Abstract
Objective: Patient support programs, such as the ASSURE Program for long-acting injectable aripiprazole, are designed to help support access to medications, including long-acting injectable (LAI) antipsychotics for patients with schizophrenia. This study was conducted to evaluate adherence to long-acting injectable aripiprazole among patients utilizing the program local care centers (LCC).
Methods: Data collected from participating LCC between October 2014 and February 2018 were utilized. Characteristics of patients receiving injections at LCC and participating in additional support services of the program, types of program offering utilized and patient cost share for long-acting injectable aripiprazole were described. Adherence, measured as the proportion of days covered (PDC) during follow-up, was estimated in patients utilizing the LCC for 6 months and 9 months. Patients with PDC ≥80% were considered adherent to treatment.
Results: Two hundred and thirty-four patients received at least one injection at participating LCC and enrolled in the patient support program. Mean (SD) age was 37.3 (13.5) years; 60.7% were male; 32.5% were covered by Medicare. In total, 157 and 87 patients were actively utilizing the LCC for at least 6 months and 9 months, respectively. PDC of 97% and 98% were reported among patients with 6 months and 9 months of follow-up, respectively, and patients were considered adherent to long-acting injectable aripiprazole during follow-up.
Conclusion: Patients utilizing the LCC demonstrated high medication adherence, suggesting that injection services provided by the centers may reduce barriers to treatment and help patients with schizophrenia remain on LAI antipsychotic treatment.
Introduction
Schizophrenia and associated disorders are complex mental illnesses with an annual prevalence in the US between 0.25% and 0.64%Citation1. Schizophrenia is an expensive and severe illness, with a direct and indirect cost in the US that exceeded $60 billion in 2002Citation2, and continued to increase, with recent estimates of $155 billion in 2013Citation3. The estimated annual costs of schizophrenia of $15,500–$22,300 per patient can increase by 3–11-fold for those who do not respond to their initial treatmentCitation4. As a chronic condition requiring long-term pharmacotherapy treatment, adherence and compliance with treatment is a critical factor in the successful treatment of schizophrenia, as non-adherence to antipsychotic treatments is associated with an increased risk of hospitalizationCitation5 and negative outcomes such as arrests, violence, victimizations, substance abuse, and alcohol problemsCitation6,Citation7.
Oral medications are the recommended first treatment for patients with schizophrenia, and a majority of patients with schizophrenia are prescribed at least one oral antipsychotic during their treatmentCitation8. Lack of adherence has been a significant issue for patients with schizophreniaCitation7, with estimates for oral medication non-adherence ranging from 50–70%Citation9–11. Non-adherence to antipsychotics may be related to side-effect profiles of medications, however it is also commonly caused by difficulties associated with obtaining the medicationCitation12. Further contributing to the issue, many physicians are not fully aware of non-adherence within their own practiceCitation13. In one study, physicians reported an estimated non-adherence rate of 7%, while data collected from their patient populations indicated a non-adherence rate of 39%Citation13.
Guidelines from the Journal of Psychiatric Practice recommend addressing issues leading to medication non-adherence initially as much as possible prior to switching treatments, including assisting with logistic difficulties and medication monitoring/environmental supportCitation14. In addition, when clinically appropriate, a longer-acting, injectable (LAI) second-generation antipsychotic is recommendedCitation9,Citation15. LAI antipsychotics usually have better adherence profiles than oral antipsychotics, with estimates for patient non-adherence to LAI antipsychotics ranging from 9–52% (48–91% adherent)Citation16–18. A recent study using insurance claims data also found higher medication adherence and lower discontinuation rates among patients treated with an LAI, in comparison to oral antipsychotic therapyCitation19. To date, a total of six long-acting, intramuscular injection formulations of second-generation antipsychotics for the treatment of schizophrenia have been approved in the USCitation20, including risperidone (2003), olanzapine palmitate (2009), paliperidone 1-month injection (2009), aripiprazole (2013), paliperidone 3-month injection (2015), and aripiprazole lauroxil (2015).
