Abstract
Objective: This retrospective database analysis complements previous research to understand treatment patterns for German patients newly-initiating or switching to subsequent GLP-1 RAs.
Methods: Adult patients (≥18 years) initiating GLP-1 RA (Cohort 1 [C1]) or switching from a previous GLP-1 RA (Cohort 2 [C2]) to exenatide twice-daily (exBID), exenatide once-weekly (exQW), dulaglutide (DULA), or liraglutide (LIRA) were included in this analysis using IQVIA LRx from January 1, 2014–March 31, 2017. Patients were required to have ≥1 oral anti-hyperglycemic prescription during the 6-month pre-index period and ≥12 months follow-up. Persistence and treatment modifications were assessed within and beyond 12 months follow-up. Average daily/weekly dosage (ADD/AWD) was calculated during persistence.
Results: C1 included 13,417 patients, while C2 included 4,264 patients. Mean ± standard deviation (SD) age was similar (57.7 ± 11.1 years [C1], 58.9 ± 10.1 years [C2]). Most patients using DULA in C2 had switched from LIRA (56.6%). For C1, mean ADD for LIRA was 1.41 ± 0.10 mg, slightly higher in C2, and increased over time. ADD for exBID was 16.9 ± 1.0 mcg, slightly greater in C2. AWD was 2.00 ± 0.05 mg for exQW users and 1.42 ± 0.03 mg for DULA users in C1, similar to C2. For C1, 27.0% exBID, 35.3% exQW, 50.9% DULA, and 48.1% LIRA users remained persistent at 12 months. Patients using DULA had a higher probability of remaining persistent over time (Kaplan-Meier) for both cohorts.
Conclusions: Patients using DULA had the highest probability of remaining persistent over time, followed by LIRA. ADD/AWD for DULA, exQW, and exBID were aligned with the recommended combination therapy dose; LIRA ADD suggests some patients use the 1.8 mg dose.
Introduction
An estimated 425 million people globally have been diagnosed with diabetes, and the number of people with type 2 diabetes mellitus (T2DM) is increasing worldwideCitation1,Citation2. In high-income countries, it has been estimated that ∼87–91% of all people with diabetes have T2DMCitation3.
In 2017, the age-adjusted prevalence of diabetes in German adults was 8.4%Citation2. Approximately 9.1 million German people have diabetes, the second largest population of people with diabetes in Europe after the Russian Federation (9.3 million)Citation2,Citation4. In Germany, per capita costs for people with T2DM totaled €5146 in 2010, with 10% of the total statutory health insurance expense (€16.1 billion) spent on diabetes managementCitation5.
T2DM is a complex and progressive metabolic disease, with a number of treatment options available for adult patients to improve glycemic control. The German treatment recommendations for T2DM published by the German Diabetes and Internal Medicine AssociationCitation6, as well as the National Treatment Guideline T2DMCitation7 and American Diabetes Association (ADA)Citation8 state that treatment options must be selected according to the pathophysiological phase of the disease at the time the treatment is begun and individualized to patients’ needsCitation6–8.
Lifestyle intervention is the first-line non-pharmacological treatment option, and is also the mainstay for all following treatment escalation steps. If the individual glycated hemoglobin (HbA1c) target is not achieved within 3–6 months, metformin is the recommended first-line pharmacotherapyCitation6,Citation8. When metformin is contraindicated or not tolerated, patients may be started on monotherapy with another anti-hyperglycemic drug, as per nationalCitation6 or international treatment recommendationsCitation8. In cases where the HbA1c value does not reach the individual treatment targetCitation6 after 3–6 months, a dual pharmacological combination strategy with metformin and another anti-hyperglycemic drug, including a glucagon-like peptide-1 receptor agonist (GLP-1 RA), is recommendedCitation6,Citation8. Treatment can be intensified further with insulin therapy or other regimens if HbA1c values do not reach the target after another 3–6 monthsCitation6,Citation8,Citation9.
