Abstract
Objectives: Voriconazole, amphotericin B (AmB) formulations, and isavuconazole are all included in guideline recommendations for treatment of patients with invasive aspergillosis (IA) but the relative efficacy of isavuconazole versus AmB formulations has not been directly compared. We aimed to estimate the relative efficacy of isavuconazole compared with AmB deoxycholate (AmB-D), liposomal AmB (L-AmB), and voriconazole for the treatment of patients with proven/probable IA.
Methods: Nine literature databases were screened for randomized controlled trials comparing treatments with any of voriconazole, AmB-D, L-AmB and isavuconazole for treatment of proven/probable IA. Articles meeting the criteria were included in a meta-analysis to determine the efficacy of AmB-D, L-AmB and voriconazole relative to isavuconazole based on all-cause mortality (ACM) and overall response using a fixed-effects model.
Results: Four articles were identified that compared L-AmB with AmB-D (Study 1), standard-dose L-AmB (3–5 mg/kg/day) with high-dose L-AmB (10 mg/kg/day; Study 2), voriconazole with AmB-D (Study 3), and isavuconazole with voriconazole (Study 4). In the network meta-analysis, isavuconazole was statistically superior to AmB-D on both ACM (odds ratio [95% credible intervals] shown as natural log, 1.00 [0.26, 1.74]) and overall response (−1.39 [−2.21, −0.63]). Differences between isavuconazole, and standard-dose L-AmB, high-dose L-AmB and voriconazole were not statistically significant for either ACM (0.18 [−1.17, 1.53], 0.50 [−1.11, 2.13] and 0.32 [−0.19, 0.84], respectively) or overall response (−0.99 [−2.21, 0.29], −0.89 [−2.41, 0.65] and 0.06 [−0.43, 0.57], respectively).
Conclusions: This data suggests that the efficacy of isavuconazole for treatment of IA is superior to AmB-D and comparable with both L-AmB and voriconazole.
Introduction
Invasive aspergillosis (IA) is a major cause of morbidity and mortality in immunocompromised patientsCitation1. Although mortality rates in patients with IA have declined within the last two decades with the substitution of amphotericin B deoxycholate (AmB-D) with voriconazole as the standard of careCitation1, treatment remains sub-optimal for patients due to adverse events and drug–drug interactions with immunosuppressive drugsCitation2,Citation3. More recently, isavuconazole was shown to have non-inferior efficacy compared with voriconazole as well as significantly fewer study drug-related adverse events and significantly fewer disorders of the hepatobiliary system, eye, and skin and subcutaneous tissuesCitation4. Guidelines for treatment of IA from the Infectious Diseases Society of America (IDSA) recommend voriconazole as primary therapy (strong recommendation; high-quality evidence), and as alternatives for primary therapy, isavuconazole or liposomal AmB (L-AmB) (3–5 mg/kg) (strong recommendation; moderate-quality evidence for both medications)Citation5. The guidelines indicate that AmB-D should only be used in resource-limited settings in which no alternative agents are available, and that triazoles are generally the preferred agents for treatment and prevention of IA in most patients. Newly issued IA treatment recommendations from the 6th European Conference on Infections in Leukemia (ECIL-6) indicate that the strongest evidence supports the use of voriconazole or isavuconazole for first-line treatment of IA in leukemia and hematopoietic stem cell transplant patients and there is weaker evidence to support the use of lipid-based formulations of AmBCitation6. Recent joint guidelines from the European Society for Clinical Microbiology and Infectious Diseases, European Confederation of Medical Mycology and the European Respiratory Society strongly support the use of voriconazole or isavuconazole as first-line treatment of pulmonary IA, whereas voriconazole is the only pharmacologic agent with a strong recommendation for extrapulmonary IA; lipid-based formulations of AmB are only moderately supportedCitation7.