While LAI antipsychotics provide better adherence for patients, there are still barriers to prescribing and taking LAI antipsychotics, including negative attitudes from clinicians and patients, logistical/transportation challenges, a lack of insurance coverage, and general-purpose clinics serving this population that are not properly equipped to administer injectionsCitation21–23. As LAI antipsychotics have additional requirements beyond those of oral medications, a center with professionals and equipment to dispense the LAI that patients can access reliably is critical to the long-term successful use of LAI antipsychotics. A lack of logistic support from clinical institutions, social, financial, and logistic support from the community, and individual social networks has been associated with medication non-adherenceCitation11,Citation23,Citation24.
Patient support programs that provide assistance in administering LAI antipsychotics can increase medication adherence, reducing the clinical and economic burden associated with under- or untreated schizophreniaCitation4–7,Citation9,Citation18,Citation25. Integrated healthcare programs have been shown to reduce the number of inpatient days and increase the quality-of-life of patients with psychotic disordersCitation25. Several patient support programs exist to provide clinical and financial support to patients and prescribers of LAIs. Patient support programs are available for patients prescribed long-acting paliperidone palmitate or risperidone, providing them with access to care centers and injection appointment reminders, and have demonstrated improved adherence among patients utilizing the centersCitation18. A patient care program is also available for long-acting olanzapine, with the aim of mitigating the risk of negative outcomes associated with post-injection delirium/sedation syndrome; all prescribers of long-acting olanzapine, pharmacy service providers, and patients are required to be enrolled in the care programCitation26. For aripiprazole lauroxil, uninsured patients can apply to the patient assistance program to receive treatment at no chargeCitation27. Lastly, the ASSURE Program for patients treated with long-acting injectable aripiprazole was designed to help patients access medication through the use of a comprehensive support program, including local care centers (LCC), nurse support for scheduling assistance and appointment reminders, as well as patient education supportCitation28. In addition, copay assistance is available for many antipsychotics prescribed, to help reduce patient out-of-pocket costs. The objectives of the study are to describe patient characteristics, cost sharing, type of services utilized, and medication adherence during the period in which patients were actively receiving long-acting injectable aripiprazole injections from the patient support program LLC.
Methods
The ASSURE program
The manufacturer of long-acting injectable aripiprazole, Otsuka America Pharmaceutical Inc., offers a support program for patients who have been prescribed long-acting injectable aripiprazole in the US. Long-acting injectable aripiprazole was approved for the treatment of adults diagnosed with schizophrenia in February 2013, and for maintenance treatment of bipolar I disorder in July 2017. The ASSURE program was developed to support the needs of both patients and physicians, ensure timely access to medication, and facilitate care coordination throughout the treatment journey. The core services, including LCC, practice reimbursement and coverage coordination, one-on-one nurse support, and patient education, work together to facilitate continuity of care. Patients can be referred to the program through their physician, or through the pharmacy when they obtain their prescription. The pilot program was first launched in four states in 2014: California, Pennsylvania, Texas, and Washington, and then became available in all 50 states in 2016. The current program offerings are summarized in .
Table 1. ASSURE program offerings.
Data source
This retrospective study utilized de-identified information collected from participating LCCs across all 50 states. The data consist of two sets of patients: patients who utilized the LCC and enrolled in the program between October 2014 and February 2018, and patients who utilized the LCC, irrespective of program enrollment and use of other support services, between February 2015 and December 2017. All data are de-identified and comply with the Health Insurance Portability and Accountability Act (HIPAA). Institutional Review Board approval was not required for this study.