In Germany, currently available GLP-1 RAs include exenatide twice-daily (Byetta, exBID), available from April 2007Citation10, liraglutide once-daily (Victoza, LIRA), available from July 2009Citation11; exenatide once-weekly (Bydureon, exQW)Citation12, available from September 2011; albiglutide once-weekly (Eperzan), available from October 2014Citation13; and dulaglutide (Trulicity, DULA) once weekly, available from February 2015Citation14.
GLP-1 RAs vary in their magnitude of HbA1c and weight reduction, and adverse event profilesCitation15,Citation16. Additionally, formulation, half-life, injection frequencies, and dose escalations of GLP-1 RAs differ. LIRA, DULA, and albiglutide can be used as monotherapy or combination therapy, while exBID and exQW should be used in combination therapy only. Patients will be initiated on 5 mcg of exBID twice-daily for at least 1 month to improve tolerability, then switched to the 10 mcg dose twice-daily to further increase glycemic controlCitation10. For better gastrointestinal tolerability, LIRA should be initiated with a dose of 0.6 mg injected once per day for at least 1 week, then increased to 1.2 mg once per day11. Some patients may benefit from a further dose increase to 1.8 mg LIRA (maximum recommended daily dose) after at least another week for additional glycemic improvementCitation11.
ExQW, albiglutide, and DULA have a longer duration of action and are all administered once per weekCitation12–14. There is only one dose of exQW available (2 mg once-weekly)Citation12, while albiglutide is initially administered as a 30 mg dose and increased to 50 mg once-weekly if needed for glycemic responseCitation13. DULA is usually recommended as 0.75 mg once-weekly for monotherapy or 1.5 mg once-weekly in combination therapy with other glucose-lowering medicationCitation14.
Only a few studies globally have evaluated treatment patterns with regards to adherence and/or persistence based on prescription data across different GLP-1 RA treatmentsCitation17–23. Previous studies indicate that better adherence and persistence with glucose lowering treatments in T2DM may result in improved clinical and economic outcomesCitation24–27. However, only limited real-world data are available on medication use with the changing spectrum of available GLP-1 RAs, including data on persistence, discontinuation, or switch dynamics, especially in Germany. The aim of this real-world study of a German prescription database was to evaluate treatment patterns, including persistence and treatment modifications, for currently available GLP-1 RAs.
Methods
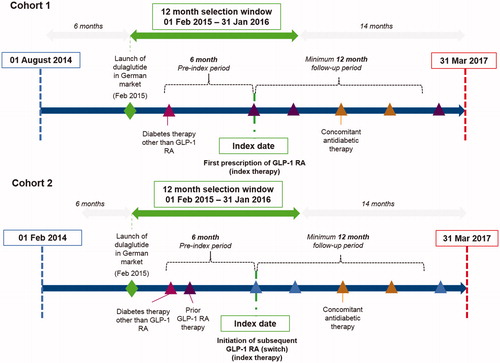
This retrospective study was performed for two cohorts of patients: those initiating their first GLP-1 RA treatment (Cohort 1; including DULA, LIRA, albiglutide, exQW, and exBID), and those switching to a subsequent GLP-1 RA during the given index time period (Cohort 2). The study period comprised a 6-month pre-index period (back to August 1, 2014) and a 12-month selection window from February 1, 2015 to March 31, 2017 (to coincide with licensing of most recently launched GLP-1 RA, DULA, and last available data). For Cohort 2, the pre-index period was extended back to February 1, 2014 for assessment of prior exposure to GLP-1 RAs only.
Patients using albiglutide were included in the analysis but not presented, as only eight patients were found in Cohort 1, and two patients in Cohort 2; however, the overall columns reflect inclusion of albiglutide users. To complement previous research, the overall study design was similar to previous GLP-1 RA use studies conducted in other European countriesCitation18,Citation19, with the addition of DULA users and patients switching to subsequent GLP-1 RAs (Cohort 2).
