Abstract
Objective: To review safety, efficacy and pharmacokinetic (PK) data from the phase 3 REJOICE trial, which evaluated a 17β-estradiol (E2) softgel vaginal insert approved in 2018 for moderate to severe dyspareunia associated with menopausal vulvar and vaginal atrophy (VVA).
Methods: REJOICE (Clinicaltrials.gov: NCT02253173) was a randomized, double-blind, placebo-controlled trial in which women with moderate to severe dyspareunia due to menopausal VVA received 4 µg, 10 µg or 25 µg of an E2 vaginal insert or placebo for 12 weeks. The published data for the recently approved 4 µg and 10 µg doses of the E2 vaginal insert, including four co-primary efficacy endpoints (change from baseline to week 12 in percentages of superficial and parabasal cells, vaginal pH and severity of dyspareunia), safety and PK (which included serum E2 levels measured by gas chromatography and tandem mass spectrometry), are summarized here.
Results: Women were randomized to receive the E2 vaginal insert (4 µg [n = 186] or 10 µg [n = 188]; Imvexxya) or placebo (n = 187) in the modified intention-to-treat population. The E2 vaginal insert (4 µg and 10 µg) significantly improved the percentages of superficial and parabasal cells (p < .0001), vaginal pH (p < .0001), and the severity score for dyspareunia (p < .05) from baseline to week 12 compared with placebo. The recently approved E2 vaginal insert was well tolerated, with no clinically significant differences in treatment-emergent or serious adverse events versus placebo. Systemic absorption of E2 with both doses was minimal.
Conclusions: The recently FDA-approved E2 softgel vaginal insert (4 µg and 10 µg) was safe and effective over 12 weeks for treating moderate to severe dyspareunia due to menopausal VVA with minimal systemic E2 levels.
Introduction
Vulvar and vaginal atrophy (VVA) involves the thinning, drying and loss of elasticity of the vaginal epithelium due to a reduction in circulating estrogens after menopauseCitation1. Nearly 70% of all postmenopausal women report clinical symptoms associated with VVA, such as vaginal dryness, irritation, itching, dysuria and pain or bleeding with sexual activityCitation2–5. Surveys of postmenopausal women found that VVA symptoms had negative consequences on their relationships, sex life, enjoyment of sex and self-esteem, and reduced their overall quality of lifeCitation4,Citation6. The negative impact of VVA symptoms emphasizes the need for initiating treatment. The Women’s EMPOWER survey found that three-quarters of current or former prescription VVA therapy users, and almost two-thirds of never users or over-the-counter/lubricant users would consider a product to alleviate VVA symptoms to improve sexual activityCitation5.
For symptomatic women with moderate to severe VVA who do not respond adequately to nonhormonal moisturizers and lubricants, the North American Menopause Society (NAMS), the International Menopause Society (IMS) and the American College of Obstetricians and Gynecologists (ACOG) recommend the use of low-dose vaginal, transdermal or systemic estrogens, and if VVA is the only menopausal symptom, vaginal estrogen is preferredCitation7–9. Although a recent study showed no difference in vaginal symptoms with vaginal estradiol, vaginal moisturizer and placebo gelCitation10, several flaws limit the conclusions of the studyCitation11. Other prospective clinical VVA studies have demonstrated superiority of vaginal estrogens compared with placeboCitation12. Despite treatment availability, multiple surveys have found that many respondents are not aware of or are unfamiliar with available vaginal estrogen treatment optionsCitation4–6,Citation13–15. Surveys have also shown that, even when women are aware of therapy, they still have concerns regarding the safety and/or side effects of systemic hormone exposureCitation5,Citation6,Citation14. Perceived risk of systemic absorption and concern over the risk for side effects were given in the 2016 EMPOWER survey as the least favored attributes of currently available products by current or former users, and never users, respectivelyCitation5.