Although comparable efficacy has been demonstrated for voriconazole and isavuconazole in the treatment of IA in a randomized controlled trial (RCT), there are no studies that directly compare the efficacy of isavuconazole with that of AmB formulations in IA. Nevertheless, information regarding the relative efficacy of different therapies can sometimes be obtained indirectly using network meta-analysis, also called mixed treatment comparison meta-analysisCitation8,Citation9. Network meta-analyses have become an established method to compare outcomes for interventions that have not been tested in head-to-head studiesCitation10. In this approach, the efficacy of an intervention relative to others against which it has not been directly compared is inferred by analyses of RCTs that have comparators in common and that are identified via a systematic literature review. The current analysis used this approach to identify RCTs that assessed clinical outcomes in patients with proven and probable IA treated with isavuconazole, L-AmB, AmB-D or voriconazole, and use data from those studies to assess the relative efficacy of isavuconazole (effects on all-cause mortality [ACM] and overall response) compared with those agents.
Methods
The systematic literature review and network meta-analysis was performed in accordance with standards set out by the National Institute for Health and Care Excellence, the Centre for Reviews and Dissemination (CRD), and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelinesCitation11–14.
Systematic review: database searches and study selection
Search strategies were designed and performed for each database screened (Supplementary Material), which included Embase, MEDLINE, CDSR, DARE, NHS EED, HTA, CENTRAL, ICTRP and NGC, and searches were supplemented by hand searching reference lists. The initial search was conducted on 6 January 2015; criteria are summarized in . Strategies were designed to identify RCTs that had used isavuconazole or any of the comparators as monotherapy for the treatment of probable or proven IA. Inclusion criteria were RCTs with human subjects published in English since 1995, treatment of patients with invasive fungal disease (IFD) caused by Aspergillus spp., and measures of clinical effectiveness of isavuconazole or any of the comparators (mortality, overall response, rate of hospitalization, discontinuation [due to adverse events or any reason], adverse events or serious adverse events). Studies excluded were those not available in English, published before 1 January 1995, not conducted as RCTs, or not including comparators or interventions of interest. Eligible studies were assessed for bias as per guidance provided by the Centre for Reviews and DisseminationCitation12.
Table 1. Parameters and criteria for the systematic review.
Network meta-analysis
Data extracted from RCTs were subjected to network analysis within a Bayesian framework using Markov-chain Monte Carlo methods as per Lu and AdesCitation15 and Dias et al. (2011Citation16 and 2013Citation17). The models were run with OpenBUGS version 3.2.3 (http://www.openbugs.net/w/FrontPage). Where possible, outcome data was obtained from patients with proven or probable IFDs as defined using the 2008 criteria of the European Organisation for Research and Treatment of Cancer (EORTC) and the Mycoses Study Group (MSG)Citation18. For studies performed and published prior to the publication of the 2008 EORTC/MSG guidelines, IFDs were categorized using the 2002 EORTC/MSG guidelinesCitation19. For studies that had been reanalyzed and republished after the publication of the 2008 guidelines, data was extracted from the parental study when not available from the re-analysis. Because only one study was found for each comparison of treatments, only a fixed-effects model was used. The outcomes assessed and compared were ACM and overall response (complete and partial responses, as defined within each study). The rates of adverse events or discontinuations (overall or due to adverse events) were not assessed because there was a lack of comparable information in the identified trials with which to perform the analysis. To address the potential role of the certainty of diagnosis, sensitivity analyses were performed that included patients previously excluded due to lack of proven or probable IFD. Statistical significance was assessed using 95% posterior credible intervals (CrIs; a Bayesian statistical term analogous to confidence intervals in frequentist statistics). Odds ratios were calculated for the main outcomes using a logistic regression model.