Data source
The IQVIA German Longitudinal Prescription (LRx) database was used, which accesses nationwide pharmacy data centers processing prescription data for ∼60% of German patients with statutory health insurance for reimbursement purposesCitation28. Data are entered at the point of sale from retail pharmacies based on prescriptions that have been dispensed, and subsequently collated and pseudonymized (de-identified). Patient-specific data include pseudonymized identification number, age, gender, insurance company (statutory insurance only), and region. Prescription information includes prescribers’ pseudonymized identification number, date, and medication at a package level. Information on diagnoses is not part of LRx. Ethical approval was not required for secondary use of these data.
Study details and cohort design
An overview of the cohort design is provided in . The index date for Cohort 1 was the date of first prescription of a GLP-1 RA, with the initiated GLP-1 RA therapy deemed the index therapy. For Cohort 2, the index date was the date of first prescription of a second or subsequent GLP-1 RA after prior exposure to GLP-1 RA during the 12 months prior to index.
Patients’ data were included in the analysis if patients had 6 months of continuous prescription activity within the database prior to index date, were aged ≥18 years, and had a minimum follow-up of 12 months after the index date. Patients were required to have received ≥1 prescription for an oral anti-hyperglycemic drug (proxy for T2DM diagnosis in absence of diagnostic codes) and no GLP-1 RA treatment in the 6-month pre-index period (Cohort 1) or prior exposure to GLP-1 RA during the 12 months prior to index date (Cohort 2). Patients’ prescription activities were analyzed until the earliest of the end of the continuous eligibility period or the end of the study period.
Patients with unknown age or who were prescribed LIRA indicated for weight management (Saxenda) were excluded from the analysis. Analyses were separated according to cohort (1 and 2) and index therapy (exBID, exQW, DULA, or LIRA).
Measures and analyses
All analyses were descriptive only, with no statistical tests performed to formally compare the treatment cohorts.
Patients’ demographics (age, gender), and details of prior exposure to anti-hyperglycemic therapy classes (other than GLP-1 RA) or non-diabetic agents within the 6 months prior to index date were described overall and by index GLP-1 RA treatment group for both cohorts. Other anti-hyperglycemic therapy classes of interest included alpha glucosidase inhibitors (AGI), biguanide (metformin), dipeptidyl peptidase (DPP)-4 inhibitors, meglitinides, sodium-glucose co-transporter (SGLT)-2 inhibitors, sulphonylurea, thiazolidinediones, fixed oral combinations (counted as two classes), short-acting insulins, basal insulins, and pre-mixed insulins. Non-diabetic agents included antidepressants, anti-emetics, weight-loss medication (including anti-obesity medications, excluding Saxenda), anti-platelet medication, cardiovascular (CV) medication (anti-hypertensive drugs, anti-arrhythmic drugs, lipid-lowering agents), and non-steroidal anti-inflammatory drugs (NSAIDs), similar to a previous studyCitation19.
Duration of follow-up (mean, standard deviation [SD]) in months was calculated by cohort and index medication group. First treatment modification (average daily dose [ADD] changes, augmentation of therapy, discontinuation of index therapy, and switch to a non-index anti-hyperglycemic therapy) was evaluated over the 12-month minimum follow-up period (n, %) and over the variable follow-up period thereafter (Kaplan-Meier).
ADD of the index therapy was assessed for all patients during the time persistent on the index therapy using previously used methodologyCitation19. A mathematical approximation of a daily dose was calculated by dividing the total amount/units of the prescribed drug by the number of days between two consecutive prescriptions of this drug during follow-up (overall and at 30-day intervals). An average weekly dose (AWD) was calculated for exQW and DULA by multiplying the approximated daily dose by 7. ADD changes for the index therapy included first ADD increase or decrease, resulting from the mathematical ADD calculation based on prescriptions over time; two consecutively lower ADDs was defined as a decrease, and two consecutively higher ADDs was an increase. ADD increase at any time was also included. Calculated ADD/AWDs more than twice the maximum licensed dosage for the respective GLP-1 RA were not considered for analyses, as these calculated doses were deemed clinically implausible.