Use of a vaginal delivery system is thought in part to minimize increases in circulating estrogen levels with lower, locally applied doses of estrogensCitation7,Citation16, possibly resulting in a lower systemic risk profileCitation7. Ultra-low-dose vaginal estrogens (≤10 µg estradiol [E2]) have been developed in an attempt to achieve these outcomes. A 17β-E2 softgel vaginal insert (Imvexxya, formerly called TX-004HR) was approved by the US Food and Drug Administration (FDA) in 2018 for the treatment of moderate to severe dyspareunia, a symptom of VVA, due to menopauseCitation17. The objective of this review was to summarize the phase 3 safety, efficacy and pharmacokinetic (PK) data of this ultra-low-dose E2 (4 µg and 10 µg) vaginal therapy.
Methods
Reviewed here are safety, efficacy and PK data of this ultra-low-dose, E2, softgel vaginal insert, which have been previously reported in separate publicationsCitation18,Citation19. One publication reported the primary safety and efficacy dataCitation18, while the second reported data from a PK substudyCitation19. While three doses of the E2 vaginal insert were evaluated (4 µg, 10 µg and 25 µg), the two lower doses are clinically available, largely due to the favorable results with the two lower doses and the recommendation for using the lowest effective dose. Therefore, this review will focus on the 4 µg and 10 µg E2 vaginal inserts, discussing the primary efficacy and safety data of the E2 vaginal insert in the context of its PK profile.
The study design of the REJOICE trial (NCT02253173) has been previously describedCitation18,Citation19; the protocol was approved by an institutional review board and written consent was obtained from the women prior to their participation. A PK substudy was also conducted in a subset of women enrolled in the REJOICE trial.
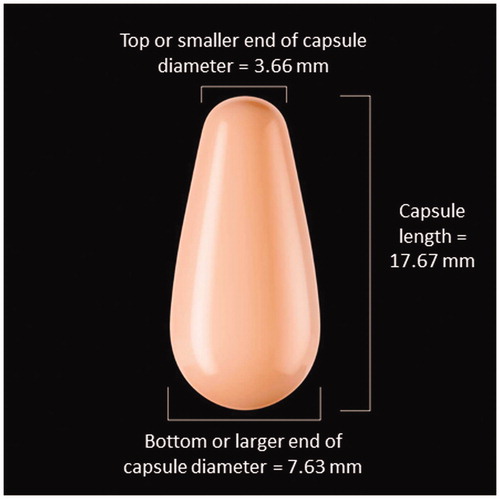
Briefly, postmenopausal women (40–75 years of age) who reported moderate to severe dyspareunia as their most bothersome symptom (MBS), and had ≤5% superficial cells on vaginal cytological smear and vaginal pH >5.0, were enrolled at 89 sites located in the US and Canada. Postmenopausal status was defined in this study as 12 months of spontaneous amenorrhea, 6 months of spontaneous amenorrhea with FSH levels >40 mIU/mL, or at least 6 weeks after bilateral oophorectomy. Women (n = 764) were randomized to one of three doses of E2 softgel vaginal inserts (4 µg, 10 µg or 25 µg) or matching placebo and were instructed to digitally self-administer one E2 vaginal insert into the vagina (about 2 inches), at approximately the same time of day, daily for 2 weeks and then twice weekly (approximately 3 to 4 days apart) for 10 weeks. The E2 vaginal insert is a small, light pink, tear-shaped capsule () that releases E2 when it comes in contact with the vaginal mucosaCitation17. Women could not have used any hormone therapy, selective estrogen receptor modulators, or vaginal lubricants or moisturizers during the study or within the required washout period prior to the study.
Consistent with the FDA’s recommendations on clinical studies of estrogen products for treating symptoms of VVACitation20, four co-primary efficacy endpoints were evaluated in the REJOICE trial and included changes from baseline to week 12 compared with placebo for: (1) percentage of vaginal superficial cells; (2) percentage of vaginal parabasal cells; (3) vaginal pH; and (4) severity of the MBS of moderate to severe dyspareunia associated with VVACitation18. Dyspareunia was defined as vaginal pain associated with sexual activity. Improvements in the objective physiological parameters would be demonstrated by increases in the percentages of superficial cells and decreases in the percentages of parabasal cells and vaginal pH. Prespecified secondary endpoints included these co-primary endpoint measures at weeks 2, 6 and 8Citation18. Adverse events reported throughout the 12-week study period were summarized to describe overall safety and tolerability in the safety population (all randomized women who had at least one dose of study medication)Citation18. Endometrial safety was evaluated based on endometrial biopsies taken by board-certified gynecologists at baseline and at week 12 or end of treatment in the safety populationCitation18. Diagnoses were based on the agreement of three independent pathologists; if no agreement, the most severe pathologic diagnosis was usedCitation20.