Results
Systematic review
The initial search identified 455 potentially eligible articles; two additional articles published after the initial search was performed were also included. After screening of titles, abstracts, and keywords to exclude duplicates and articles that did not meet inclusion criteria (), 42 articles were included for full-text screening (; Supplementary Material). Of these, eight articles covering four clinical trials were identified as meeting the eligibility criteria (). For trials with multiple published articles in which different diagnostic criteria had been applied, articles selected for inclusion in the meta-analysis were those that analyzed outcomes in patients assessed as having probable or proven IFD or IA using 2008 criteria set out by the EORTC/MSGCitation18. Thus, the final data set included one article each from four studies (). Study 1 was a clinical trial that compared AmB-D (1 mg/kg) with L-AmB (5 mg/kg) in patients with neutropenia-associated IFD, including most with documented or highly suspected IA (AmB-D, 76.5%; L-AmB, 78.1%)Citation20. Study 2 was a clinical trial that compared standard and high doses of L-AmB (3 mg/kg vs. 10 mg/kg) for treatment of patients with IFD and for which two articles were foundCitation21,Citation22. In the parent study, 97% of patients met the protocol-specific criteria for probable or proven IA. Outcomes for patients with proven and probable IFD using 2008 EORTC/MSG criteria were published in a re-analysis of the original study dataCitation22. Study 3 was a clinical trial that compared voriconazole (4 mg/kg BID maintenance dose) with AmB-D (1.0–1.5 mg/kg) in patients with IA and for which three articles were foundCitation23–25. Outcomes for patients with proven and probable IA using 2008 EORTC/MSG criteria were published in a re-analysis of the original study dataCitation25. Study 4 was a clinical trial of isavuconazole (200 mg QD maintenance dose) versus voriconazole (4 mg/kg BID maintenance dose) in patients with IFD and for which two articles were foundCitation4,Citation26. In the primary publication, most patients with proven or probable IFD (determined by an independent data review committee) had documented IA or positive serum galactomannan test results (isavuconazole group, 86.7%; voriconazole group, 83.7%)Citation4. For the purposes of this analysis, all patients from Study 1 (AmB-D vs. L-AmB) were included, whereas for Studies 2–4, only patients with probable or proven IFD or IA who received at least one dose of study drug (modified intent-to-treat [mITT] populations) were included.
Figure 1. Flow diagram of studies included and excluded at each step. aTwo articles published after the performance of the systematic search were also included: Herbrecht et al. 2015Citation25 and Maertens et al. 2016Citation4.
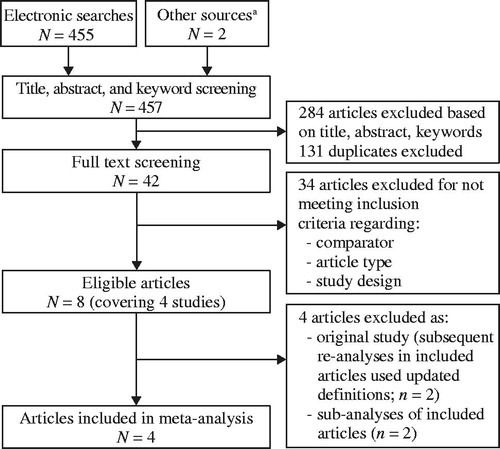
Figure 2. Comparisons in studies identified in the systematic review included in the network analysis. Abbreviations. AmB-D, amphotericin B deoxycholate; ISAV, isavuconazole; L-AmB, liposomal amphotericin B; VRC, voriconazole. aMaertens et al. 20164. bHerbrecht et al. 201525. cLeenders et al. 199820. dCornely et al. 201122.
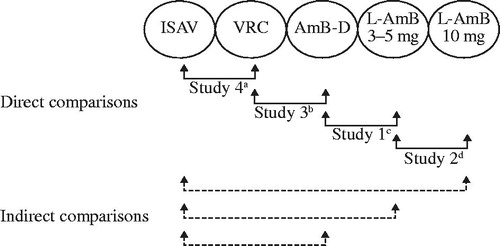
Table 2. Studies identified for the analysis.