Patients were considered persistent (i.e. remaining on index therapy) until evidence of discontinuation or switch; this was defined as the occurrence of a gap in successive prescriptions that was >2-times the expected duration of a single prescription, or prescription of a new non-index therapy within 30 days prior to or following a discontinuation. Treatment augmentation was defined as ≥2 prescriptions for ≥1 new non-index anti-hyperglycemic prescription (other than GLP-1 RAs), started >30 days prior to the end of follow-up or discontinuation date.
All analyses were conducted using SAS software version 9.4 (Cary, NC).
Results
Cohort 1
After inclusion and exclusion criteria were applied, Cohort 1 comprised prescription data from 13,417 patients. The greatest proportion of GLP-1 RA initiators were prescribed LIRA (47.9%), followed by DULA (34.1%); 9.1% of patients received prescriptions of exQW, and 8.8% recorded exBID. The mean (±SD) age of the cohort was 57.7 ± 11.1 years. Females and males accounted for 37.3% of the cohort each, and sex information was missing for 25.4%. Patients were followed for an average of 20.8 ± 3.5 months ().
Table 1. Baseline characteristics for Cohort 1.
Prior to index date, 56.1% of the patients recorded only one anti-hyperglycemic therapy class, the most common of which was metformin (63.3%); this ranged from 51.9% for DULA users to 70.7% for LIRA users. A larger proportion of DULA users (40.2%) recorded fixed oral combinations than LIRA (26.0%) and exBID/QW users (21.2% and 36.4%, respectively). The greatest proportion of Cohort 1 recorded 1–4 non-diabetic therapies (44.7%), which was similar across GLP-1 RA molecules. Cardiovascular medication (82.1%) was the most commonly used non-diabetic therapy class for all GLP-1 RA treatments ().
Mean ADD for LIRA was calculated to be 1.41 ± 0.10 mg (n = 4676) and 16.9 ± 1.0 mcg (n = 703) for exBID. AWD was calculated for DULA and exQW users, where exQW users recorded a mean AWD of 2.00 ± 0.05 mg (n = 853) and DULA users had a mean AWD of 1.42 ± 0.03 mg (n = 3710). When examining ADD over time, mean ADD tended to increase for LIRA users (1.29 ± 0.52 mg [n = 4651] for days 0–30 to 1.58 ± 0.53 mg [n = 315] by days 601–630). For DULA users, AWD ranged from 1.39–1.46 mg (up to day 630; n = 3702 to n = 338) before patient numbers steeply declined beyond day 630.
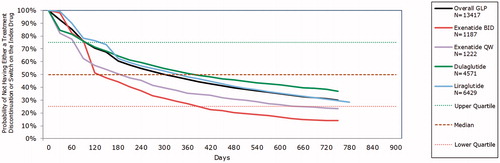
Within the minimum 12-month follow-up period, 46.0% of Cohort 1 remained persistent (). The proportion of patients persistent with the index therapy was greatest for patients prescribed DULA (50.9%) and LIRA (48.1%) and lowest for patients prescribed exBID (27.0%). Similar proportions of DULA (73.0%), LIRA (73.4%), and exQW (71.3%) recorded a first treatment modification during 12 months of follow-up, while 84.5% of exBID users modified therapy.
Table 2. Index therapy treatment modifications over the 12-month follow-up period, Cohort 1.
In Kaplan-Meier analyses, patients using DULA had the greatest probability of persistence beyond day 180, while for exBID and exQW users, persistence was lowest at and beyond this point (). At day 630, the probability of remaining on index therapy was 0.41 for DULA users (95% CI = 0.39–0.42), and 0.34 (95% CI = 0.33–0.35) for LIRA users.