A separate randomization protocol was performed for the women who were included in the PK substudyCitation19. Briefly, the PK substudy assessed serum hormone concentrations of E2 and estrone (E1) using gas chromatography/tandem mass spectrometry (GC/MS/MS)Citation19. Serum samples were obtained at screening, and at day 1 and day 14 before dosing, and then at five post-dose time points (2, 4, 6, 10, and 24 h) on days 1 and 14, and one final sample at day 84, approximately 4 days after the last doseCitation19. PK parameters analyzed included area under the serum concentration–time curve (AUC0–24), average concentration (Cavg) and peak concentration (Cmax) for each E2 vaginal insert on days 1 and 14Citation19.
Results
Participant disposition and baseline demographics
Of the 764 postmenopausal women who were randomized to treatment in the REJOICE trial, 186 were randomized to the 4 µg E2 vaginal insert, 188 to the 10 µg insert and 187 to placeboCitation18, contributing 561 women to the modified intent-to-treat population (MITT)Citation18. The MITT population had a mean age of 59 years and a mean BMI of 27 kg/m2. The majority (87%) of women were white and 12% were African American. No differences in demographic and baseline characteristics were noted among the treatment groupsCitation18. Over 90% of the women also reported moderate to severe dryness as part of their menopausal VVACitation18.
Efficacy
Significant improvements from baseline in the percentage of superficial cells (increases) and parabasal cells (decreases) were observed as early as week 2 and sustained up to week 12 with both vaginal E2 doses compared with placebo (p < .0001 at weeks 2, 6, 8 [secondary endpoints] and 12)Citation18. The mean percentage increases from baseline to week 12 in the superficial cell percentages were 18% with the 4 µg vaginal E2 insert and 17% with the 10 µg insert versus 6% with placebo (p < .0001; )Citation18. Mean percentage decreases from baseline to week 12 in parabasal cell percentages were −41% and −44% with the 4 µg and 10 µg doses, respectively, versus −7% with placebo (p < .0001; )Citation18. Significant decreases in vaginal pH with the 4 µg (-1.3 points) and 10 µg (-1.4 points) E2 vaginal inserts were also observed up to week 12 () compared with placebo (-0.3 points) and were seen as early as week 2 (p < .0001 at weeks 2, 6, 8 [secondary endpoints] and 12)Citation18.
Figure 2. Changes from baseline over 12 weeks in: (A) percentage of superficial cells; (B) percentage of parabasal cells; (C) vaginal pH; and (D) dyspareunia severity scores in the REJOICE trial for the 4 µg (n = 186) and 10 µg (n = 188) E2 vaginal inserts versus placebo (n = 187). *p < .05; †p < .01; ‡p < .0001 vs. placebo.
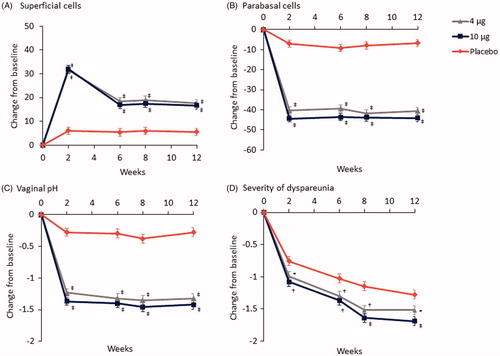
A significant reduction in the mean severity score for dyspareunia was reported with the 4 µg and 10 µg E2 vaginal inserts versus placebo as early as week 2 (secondary endpoint) and sustained up to week 12 (p < .05)Citation18. The mean decrease in the dyspareunia score from baseline to week 12 was −1.5 for the 4 µg dose (p < .05) and −1.7 for the 10 µg dose (p < .0001) compared with −1.3 for placebo ()Citation18.