LCCs consist of participating retail pharmacies or urgent clinics which receive on-boarding training to help understand patient needs as well as to provide information on the support options available to assist patients prescribed with long-acting injectable aripiprazole. Patients receiving injection service at the LCC comprise of individuals who enrolled in the patient support program to receive support services, and those who did not enroll to receive other offerings of the program, but opted to receive their injection at the LCC. Upon patient referral and before treatment, LCC personnel are required to verify patient benefits and work directly with patients or caregivers to schedule and confirm appointments. A patient signed consent form or Patient Data Report (PDR) with patient, prescriber, and injection information was faxed to program administrators within 24 hours of each injection. Once received, the program notifies the provider that the injection was administered. Providers are also alerted when their patients are “no-shows” for injection appointments to monitor adherence and compliance to the prescribed medication.
Data collection and study sample
De-identified patient data utilized in this study were collected by the LCC and ASSURE program to monitor and support patients, and not specifically for research purposes. Injection data were collected from PDR; demographic data were self-reported by the patient, and other data were collected during support phone calls.
All patients receiving at least one long-acting injectable aripiprazole at a participating LCC during the study period were selected into the study. Of this population, sub-sets of patients who were enrolled in the program with additional support services and those who were actively receiving injections from the LCC during follow-up were included in subsequent analyses. During the study observational period, schizophrenia was the only approved indication for long-acting injectable aripiprazole, and it was assumed that patients in the study were treated for schizophrenia. Injections from facilities other than the participating program LCCs were not included in the study.
Measures
Characteristics of patients who received injections at an LCC and were enrolled in the program with additional support services were described. Types of other services utilized and patient cost share for long-acting injectable aripiprazole were also reported for this patient cohort.
Adherence to long-acting injectable aripiprazole was measured among patients actively receiving injections from the LCC for a minimum of 6 months and 9 months. Patients were considered adherent if their proportion of days covered (PDC) during follow-up was 80% or greater. This definition is based on expert opinion that patients with schizophrenia taking 80% or less of the medication are non-adherent to their medication regimenCitation9. PDC was defined as the total number of days covered (i.e. each injection lasts 30 days), divided by the total days of the fixed follow-up period (i.e. 182 days for 6 months and 273 days for 9 months of follow-up). Overlapping days were not counted twice. The number of injections received was also reported, in addition to patient age, gender, and preferred contact methods for patients with at least 6 months of follow-up.
Analyses
Descriptive analyses of patient data were performed. For categorical measures, data included the frequency (number of cases [n] and percentage [%]) for each cohort. For continuous variables, findings were reported as mean, standard deviation (SD), median, Q1, and Q3. When necessary, continuous variables were categorized into intervals, with the distribution of patients (n, %) for each interval provided. All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Overall program utilization
Of 1424 patients who received at least one injection at a participating LCC, 234 patients (16.4%) were enrolled to receive additional support services from the program, mostly through a clinic (59.0%) (). Mean (SD) age of the enrolled population was 37.3 (13.5) years. Of these, the majority were male (n = 142, 60.7%), and from the western (n = 93, 39.7%) and southern (n = 84, 35.9%) regions of the US. The most common payer type was Medicare (n = 76, 32.5%), followed by private commercial (n = 70, 29.9%) and Medicaid (n = 59, 25.2%). The most common plan type was preferred provider organization (PPO) (n = 48, 20.5%), followed by Medicaid (n = 45, 19.2%) and Medicare Part D (n = 35, 15.0%).
Table 2. Characteristics of patients enrolled in the patient support program.
Use of other program support services is reported in . The majority of patients who enrolled in the program completed their benefit verification (n = 203, 86.8%) and/or received nurse support services (n = 170, 72.6%). Of the patients who utilized the nurse support offering, 99 patients (58.2%) received a call reminder for their injection. Prior authorization assistance, copay assistance, and patient assistance program referral was utilized by 14.1%, 13.2%, and 12.0% of patients, respectively.
Table 3. Other patient support program offerings utilized.