Cohort 2
Cohort 2 included prescription data for 4264 patients. Most patients (76.3%) initiated DULA as a second or subsequent therapy (). Nearly half (49.5%) of Cohort 2 had switched from LIRA; amongst DULA users, 56.6% were previously treated with LIRA. Patients were, on average, 58.9 ± 10.1 years of age overall. The cohort was 36.4% male, 40.0% female, and 23.6% had missing sex information. Patients were followed for a mean of 18.7 ± 4.8 months, overall. During the 6 months prior to index, most patients recorded three or more (64.2%) anti-hyperglycemic classes, the most common being metformin (72.9–81.1%), aside from previous GLP-1 RA exposure. A smaller proportion of exQW users tended to record three or more classes of anti-hyperglycemic treatment during the pre-index period (51.6%), compared to >63% for other GLP-1 RA users. Overall, the largest proportion of patients (46.8%) recorded 5–9 non-diabetic therapy classes; cardiovascular medications were the most common (88.6%) ().
Table 3. Baseline characteristics for Cohort 2.
Mean overall ADD for LIRA was calculated at 1.52 ± 0.09 mg (n = 314) and 18.4 ± 1.9 mcg (n = 141) for exBID. For exQW users, the calculated mean AWD was 1.99 ± 0.07 mg (n = 229) and for DULA users, the mean AWD was 1.47 ± 0.03 mg (n = 2684). LIRA ADD tended to increase over time, from a mean of 1.41 ± 0.56 mg (n = 312) at baseline to a mean of 1.62 ± 0.53 mg (n = 70) at days 121–150 post-initiation. For DULA users, AWD ranged from 1.43–1.45 mg (n = 2679 to n = 766) over the same time period.
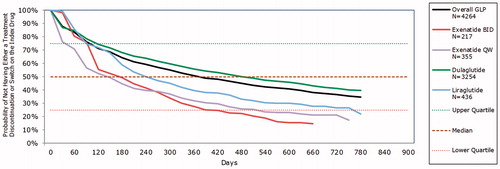
Over half (51.0%) of Cohort 2 remained persistent during the 12-month follow-up period; this ranged from 28.1% of exBID users to 56.0% of DULA users (). Most LIRA users (81.0%) and 83.9% of exBID users modified treatment, compared with 69.4% of DULA users. ADD decrease was the most common first modification (15.5%) aside from discontinuation (38.1%), and was most frequently recorded in DULA users (17.8%). A further switch was recorded infrequently (3.3% overall).
Table 4. Index therapy treatment modifications over the 12-month follow-up period, Cohort 2.
During the variable follow-up, Kaplan-Meier analyses revealed that patients using DULA had the highest probability of remaining persistent, while those using exBID and exQW were most likely to discontinue index therapy (). The probability of remaining on therapy for DULA was 0.45 (95% CI = 0.43–0.46) at day 630, which was substantially greater than for LIRA users (0.29, 95% CI = 0.25–0.34).
Discussion
The results of this retrospective, observational real-world drug utilization study provide insights into treatment patterns for new users of GLP-1 RAs and those switching from an initial to another GLP-1 RA treatment. Treatment patterns varied, but trends were apparent: patients using DULA either as first or subsequent GLP-1 RA tended to persist on treatment longer than other GLP-1 RAs, including LIRA, with greater differences emerging beyond 12 months post-initiation. Patients using exBID and exQW were most likely to modify or discontinue treatment.
The findings of this study complement previous research conducted using German LRx prior to licensing of DULACitation18,Citation19. Similar to Divino et al.Citation18, the proportion of patients remaining persistent at 12 months was lowest for exBID users (29.0% in Divino et al.Citation18; 27.0% in Cohort 1 of the present study) and higher for LIRA users (43.1% remaining persistent in Divino et al.Citation18; 48.1% in Cohort 1). A study by Alatorre et al.Citation17 using American claims data also found that patients using DULA were more likely to persist on therapy than those using LIRA or exQW over a 6-month follow-up period. Improved persistence and adherence are therapeutic goals in T2DM as discontinuation rates of therapies are high, regardless of treatment groupCitation29. Better persistence to GLP-1 RAs has been associated with greater reductions in HbA1c and decreased healthcare costsCitation22, suggesting that continuous treatment may improve patient outcomes and reduce T2DM-related expenditure.