Safety and tolerability
Overall, the ultra-low-dose E2 softgel vaginal insert was found to be safe and well tolerated ()Citation18. Headache was the most common treatment-emergent adverse event (TEAE) observed in all treatment groups (4 µg E2 [6.3%] and 10 µg E2 [7.3%]; placebo [7.8%])Citation18. Other TEAEs reported in ≥3% of women included vaginal discharge, nasopharyngitis, vulvovaginal pruritus, back pain, urinary tract infection, upper respiratory tract infection and oropharyngeal pain in descending order, and were reported at an incidence of < 5% ()Citation18. Headache was the only treatment-related TEAE occurring in ≥3% and was reported numerically more in women who received the E2 vaginal insert (3.7% with 4 µg; 2.6% with 10 µg) than in women who received placebo (3.1%)Citation18. The treatment-related TEAE of vaginal discharge was reported by numerically fewer women with the E2 vaginal insert (2.6% with 4 µg; 3.1% with 10 µg) than with placebo (6.3%)Citation18. Most TEAEs were mild to moderate in severity, and few study discontinuations due to adverse events (1.0% [4 µg]; 1.6% [10 µg]; 2.6% [placebo]) were reportedCitation18.
Table 1. Treatment-emergent adverse events reported for ≥3% in any treatment arm (safety population).
No serious TEAEs were reported in the 4 µg E2 group, 3 women reported 4 serious TEAEs (1 woman had sinus node dysfunction and an ankle fracture, 1 had arthralgia and 1 had malignant melanoma) in the 10 µg E2 group, and 1 woman reported cervical myelopathy in the placebo groupCitation18. No serious TEAEs were considered related to treatment, and no deaths occurredCitation18.
No diagnoses of endometrial hyperplasia or malignancy were made at week 12 in the safety population (n = 574 [placebo, 4 µg and 10 µg groups])Citation18.
Pharmacokinetics
A total of 72 postmenopausal women were randomized in the PK substudyCitation19. Of these, 54 were randomized with allocation to the 4 µg E2 vaginal insert (n = 18), 10 µg insert (n = 19) and placebo (n = 17)Citation19. Mean serum baseline levels of estradiol (E2) ranged from 3.9 to 4.9 pg/mL and estrone (E1) from 15.3 to 20.3 pg/mL in the 4 µg, 10 µg dose and placebo groups. illustrates the mean serum E2 concentrations over time for each dose versus placebo on days 1 and 14. Mean serum E2 levels were ≤5.0 pg/mL for each treatment group at baseline and remained low over the 24 hours on measurement days 1 and 14, except for slightly higher levels at 2 hours on day 1 that were not observed on day 14Citation19. Day 84 mean serum E2 concentrations with the E2 vaginal inserts were similar to those observed at baseline and with placeboCitation19.
Figure 3. Unadjusted mean serum E2 concentrations with E2 softgel vaginal inserts: (A) 4 µg E2 (n = 18) and (B) 10 µg E2 (n = 19) compared with placebo (n = 17) on days 1 and 14. Reprinted with permission from Wolters Kluwer from Archer DF, Constantine GD, Simon J, et al. TX-004HR vaginal estradiol has negligible to very low systemic absorption of estradiol. Menopause 2017;24:510-16.
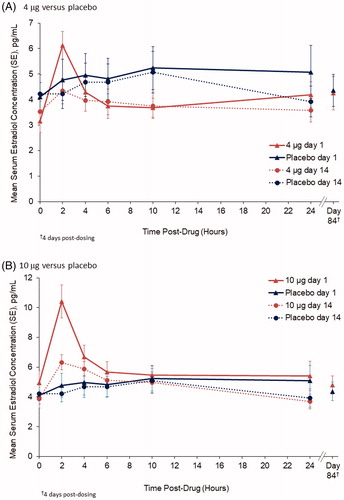
No significant differences in unadjusted E2 measures for AUC, Cmax or Cavg were noted between the 4 µg E2 dose and placebo on days 1 or 14 ()Citation19. The only significant difference between the 10 µg E2 dose and placebo was Cmax on day 1 (mean 10.9 pg/mL vs. 6.6 pg/mL; p < .05)Citation19. Unadjusted estrone AUC, Cmax and Cavg values were all significantly lower (p < .05) than or similar to placebo with the 4 µg and 10 µg E2 dose on days 1 and 14 ()Citation19, and hourly data does not show a similar pattern to that of E2 at 2 hours on day 1.