Baseline and treatment details
The average ages of treatment groups (means or medians) were all within a range of 42–52.5 years (). Study 1 included predominantly females, whereas more than half of patients in Studies 2–4 were males. Data on body weight were only available for Studies 2 and 3, and were similar ().
Available data regarding certainty of diagnosis was variable due to the evolution of diagnostic criteria over time and approaches to analysis of data across Studies 1–4 (). Data regarding infection sites and underlying diseases was largely similar. In more than half of patients in each study, the most common infection site was the lungs and the most common underlying disease was hematologic malignancy. In Study 1, neutropenia within 14 days of enrollment was a study inclusion criterion, and so almost all patients were neutropenic at baseline. In Studies 2–4, between approximately half and two-thirds of patients in each study group were neutropenic ().
Table 3. Infection sites and underlying diseases in patients with proven or probable invasive fungal diseasesTable Footnotea.
Data regarding the average dose of study drug and concomitant medications were not consistently reported (). For AmB-D and L-AmB treatment arms in Studies 1–3, the median treatment duration ranged from 10–15 days, whereas the treatment duration with triazole antifungal drugs in Studies 3 and 4 (voriconazole and isavuconazole) ranged from 58–87 days.
Risk of bias assessment within individual studies
In the risk of bias assessments for each study, randomization was appropriate for all studies and therefore posed a low risk (). Treatment groups in each study were reported as having similar baseline characteristics, suggesting a low risk of bias due to imbalances. Risks of bias were identified for Study 1 because of the open-label trial design (no blinding or concealment) and primary analysis of the per-protocol population instead of the ITT population. For Study 3, although assessors of outcomes were blinded to treatment allocation, the study was otherwise unblinded. In addition, baseline characteristics were reported for the mITT population rather than the ITT population, so it could not be established unambiguously that there was not an imbalance in study dropouts. There was no evidence that any of the studies had a risk of bias attributable to unreported outcomes, and no other risks of bias were detected.
Efficacy outcomes of individual studies and network meta-analysis
Studies 2–4 included data on ACM at 12 weeks (84 days)Citation4,Citation22,Citation25, whereas Study 1 included ACM data at the end of study (treatment period plus 4 week follow-up period) and used logistic regression to factor in the influence of malignancy statusCitation20. A significant difference between treatment arms was reported in Study 1 (favoring L-AmB over AmB-D) and in Study 3 (favoring voriconazole over AmB-D), whereas the treatment difference was not significant in Study 4 (isavuconazole vs. voriconazole) and significance was not reported in Study 2 (L-AmB 3 mg/kg vs 10 mg/kg) (). In the network meta-analysis, isavuconazole demonstrated a statistically significant advantage over AmB-D, whereas differences with L-AmB (3 mg/kg and 10 mg/kg) and voriconazole did not reach significance (). To determine whether the certainty of diagnosis affected comparisons, sensitivity analyses were performed that also included patients with possible IFD (Studies 2 and 3) or the ITT population (Study 4; all randomized patients who received at least one dose of study medication, irrespective of diagnosis) (). Overall treatment comparisons were similar to those for patients with proven or probable IFD/IA (Supplementary Figure S1).
Figure 3. All-cause mortality in individual studies (A) and odds ratiosa for comparisons with isavuconazole in the network meta-analysis (B). Odds ratio shown as natural log. aCalculated using exact logistic regression factoring in malignancy status. Abbreviations. AmB-D, amphotericin B deoxycholate; CrI, credible interval; ISAV, isavuconazole; L-AmB, liposomal amphotericin B; NR, not reported; NS, not significant; VRC, voriconazole.