By analysing prescriptions dispensed over time, ADD was within the licensed dosage for LIRA initiators overall (1.41 mg), but tended to increase over time. AWD for DULA initiators was 1.42 mg. For patients initiating LIRA as their subsequent GLP-1 RA, a greater mean ADD (1.52 mg) was calculated, which also increased over time. The trend of increased use of the 1.8 mg LIRA dose is confirmed in an observational study conducted in Belgium, where the proportion of patients recording the 1.8 mg dose increased from <11% at 3 months to 32.7% at 12 monthsCitation29. Furthermore, the EVIDENCE prospective observational study in France found that ∼13% of patients were receiving 1.8 mg LIRA at 3 months, which increased to ∼37% at 12 monthsCitation31.
The authors acknowledge some limitations in relation to the study design and data source. As diagnoses were not available within the data set, a proxy of prior prescription with oral anti-hyperglycemic treatment was used. Furthermore, other clinical data to estimate effectiveness of treatments (e.g. HbA1c levels) were not available within LRx. As such, the authors were unable to perform adjusted analyses due to the lack of data capture of key demographic and clinical characteristics which could potentially influence patient or physician choice of GLP-1 RAs. With regards to prescription data, those received or purchased outside the LRx panel pharmacies were not recorded, which could lead to under-estimation of drug utilization; inclusion of these pharmacies only could also lead to selection bias, but German LRx coverage is substantial (∼ 60% of prescriptions for patients with statutory health insurance). Although treatment modifications were assessed based on available data, the reasons for these decisions were not available as part of the database characteristics. Frequency of treatment administration (i.e., weekly, daily) may have impacted the choice to initiate or discontinue a therapyCitation33. Injection frequency and application characteristics may become more important when the clinical profile of treatments is similarCitation34. ADD/AWD were calculated using packs dispensed over time, which means that stockpiling of prescriptions or prescription fills prior to run-out could lead to over-estimations. In the future, analyses could be conducted over longer periods to assess treatment patterns over time, or using other data sources inclusive of clinical parameters.
Conclusions
The findings of this study indicate that use patterns of GLP-1 RAs have changed over time following the licensing of new therapies. Naïve and GLP-1 RA experienced patients initiating DULA had the highest probability of remaining persistent over time, while users of exBID were more likely to discontinue or modify therapy. ADD for LIRA suggests that, over time, a greater proportion of patients may have received the higher dose of 1.8 mg indicatedCitation11 if additional glycemic control is needed. The results align with previously conducted research into GLP-1 RA treatment patterns across various countries. An analysis of the association between treatment patterns and clinical characteristics could provide further insights.
Transparency
Declaration of funding
Sponsorship for this study and article processing charges were funded by Eli Lilly and Company, Indianapolis, IN.
Declaration of financial/other interests
Trulicity® (dulaglutide) is a registered trademark owned and licensed by Eli Lilly and Company, its subsidiaries, or affiliates. IQVIA received consulting fees from Eli Lilly and company for the conduct of this study. Kirsi Norrbacka is an employee and a shareholder of Eli Lilly and Company. Thorsten Otto is an employee of Eli Lilly and Company. Heike Jung is an employee and shareholder of Eli Lilly and Company. Jeremie Lebrec is an employee of Eli Lilly and Company. Hartmut Richter and Melissa Myland are employees of IQVIA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. CMRO peer reviewers on this manuscript have no relevant financial relationships to disclose.