Table 2. Unadjusted pharmacokinetic parameters.
Discussion
Clinical safety and efficacy data reviewed here from the REJOICE trial show that the ultra-low-dose E2 softgel vaginal inserts (4 µg and 10 µg) are safe and effective for treating moderate to severe dyspareunia associated with VVA in postmenopausal women. Supporting data include significant improvements from baseline in the four measured co-primary endpoints from week 2 (secondary endpoint) to week 12, with no significant safety concerns noted with either dose. These ultra-low-dose E2 vaginal inserts provide a new option for treating women with moderate to severe dyspareunia associated with menopausal VVA, and may help alleviate concerns of women pertaining to systemic hormone absorption and associated side effects.
The early onset of action on moderate to severe dyspareunia as an MBS was demonstrated by statistically significant, patient-reported improvements with the E2 softgel vaginal insert versus placebo at week 2 as a secondary endpointCitation18. Dyspareunia severity reductions with this E2 vaginal insert were consistent with early significant improvements in superficial cell and parabasal cell percentages, and vaginal pHCitation18.
The efficacy reviewed here with the 4 µg and 10 µg E2 vaginal inserts was achieved with minimal systemic absorption of E2 as measured by the highly sensitive GC/MS/MS method. Mean E2 levels with 4 µg and 10 µg vaginal E2 doses were similar to those seen at baseline and with placebo, and remained within the range of untreated, postmenopausal women ( < 10 pg/mLCitation21), when given daily for 14 daysCitation19. No significant differences in the mean AUC and Cavg for E2 were observed with the 4 µg and 10 µg E2 doses versus placebo at days 1 or 14, although Cmax was higher with the 10 µg E2 dose compared with placebo at day 1Citation19. This initial increase in E2 absorption on day 1 is likely due to higher E2 absorption through untreated, thin, atrophic vaginal tissues as, after 14 days of daily treatment and E2 exposure, no significant difference in E2 levels between the 10 µg vaginal insert and placebo were foundCitation19, which is similar to what has been shown in other studiesCitation22–24. In addition, no accumulation of E2 was supported by day 84 E2 levels. Thus, in conjunction with the efficacy data showing early symptom resolution, the PK data demonstrating minimal systemic E2 absorption are consistent with goals of local vaginal estrogen therapy for treating symptoms of menopausal VVA.
Adverse event reporting and endometrial biopsy assessments demonstrated that the 4 µg and 10 µg softgel E2 vaginal inserts were safe and well toleratedCitation18. No clinically significant differences were noted between TEAEs reported with the E2 vaginal insert and placebo, most of the TEAEs were mild or moderate, and very few women discontinued treatment due to adverse eventsCitation18. Lastly, no cases of endometrial hyperplasia or malignancy were reported with 12 weeks of the E2 vaginal insert useCitation18. Further study would be needed to determine the risk of endometrial hyperplasia or cancer with use of low-dose vaginal estrogens unopposed by a progestogen for durations longer than 12 weeks. Approval of this E2 vaginal insert by the FDA for treating moderate to severe dyspareunia, due to menopause, does not limit duration of useCitation17.