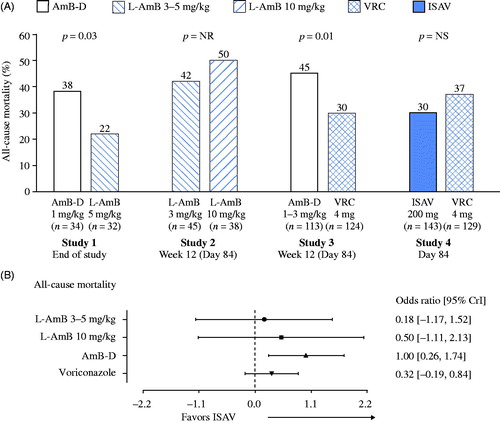
Table 4. Clinical/pharmacologic aspects of isavuconazole, voriconazole, amphotericin B deoxycholate and liposomal amphotericin B in the treatment of invasive mold diseaseTable Footnotea.
For assessments of overall response, Studies 1, 2 and 4 included assessments at end of treatment, whereas detailed data on complete and partial responses for Study 3 was available only at 12 weeks. A significant difference favoring voriconazole over AmB-D was reported in Study 3, whereas the treatment difference between isavuconazole and voriconazole was not significant in Study 4, and significance of differences for combined complete and partial responses was not reported for Studies 1 (L-AmB vs. AmB-D) and 2 (L-AmB 3 mg/kg vs. 10 mg/kg) (). In the network meta-analysis, isavuconazole again demonstrated a statistically significant advantage over AmB-D but not L-AmB (3 mg/kg and 10 mg/kg) or voriconazole (). In sensitivity analyses to assess the potential role of the certainty of diagnosis, patients with possible IFD/IA from Studies 2 and 3 were also included (Supplementary Table S5). Again, isavuconazole was found to have a significant advantage over AmB-D but not L-AmB or voriconazole (Supplementary Figure S2).
Figure 4. Overall response in individual studies (A) and odds ratios for comparisons with isavuconazole in the network meta-analysis (B). Odds ratio shown as natural log. Abbreviations. AmB-D, amphotericin B deoxycholate; CrI, credible interval; ISAV, isavuconazole; L-AmB, liposomal amphotericin B; NR, not reported; NS, not significant; VRC, voriconazole.
An analysis of the risk of bias across studies could not be performed because of the limited number of studies available for comparison.
Discussion
In this study, network meta-analysis of RCTs allowed the clinical outcomes of isavuconazole relative to AmB-D, L-AmB and voriconazole to be assessed. Outcomes of mortality and overall response were statistically significantly better for isavuconazole than AmB-D, whereas differences between isavuconazole and other comparators were not statistically significant. Thus, these results support the comparable efficacy of voriconazole, isavuconazole and L-AmB as first-line treatment for patients with IA.
Given the similar efficacy of these agents for treatment of IA demonstrated in this network meta-analysis, selection of an appropriate therapy may be best guided by consideration of other factors associated with each, including flexibility in the route of administration (intravenous and oral), safety/tolerability and potential for drug–drug interactions (). The availability of oral formulations for voriconazole and isavuconazole may be an advantage over L-AmB, particularly for patients requiring prolonged treatment courses or outpatient treatment. The median duration of therapy with AmB formulations in Studies 1, 2 and 3 (range, 10.0–16.5 days)Citation20,Citation22,Citation23 was considerably shorter than those for voriconazole or isavuconazole in Studies 3 and 4 (range, 45–77 days)Citation4,Citation23, but approximately 80% of patients who received AmB-D in Study 3 switched to other licensed antifungal agents due to intolerance or insufficient responseCitation24. Although L-AmB is less nephrotoxic than AmB-D and has fewer infusion-related reactions, its use is associated with potential hepatotoxicityCitation27. Triazoles are recognized as preferred treatment options in both IDSA and ECIL-6 guidelines based in large part on better safety/tolerabilityCitation5,Citation6, but differences exist in the safety/tolerability of the different triazoles, including for potential hepatotoxicity. Although clinical experience with isavuconazole is more limited than with voriconazole, the IDSA guidelines recognize the lower rates of photosensitivity, skin disorders, and hepatobiliary and visual disorders with isavuconazole, and the ECIL-6 guidelines cite its better safety profile compared with voriconazoleCitation6. Isavuconazole also has a lesser propensity than voriconazole for drug–drug interactions mediated by cytochrome P450 3A4Citation28. Voriconazole also displays highly variable, non-linear pharmacokinetics and variability in plasma concentrations associated with polymorphisms in the cytochrome P450 2C19 enzyme, which necessitates frequent therapeutic drug monitoring to maintain plasma concentrations in a safe and efficacious rangeCitation29.