Supplemental Material
Download TIFF Image (146.9 KB)Supplemental Material
Download TIFF Image (153.4 KB)Acknowledgments
The authors thank Sarah Jenner, employee of IQVIA, who has been involved in the study set up and protocol writing.
References
- World Health Organization. Global report on diabetes [Internet]. Geneva, Switzerland: WHO; 2016. Available at: http://www.who.int/about/licensing/\n http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf [Last accessed 1 September 2017]
- IDF. IDF diabetes atlas, 8th edition [Internet]. Brussels: International Diabetes Federation; 2017. Available at: http://www.diabetesatlas.org/ [Last accessed November 17, 2017]
- Jaacks LM, Siegel KR, Gujral UP, et al. Type 2 diabetes: a 21st century epidemic. Best Pract Res Clin Endocrinol Metab 2016;30:331–43
- Tamayo T, Brinks R, Hoyer A, et al. The prevalence and incidence of diabetes in Germany. Dtsch Arztebl Int 2016;113:177–82
- Jacobs E, Hoyer A, Brinks R, et al. Healthcare costs of Type 2 diabetes in Germany. Diabet Med 2017;34:855–61
- Landgraf R, Kellerer M, Fach E, et al. Praxisempfehlungen DDG/DGIM. Diabetol Stoffwechsel 2016;11:S117–S129
- Gemeinsamer Bundesausschuss. des Gemeinsamen Bundesausschusses über eine Änderung der Arzneimittel-Richtlinie (AM-RL): Anlage XII - Beschlüsse über die Nutzenbewertung von Arzneimitteln mit neuen Wirkstoffen nach § 35a SGB V – Dulaglutid [Internet]. Berlin: Gemeinsamer Bundesausschus; 2015. Available at: https://www.g-ba.de/downloads/39-261-2290/2015-07-16_AM-RL-XII_Dulaglutid_2015-02-01-D-154_BAnz.pdf [Last accessed November 17, 2017]
- American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40:S64–S74
- VersorgungsLeitlinie N. Therapiedes Tyap 2 Diabetes [Internet]. Berlin: NATIONALE VERSORGUNGSLEITLINIEN; 2014. Available at: http://www.deutsche-diabetes-gesellschaft.de/fileadmin/Redakteur/Leitlinien/Evidenzbasierte_Leitlinien/NVL_Typ-2_Therapie-lang_Apr_2014.pdf [Last accessed: 22 March 2018]
- EMA. Summary of product characteristics: Byetta, exenatide BID [Internet]. London: EMA; 2018. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000698/WC500051845.pdf [Last accessed March 29, 2018]
- EMA. Summary of product characteristics: Victoza, liraglutide [Internet]. London: EMA; 2018. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001026/WC500050017.pdf [Last accessed March 29, 2018]
- EMA. Summary of product characteristics: Bydureon, exenatide QW [Internet]. London: EMA; 2018. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002020/WC500108241.pdf [Last accessed March 29, 2018]
- EMA. Summary of product characteristics: Eperzan, albiglutide [Internet]. London: EMA; 2017. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002735/WC500165117.pdf [Last accessed March 29, 2018]
- EMA. Summary of product characteristics: Trulicity, dulaglutide [Internet]. London: EMA; 2018. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/0028 25/WC500179470.pdf [Last accessed February 19, 2018]
- Lovshin JA. Glucagon-like peptide-1 receptor agonists: a class update for treating type 2 diabetes. Can J Diabetes 2017;41:524–35
- Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2012;8:728–42
- Alatorre C, Fernández Landó L, Yu M, et al. Treatment patterns in patients with type 2 diabetes mellitus treated with glucagon-like peptide-1 receptor agonists: higher adherence and persistence with dulaglutide compared with once-weekly exenatide and liraglutide. Diabetes Obes Metab 2017;19:953–61
