asakura T, Hoshino T, Niemeyer M. Comparison between FlexTouch® and InnoLet® in Japanese patients with diabetes stratified by disability in finger functions. Prog Med 2013;33:2245-54. Japanese
- Yu M, Brunt KV, Milicevic Z, et al. Patient-reported outcomes in patients with type 2 diabetes treated with dulaglutide added to titrated insulin glargine (AWARD-9). Clin Ther 2017;39:2284-95