This analysis has some important limitations. The limited number of eligible studies did not permit an assessment of the risk of bias across studies. The time span over which these studies were performed was likely to have introduced some differences in trial characteristics. For example, the 1998 study comparing AmB-D and L-AmB did not use EORTC/MSG criteria for diagnosis of IFD, and included 10 patients with infections due to Candida spp. or other yeastCitation20, which were excluded from our analysis. Some differences between studies were evident with respect to sites of infection. For example, Studies 2–4 included a greater percentage of patients with pulmonary infections (approximately 80–90%) compared with Study 1 (approximately 55–65%), and that may have affected comparisons. Standards of clinical trial reporting have evolved over the last 25 years and so the baseline data available from past studies does not always permit comparison. In addition, changes in healthcare related to underlying diseases probably introduced differences in concomitant medications and may have had different effects on outcomes of ACM between studies. Nevertheless, comparisons of risk ratios in the current analysis are unlikely to have been unduly influenced by absolute differences in ACM. Finally, recommendations regarding time points for efficacy assessments have also been transformed over the years, and were not identical in all of these studies. Nevertheless, all studies in the analysis were RCTs with sufficient numbers of enrolled patients to permit an estimation of relative efficacy. Additionally, the similarities observed in this analysis for the overall response (a measurement subject to changes over time) and ACM (an objective, unchanging measurement) support the validity of the results.
Conclusion
Notwithstanding any limitations, the results of this analysis support the notion that the comparable efficacy of isavuconazole and voriconazole observed in the SECURE trial might be extended to imply superiority of isavuconazole to AmB-D. The results also suggest that isavuconazole is at least as efficacious as L-AmB, although any comparisons with AmB formulations would need to be confirmed in an RCT. Taken together, these findings tend to support the use of isavuconazole as primary therapy for IA and might help to inform clinicians’ decisions when considering therapeutic options.
Transparency
Declaration of funding
This study was funded by Basilea Pharmaceutica International Ltd. The funder was involved in the study design, data collection and data analysis.
Author contributions: N.P. performed data analysis; all authors were involved in the design of the study, interpretation of the data, drafting the paper and revising it critically for intellectual content. All authors agree to be accountable for all aspects of the work. All authors had full access to all study data and had final responsibility for the decision to submit for publication.
Declaration of financial/other relationships
R.H. has disclosed that he has received honoraria from Astellas, Basilea, Gilead, MSD and Pfizer, and a research grant from Pfizer. D.K. and J.P. have disclosed that they are full-time employees of Basilea Pharmaceutica International Ltd, Basel, Switzerland and both hold stock options with Basilea. C.E. has disclosed that he was a full-time employee of Basilea Pharmaceutica International Ltd at the time the analysis was performed. N.P. has disclosed that he was a full-time employee of PHMR, London, UK, at the time the analysis was performed (PHMR was commissioned by Basilea Pharmaceutica International Ltd to carry out statistical analyses).
CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download PDF (120.8 KB)Acknowledgements
Medical writing support was provided by Ed Parr PhD CMPP of Envision Scientific Solutions and funded by Basilea Pharmaceutica International Ltd.