Previously reported low uptake of prescription vaginal estrogens by postmenopausal women (∼7%)Citation4,Citation25 may be attributed to the lack of information about available and/or effective treatments, concerns over systemic hormone absorption, or dissatisfaction with available productsCitation4–6,Citation26. Some of the least favored attributes of available VVA therapies were recently reported by the Women's EMPOWER survey respondents as messiness of vaginal cream and the need to use an applicatorCitation5. The E2 vaginal insert is expected to provide women with moderate to severe dyspareunia a novel softgel treatment choice with minimal systemic absorption of E2, which may alleviate some women’s concerns of systemic exposure. In addition, eliminating the need for an applicator or the need to measure individual doses with this E2 vaginal insert may improve user experience of a vaginal product. As part of the phase 3 REJOICE study reviewed here, 574 participants who used 4 µg of E2 (n = 191), 10 µg of E2 (n = 191) or placebo (n = 192) were surveyed at the end of the study with five questions that assessed acceptability of the softgel vaginal insert. The survey data showed that most women found the vaginal insert easy to use (85%–90%) and easy to insert (75%–82%). More women were also satisfied with the E2 vaginal insert (69%–73%) than they were with placebo (57%; p < .001), and satisfaction with the product was correlated (r=-.551; p < .0001) with reductions in moderate to severe dyspareuniaCitation27.
Conclusions
Based on the REJOICE study, the FDA recently approved (May 2018) the E2 softgel vaginal inserts (4 µg and 10 µg doses) as safe and effective when used as labeled for treating moderate to severe dyspareunia, due to menopause, with improvements in objective vaginal markers associated with VVA, an early onset of effectiveness (2 weeks) and minimal systemic E2 absorption.
Transparency
Author contributions: All authors participated in the conception and design, or analysis and interpretation of the data for this review; drafting the paper or revising it critically for intellectual content; and final approval of the version to be published. All authors agreed to be accountable for all aspects of the work.
Declaration of financial/other relationships
G.D.C. has disclosed that she consults for multiple pharmaceutical companies, including but not limited to TherapeuticsMD, and has stock options with TherapeuticsMD. J.A.S. has disclosed that he has served (within the last year or currently) as a consultant/advisor for AbbVie, Allergan, AMAG, Amgen, Ascend Therapeutics, Azure Biotech, Bayer Healthcare, CEEK Enterprises, Covance, Millendo Therapeutics, Mitsubishi Tanage, ObsEva SA, Radius Health, Sanofi SA, Sebela, Sermonix, Shionogi, Symbiotec Pharmalab, TherapeuticsMD and Valeant; has served (within the last year or currently) on the speaker’s bureaus of Duchesnay, Novo Nordisk, Shionogi and Valeant; has received (within the past year or currently) grant/research support from AbbVie, Agile Therapeutics, Allergan, Bayer Healthcare, New England Research Institute, ObsEva SA, Palatin Technologies, Symbio Research and TherapeuticsMD; and is a stockholder (direct purchase) in Sermonix Pharmaceuticals. J.H.P. has disclosed that he has consulted for Pfizer, Shionogi and TherapeuticsMD (stock options). D.F.A. has disclosed that he serves as a consultant for Abbvie (previously Abbott Laboratories), Actavis (previously Allergan, Watson Pharmaceuticals, Warner Chilcott), Agile Therapeutics, Bayer Healthcare, Endoceutics, Exeltis (previously CHEMO), InnovaGyn, Merck (previously Schering Plough, Organon), Pfizer, Radius Health, Sermonix, Shionogi, Teva Women’s Healthcare and TherapeuticsMD; and has received research support from Actavis (previously Allergan, Watson Pharmaceuticals, Warner Chilcott), Bayer Healthcare, Endoceutics, Glenmark, Merck (previously Schering Plough, Organon), Radius Health, Shionogi and TherapeuticsMD. B.B. has disclosed that he is a Board member and an employee of TherapeuticsMD with stock/stock options. S.G. and S.M. have disclosed that they are employees of TherapeuticsMD with stock/stock options.
Acknowledgements
The authors acknowledge the medical writing assistance of Chastity Bradley PhD and Kathleen Ohleth PhD CMPP of Precise Publications LLC, which was supported by TherapeuticsMD.