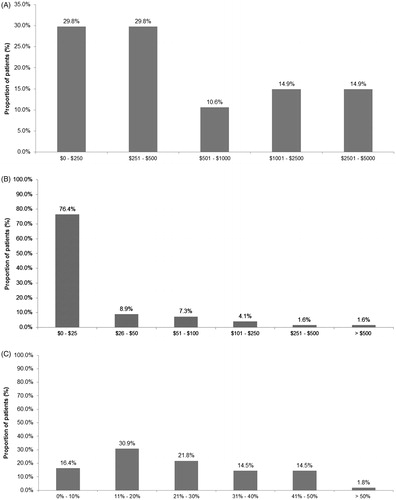
Patient cost share for long-acting injectable aripiprazole was summarized for patients with reported information. Deductibles were reported for 47 out of 234 patients; the mean (SD) and median deductibles were $964 ($1,090) and $400, respectively (). Copayment for long-acting injectable aripiprazole was reported for 123 patients, of which the majority had a copayment of $0–$25 per prescription (n = 94, 76.4%) (). Coinsurance was reported for 55 patients (). Nearly half of patients (47.3%) had coinsurance per prescription of 20% or lower.
LAI antipsychotic adherence
Of the patients who received an injection at the LCC, regardless of their enrollment in other aspects of the program, 157 patients had 6 months of follow-up, and 87 patients had 9 months of follow-up. Patients without sufficient follow-up were excluded from the adherence analyses. The demographic characteristics of each group are reported in . Patients were 41 years old, on average, and the largest group of patients was 34 years of age or younger, followed by patients between the ages of 35 and 44. The majority of patients were male, and preferred to be contacted by the LCC via phone.
Table 4. Characteristics of patients in the adherence analyses.
Adherence to long-acting injectable aripiprazole is reported in . On average, patients received 6.74 injections during the 6 months of follow-up, with a mean PDC of 0.98. Patients were treated for an average of 177.67 days (out of a maximum of 182 days) during the 6 months of follow-up (). For the cohort of patients with at least 9 months of follow-up, patients received an average of 9.78 injections, with a mean PDC of 0.97. Patients were treated for an average of 266.08 days (out of a maximum of 273 allowed days) during the 9 months follow-up (). All patients included in the analyses were considered adherent to treatment (PDC ≥80%) during both 6 months and 9 months of follow-up periods.
Table 5. Adherence to long-acting injectable aripiprazole.
Discussion
This study is the first to evaluate the ASSURE patient support program and investigate real-world adherence to long-acting injectable aripiprazole among patients receiving injections at nationwide ASSURE program LCCs. We found patients utilizing LCCs and with at least 6 months and 9 months of follow-up have between 97% and 98% of days covered by long-acting injectable aripiprazole. Our estimates among patients utilizing the LCC are substantially higher than those reported in the general population of patients with schizophrenia using oral antipsychotics (30–50%)Citation9,Citation10 or LAI antipsychotics (48–91%)Citation8,Citation16–18.
Increasing adherence and persistence among patients with schizophrenia is critical to reducing the economic and clinical burden associated with under- or untreated schizophreniaCitation4–7,Citation9. Our findings are corroborated by a prior study that indicated a positive effect of patient support programs on adherence for patients with schizophreniaCitation18. The effect of patient support programs is not limited to schizophrenia; a previous meta-analysis found that patient support programs in general improve adherence and persistence for patients, with programs that incorporate both behavioural and informational strategies having the greatest impactCitation29. The ASSURE Program, in addition to providing patient and physician education/information, also includes behavioural strategies such as individualized planning and one-on-one nurse support, which may explain some of the benefits of the program on adherence and persistence. Enabling patients to access a medication by removing barriers to treatment (e.g. costs, lack of facility support) also influences medication adherence and persistence by making it easier for patients to remain on treatment. It is also possible that patients who enroll and maintain contact with a patient support program are more aware of their disease and treatment, and awareness of their disease is a factor that is associated with increased medication adherenceCitation30.