References
- Gregg KS, Kauffman CA. Invasive aspergillosis: epidemiology, clinical aspects, and treatment. Semin Respir Crit Care Med 2015;36:662-72
- Neofytos D, Avdic E, Magiorakos AP. Clinical safety and tolerability issues in use of triazole derivatives in management of fungal infections. Drug Healthc Patient Saf 2010;2:27-38
- Andes D, Azie N, Yang H, et al. Drug-drug interaction associated with mold-active triazoles among hospitalized patients. Antimicrob Agents Chemother 2016;60:3398-406
- Maertens JA, Raad, II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet 2016;387:760-9
- Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016;63:e1-60
- Tissot F, Agrawal S, Pagano L, et al. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica 2017;102:433-44
- Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect 2018;24 (Suppl 1):e1-38
- Salanti G, Higgins JP, Ades AE, et al. Evaluation of networks of randomized trials. Stat Methods Med Res 2008;17:279-301
- Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med 2002;21:2313-24
- Lee AW. Review of mixed treatment comparisons in published systematic reviews shows marked increase since 2009. J Clin Epidemiol 2014;67:138-43
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097
- Centre for Reviews and Dissemination. Systematic Reviews; CRD’s Guidance for Undertaking Reviews in Health Care. York, UK: CRD, University of York, 2009
- National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. Available at: https://www.nice.org.uk/process/pmg9/chapter/foreword [Last accessed 6 June 2017]
- The Cochrane Collaboration. The Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 2011. Available at: http://handbook.cochrane.org/ [Last accessed 6 June 2017]
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105-24
- Dias S, Welton NJ, Sutton AJ, et al. NICE DSU Technical Support Document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials 2011. Available at: http://www.nicedsu.org.uk [Last accessed 26 February 2018]
- Dias S, Sutton AJ, Ades AE, et al. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making 2013;33:607-17
- De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008;46:1813-21
- Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 2002;34:7-14
- Leenders AC, Daenen S, Jansen RL, et al. Liposomal amphotericin B compared with amphotericin B deoxycholate in the treatment of documented and suspected neutropenia-associated invasive fungal infections. Br J Haematol 1998;103:205-12
- Cornely OA, Maertens J, Bresnik M, et al. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial). Clin Infect Dis 2007;44:1289-97
- Cornely OA, Maertens J, Bresnik M, et al. Efficacy outcomes in a randomised trial of liposomal amphotericin B based on revised EORTC/MSG 2008 definitions of invasive mould disease. Mycoses 2011;54:e449-55
- Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med 2002;347:408-15
- Patterson TF, Boucher HW, Herbrecht R, et al. Strategy of following voriconazole versus amphotericin B therapy with other licensed antifungal therapy for primary treatment of invasive aspergillosis: impact of other therapies on outcome. Clin Infect Dis 2005;41:1448-52
- Herbrecht R, Patterson TF, Slavin MA, et al. Application of the 2008 definitions for invasive fungal diseases to the trial comparing voriconazole versus amphotericin B for therapy of invasive aspergillosis: a collaborative study of the Mycoses Study Group (MSG 05) and the European Organization for Research and Treatment of Cancer Infectious Diseases Group. Clin Infect Dis 2015;60:713-20
- Heinz WJ, Cornely OA, Franks B, et al. A phase III, randomized, double-blind trial to evaluate efficacy and safety of isavuconazole versus voriconazole in patients with invasive mold disease (SECURE): outcomes in hematopoietic stem cell transplant patients with invasive aspergillosis [abstract]. Blood 2014;124:1133
- Stone NR, Bicanic T, Salim R, et al. Liposomal amphotericin B (AmBisome): a review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions. Drugs 2016;76:485-500
- Townsend R, Dietz A, Hale C, et al. Pharmacokinetic evaluation of CYP3A4-mediated drug–drug interactions of isavuconazole with rifampin, ketoconazole, midazolam, and ethinyl estradiol/norethindrone in healthy adults. Clin Pharmacol Drug Dev 2017;6:44-53
- Dolton MJ, McLachlan AJ. Voriconazole pharmacokinetics and exposure-response relationships: assessing the links between exposure, efficacy and toxicity. Int J Antimicrob Agents 2014;44:183-93