Notes
Additional information
Funding
Notes
a Imvexxy is a trademark of TherapeuticsMD, Boca Raton, FL, USA
References
- Mac Bride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc 2010;85:87-94
- Gass ML, Cochrane BB, Larson JC, et al. Patterns and predictors of sexual activity among women in the hormone therapy trials of the Women’s Health Initiative. Menopause 2011;18:1160-71
- Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med 2009;6:2133-42
- Simon JA, Kokot-Kierepa M, Goldstein J, Nappi RE. Vaginal health in the United States: results from the Vaginal Health: Insights, Views & Attitudes survey. Menopause 2013;20:1043-8
- Kingsberg S, Krychman M, Graham S, et al. The Women’s EMPOWER survey: identifying women’s perceptions on vulvar and vaginal atrophy (VVA) and its treatment. J Sex Med 2017;14:413-24
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med 2013;10:1790-9
- The North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013;20:888-902
- de Villiers TJ, Hall JE, Pinkerton JV, et al. Revised global consensus statement on menopausal hormone therapy. Climacteric 2016;19:313-15
- American College of Obstetricians and Gynecologists. Management of menopausal symptoms. Practice Bulletin No. 141. Obstet Gynecol 2014;123:202-16
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms: a randomized clinical trial. JAMA Intern Med 2018;178:681-90
- Pinkerton JV, Kaunitz AM, Manson JE. Not time to abandon use of local vaginal hormone therapies. Menopause 2018;25:855-8
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 2017;24:728-53
- Nappi RE, Kokot-Kierepa M. Vaginal health: Insights, Views & Attitudes (VIVA) – results from an international survey. Climacteric 2012;15:36-44
- Nappi RE, Palacios S, Panay N, et al. Vulvar and vaginal atrophy in four European countries: evidence from the European REVIVE Survey. Climacteric 2016;19:188-97
- Nappi RE, Mattsson LA, Lachowsky M, et al. The CLOSER survey: impact of postmenopausal vaginal discomfort on relationships between women and their partners in Northern and Southern Europe. Maturitas 2013;75:373-9
- American College of Obstetricians and Gynecologists. The use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Committee Opinion No. 659. Obstet Gynecol 2016;127:e93-6
- Imvexxy (estradiol vaginal inserts). Boca Raton, FL: Therapeutics MD, 2018
- Constantine G, Simon JA, Pickar JH, et al. The REJOICE trial: a phase 3 randomized, controlled trial evaluating the safety and efficacy of a novel vaginal estradiol soft-gel capsule for symptomatic vulvar and vaginal atrophy. Menopause 2017;24:409-16
- Archer DF, Constantine GD, Simon J, et al. TX-004HR vaginal estradiol has negligible to very low systemic absorption of estradiol. Menopause 2017;24:510-16
- US Food and Drug Administration. Guidance for industry: estrogen and estrogen/progestin drug products to treat vasomotor symptoms and vulvar and vaginal atrophy symptoms – recommendations for clinical evaluation. Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/informationbyDrugClass/UCM135338.pdf [Last accessed 15 August 2015]
- Mayo Medical Laboratories. Test ID:ESTS Estradiol, Rapid, Immunoassay, Serum. 2018. Available at: https://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/8575 [Last accessed 14 August 2018]
- Eugster-Hausmann M, Waitzinger J, Lehnick D. Minimized estradiol absorption with ultra-low-dose 10 microg 17beta-estradiol vaginal tablets. Climacteric 2010;13:219-27
- Nilsson K, Heimer G. Low-dose oestradiol in the treatment of urogenital oestrogen deficiency – a pharmacokinetic and pharmacodynamic study. Maturitas 1992;15:121-7
- Holmgren PA, Lindskog M, von Schoultz B. Vaginal rings for continuous low-dose release of oestradiol in the treatment of urogenital atrophy. Maturitas 1989;11:55-63
- US Census Bureau. Age and sex composition: 2010 Census Briefs. Available at: http://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf [Last accessed 14 April 2015]
- Nappi RE, Kokot-Kierepa M. Women’s voices in the menopause: results from an international survey on vaginal atrophy. Maturitas 2010;67:233-8
- Kingsberg S, Kroll R, Goldstein I, et al. Patient acceptability and satisfaction with a low-dose solubilized vaginal estradiol softgel capsule, TX-004HR. Menopause 2017;24:894-9