Study limitations should be considered when interpreting these results. First, our data were collected for program implementation and not for research purposes, and there is the potential for missing data, particularly clinical information. Information regarding the specific diagnosis for which patients were receiving injections was not available in this dataset, however during the time these data were collected, long-acting injectable aripiprazole was only indicated for the treatment of schizophrenia. Given the study period and FDA approval date for long-acting injectable aripiprazole, it is reasonable to assume that the patients in our study were seeking treatment support for schizophrenia. Second, our sample size for this study was limited, and future studies should be performed with a larger sample to confirm our findings. Third, results only reflect medication adherence of patients who seek treatment and support from the LCC and have sufficient follow-up in the program, and findings may not be generalizable to all patients. Specifically, patients who actively seek care from the LCC may be more inclined to adhere to treatment compared to those with shorter follow-up. Moreover, since treatment data outside of the program were not available and the study was not designed to compare across different patient support programs or between patients utilizing the LCC and those who did not, our findings should not be interpreted in such context. Despite a lack of a control group, previous studies examining adherence among patients with schizophrenia can be used as a benchmark to understand the adherence and persistence of patients in this patient support program. Additional research is needed to validate our findings, and future studies should assess the economic and clinical benefits of the patient assistance program by comparing outcomes in patients utilizing the LCC to patients not utilizing the LCC.
Conclusion
Patients prescribed long-acting injectable aripiprazole who received injections at local care centers demonstrate high adherence to medication over 6 and 9 months. Our findings suggest that injection services provided by the local care centers may help patients remain on long-acting injectable antipsychotic treatment. Enhancing availability of local care centers and nurse support, as well as promoting patient awareness of such a program, may be key to further facilitate adherence and persistence to long-acting injectable antipsychotic treatments.
Transparency
Declaration of funding
This study was funded by Otsuka Pharmaceutical Development & Commercialization, Inc. Employees of Otsuka Pharmaceutical Development & Commercialization, Inc. were involved in the data analysis and interpretation, and in the preparation of the manuscript.
Declaration of financial/other relationships
CB, MB, and AS were contracted by Otsuka Pharmaceutical Development & Commercialization, Inc. to conduct this study. MG and HK are employees of Otsuka Pharmaceutical Development & Commercialization, Inc. Medical writing of the manuscript was provided by CB, MB, and AS, and was funded by Otsuka Pharmaceutical Development & Commercialization, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. A peer review on this manuscript has received compensation for serving on an advisory board for Alkermes related to their Aristada product. Their university receives research funds on their behalf from Alkermes, Otsuka, Janssen.
Acknowledgments
Programming and statistical support was provided by Kainan Sun of IQVIA.
References
- NIMH: Schizophrenia. Bethesda, MD; 2018. Available at: https://www.nimh.nih.gov/health/statistics/schizophrenia.shtml [Accessed January 28, 2018]
- Wu EQ, Birnbaum HG, Shi L, et al. The economic burden of schizophrenia in the United States in 2002. J Clin Psychiatry 2005;66:1122-9
- Cloutier M, Aigbogun MS, Guerin A, et al. The economic burden of schizophrenia in the United States in 2013. J Clin Psychiatry 2016;77:764-71
- Kennedy JL, Altar CA, Taylor DL, et al. The social and economic burden of treatment-resistant schizophrenia: a systematic literature review. Int Clin Psychopharmacol 2014;29:63-76
- Weiden PJ, Kozma C, Grogg A, et al. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv 2004;55:886-91