- Divino V, DeKoven M, Khan FA, et al. GLP-1 RA treatment patterns among type 2 diabetes patients in five European countries. Diabetes Ther 2017;8:1–14
- Divino V, DeKoven M, Hallinan S, et al. Glucagon-like peptide-1 receptor agonist treatment patterns among type 2 diabetes patients in six European countries. Diabetes Ther 2014;5:499–520
- McDonell A, Kiiskinen U, Zammit D, et al. Estimating the real world daily usage and cost for exenatide twice daily and liraglutide in Germany, the Netherlands, and the UK based on volumes dispensed by pharmacies. Clin Outcomes Res 2015;7:95
- Fuchs S, Kostev K, Seitz L, et al. Ermittlung der tatsächlichen Tagesdosierung von Liraglutid (PDD) unter realen Versorgungsbedingungen im Hinblick auf die Berechnung von Tagestherapiekosten. Diabetol Stoffwechsel 2011;6:P234
- Lin J, Lingohr-Smith M, Fan T. Real-world medication persistence and outcomes associated with basal insulin and glucagon-like peptide 1 receptor agonist free-dose combination therapy in patients with type 2 diabetes in the US. Clinicoecon Outcomes Res 2017;9:19–29
- Miller L-A, Burudpakdee C, Zagar A, et al. Exenatide BID and liraglutide QD treatment patterns among type 2 diabetes patients in Germany. J Med Econ 2012;15:746–57
- Rhee MK, Slocum W, Ziemer DC, et al. Patient adherence improves glycemic control. Diabetes Educ 2005;31:240–50
- Lee WC, Balu S, Cobden D, et al. Medication adherence and the associated health-economic impact among patients with type 2 diabetes mellitus converting to insulin pen therapy: an analysis of third-party managed care claims data. Clin Ther 2006;28:1712–25
- Stuart BC, Simoni-Wastila L, Zhao L, et al. Increased persistency in medication use by U.S. Medicare beneficiaries with diabetes is associated with lower hospitalization rates and cost savings. Diabetes Care 2009;32:647–9
- Nagrebetsky A, Griffin S, Kinmonth AL, et al. Predictors of suboptimal glycaemic control in type 2 diabetes patients: the role of medication adherence and body mass index in the relationship between glycaemia and age. Diabetes Res Clin Pract 2012;96:119–28
- Richter H, Dombrowski S, Hamer H, et al. Use of a German longitudinal prescription database (LRx) in pharmacoepidemiology. Ger Med Sci 2015;13:Doc14
- Bell KF, Cappell K, Liang M, et al. Comparing Medication Adherence and Persistence Among Patients with Type 2 Diabetes Using Sodium-Glucose Cotransporter 2 Inhibitors or Sulfonylureas. Am Heal drug benefits 2017;10:165–174
- Buysschaert M, D’Hooge D, Preumont V. ROOTS: a multicenter study in Belgium to evaluate the effectiveness and safety of liraglutide (Victoza®) in type 2 diabetic patients. Diabetes Metab Syndr Clin Res Rev 2015;9:139–42
- Gautier J-F, Martinez L, Penfornis A, et al. Effectiveness and persistence with liraglutide among patients with type 2 diabetes in routine clinical practice—EVIDENCE: a prospective, 2-year follow-up, observational, post-marketing study. Adv Ther 2015;32:838–53
- Guo X-H The value of short- and long-acting glucagon-like peptide-1 agonists in the management of type 2 diabetes mellitus: experience with exenatide. Curr Med Res Opin 2016;32:61–76
- Otto T, Stralka R, Schimmelpfennig H, et al. Treatment options for patients with progressing type 2 diabetes: What are patients' preferences in Germany when switching from oral to injectable antidiabetic treatment? Gesundh ökon Qual Manag 2016;21:181–198
- Gelhorn H, Poon J-L, Davies E, et al. Evaluating preferences for profiles of GLP-1 receptor agonists among injection-naïve type 2 diabetes patients in the UK. Patient Prefer Adherence 2015;9:1611