- Ascher-Svanum H, Faries DE, Zhu B, et al. Medication adherence and long-term functional outcomes in the treatment of schizophrenia in usual care. J Clin Psychiatry 2006;67(3):453–60
- Lindenmayer J-P, Liu-Seifert H, Kulkarni PM, et al. Medication nonadherence and treatment outcome in patients with schizophrenia or schizoaffective disorder with suboptimal prior response. J Clin Psychiatry 2009;70:990-6
- Patel KR, Cherian J, Gohil K, et al. Schizophrenia: overview and treatment options. Pharm Ther 2014;39:638
- Bellack AS, Bowden CL, Bowie CR, et al. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry 2009;70(Suppl 4):1-48
- Morken G, Widen JH, Grawe RW. Non-adherence to antipsychotic medication, relapse and rehospitalisation in recent-onset schizophrenia. BMC Psychiatry 2008;8:32
- Perkins DO. Predictors of noncompliance in patients with schizophrenia. J Clin Psychiatry 2002;63:1121-8
- Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry 2002;63:892-909
- Stephenson J, Tuncelli O, Gu T, et al. Adherence to oral second‐generation antipsychotic medications in patients with schizophrenia and bipolar disorder: physicians’ perceptions of adherence vs. pharmacy claims. Int J Clin Pract 2012;66:565-73
- Velligan DI, Weiden PJ, Sajatovic M, et al. Strategies for addressing adherence problems in patients with serious and persistent mental illness: recommendations from the expert consensus guidelines. J Psychiatr Pract 2010;16:306-24
- Přikryl R, Přikrylová Kučerová H, Vrzalová M, et al. Role of long-acting injectable second-generation antipsychotics in the treatment of first-episode schizophrenia: a clinical perspective. Schizophr Res Treat 2012;2012;2012:764769
- Pilon D, Tandon N, Lafeuille M-H, et al. Treatment patterns, health care resource utilization, and spending in Medicaid beneficiaries initiating second-generation long-acting injectable agents versus oral atypical antipsychotics. Clin Ther 2017;39:1972-85.e1972
- Marcus SC, Zummo J, Pettit AR, et al. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Special Pharm 2015;21:754-69
- Benson CJ, Joshi K, Lapane KL, et al. Evaluation of a comprehensive information and assistance program for patients with schizophrenia treated with long-acting injectable antipsychotics. Curr Med Res Opin 2015;31:1437-48
- Greene M, Yan T, Chang E, et al. Medication adherence and discontinuation of long-acting injectable versus oral antipsychotics in patients with schizophrenia or bipolar disorder. J Med Econ 2018;21:127-34
- Raedler LA. Aripiprazole lauroxil (Aristada): long-acting atypical antipsychotic injection approved for the treatment of patients with schizophrenia. Am Health Drug Benef 2016;9(Spec Feature):40-3
- Velligan DI, Medellin E, Draper M, et al. Barriers to, and strategies for, starting a long acting injection clinic in a community mental health center. Commun Ment Health J 2011;47:654-9
- Patel MX, Taylor M, David AS. Antipsychotic long-acting injections: mind the gap. Br J Psychiatry Suppl. 2009;52:S1-4
- Getzen H, Beasley M, D’mello DA. Barriers to utilizing long-acting injectable antipsychotic medications. Ann Clin Psychiatry 2013;25:E1-E6
- Velligan DI, Mueller J, Wang M, et al. Use of environmental supports among patients with schizophrenia. Psychiatr Serv 2006;57:219-24
- Hamann J, Heres S, Seemann U, et al. Effects of an integrated care program for outpatients with affective or psychotic disorders. Psychiatry Res 2014;217:15-19
- ZYPREXA RELPREVV patient care program instructions brochure. 2016. Available at: https://www.zyprexarelprevvprogram.com/PDF/Lilly%20Zyprexa%20Relprevv%20Folder%20Booklet.pdf [Last accessed September 13, 2018]
- ARISTADA Care Program. 2018. Available at: https://www.aristadacaresupport.com/assistance-programs [Last accessed October 10, 2018]
- The ASSURE Program from Otsuka. 2018. Available at: https://www.assure.com/ [Last accessed September 10, 2018]
- Burudpakdee C, Khan ZM, Gala S, et al. Impact of patient programs on adherence and persistence in inflammatory and immunologic diseases: a meta-analysis. Patient Prefer Adher 2015;9:435
- Olfson M, Marcus SC, Wilk J, et al. Awareness of illness and nonadherence to antipsychotic medications among persons with schizophrenia. Psychiatr Serv 2006;57:205-11