Abstract
Objective: Achieving and maintaining recommended glycemic targets, including those for glycated hemoglobin A1c (A1C), is key to improving outcomes in patients with type 2 diabetes (T2D). As fasting plasma glucose and postprandial glucose contribute to overall A1C, targeting both is essential for sustaining glycemic control.
Methods: This review examines the complementary mechanisms of action of glucagon-like peptide 1 (GLP-1) receptor agonists and basal insulin; they both enhance glucose-stimulated insulin release and suppress glucagon secretion. GLP-1 receptor agonists also slow gastric emptying and increase satiety.
Results: Adding a GLP-1 receptor agonist to therapy with a basal insulin analog has been associated with improved overall glycemic control, with comparable risk of hypoglycemia and no weight gain. Titratable fixed-ratio co-formulations of basal insulin and a GLP-1 receptor agonist have been shown to improve glycemic control, with less complex dosing schedules, possibly increasing treatment adherence. The slow titration of fixed-ratio co-formulations has been shown to reduce the occurrence and severity of gastrointestinal adverse events associated with the use of a separate GLP-1 receptor agonist. Titratable fixed-ratio co-formulations also mitigate insulin-associated weight gain, and show a comparable risk of hypoglycemia to basal insulin use alone.
Conclusions: The efficacy and safety of titratable fixed-ratio co-formulations have been demonstrated for insulin degludec/liraglutide and insulin glargine/lixisenatide in the DUAL and LixiLan trials, respectively, in both insulin-naive and -experienced patients. Titratable fixed-ratio co-formulations represent an attractive treatment option for many patients with T2D.
Introduction
Type 2 diabetes (T2D) is a chronic metabolic disease characterized by an insulin secretory defect in pancreatic β-cells that, in most individuals, is combined with insulin resistance in peripheral tissues, such as skeletal muscle, fat, and liver. This is clinically manifested by increases in fasting plasma glucose (FPG) and postprandial glucose (PPG) levels, both of which independently contribute to an overall increase in glycated hemoglobin (A1C)Citation1,Citation2.
Achieving glycemic control is central to preventing diabetes-associated microvascular and macrovascular complications in those with T2DCitation3. As their disease progresses and β-cell function declines, most patients with T2D will require treatment escalation from initial metformin monotherapy to combinations of metformin with other oral anti-hyperglycemic drugs (OADs) and/or injectable therapies—commonly basal insulin and/or, more recently, a glucagon-like peptide 1 (GLP-1) receptor agonistCitation3.
Basal insulin analogs are intended to mimic the basal component of normal physiological endogenous insulin secretion by pancreatic β-cells, and are primarily effective in controlling A1C via reductions in FPG levels. However, clinical trials and real-world studies have demonstrated that many patients with T2D do not reach (or fail to maintain) glycemic targets with basal insulin alone, and are in need of therapeutic escalationCitation4,Citation5. This may be related to a number of factors, including clinical inertiaCitation6, a plateau effect on FPG with basal insulinCitation7,Citation8, and the contribution of PPG levels to A1CCitation1,Citation2.
Treatment guidelines recommend intensification of therapy for patients on basal insulin when A1C remains above target, despite acceptable FPG levels (suggesting PPG is above goal) or where basal insulin has been titrated to >0.5 units (U)/kg/day and FPG is still not at goalCitation3,Citation9,Citation10. Under this circumstance, the American Diabetes Association (ADA) recommends consideration of initiation of combination injectable therapyCitation3. GLP-1 receptor agonists, short- or rapid-acting insulin preparations, dipeptidyl peptide-4 (DPP-4) inhibitors, and sodium-glucose co-transporter type 2 inhibitors are among the recommended strategies to control PPG levelsCitation3,Citation10. However, patients and clinicians are sometimes reluctant to escalate therapy because of concerns which may include weight gain, hypoglycemia, and tolerability issues.
Strategies which can improve glycemic control while minimizing adverse events have clear advantages. The combination of a basal insulin analog and a GLP-1 receptor agonist has theoretical advantages based on the complementary mechanisms of action of the two agents affecting both FPG (predominantly controlled by basal insulins) and PPG (predominantly controlled by GLP-1 receptor agonists). In addition, the combination has potential to reduce hypoglycemia and weight gain that can be associated with basal insulin therapy aloneCitation11–18. The aim of this review is to explore the rationale and benefits of using a once-daily, titratable fixed-ratio co-formulation (combination) of a basal insulin analog and a GLP-1 receptor agonist for the management of T2D.
The importance of adherence in the treatment of those with T2D
Adherence to therapy is critical for achieving optimal glycemic control in patients with T2D. However, it is widely recognized that there are a range of patient- and treatment-related factors that can negatively impact adherence ()Citation19–21. For patients treated with insulin-based regimens, the most commonly cited concerns are hypoglycemia and weight gainCitation22. Other adverse events associated with additional agents may also raise concerns and/or impact on adherence; for example, GLP-1 receptor agonists are associated with an increased risk of gastrointestinal adverse eventsCitation23, while the addition of some DPP-4 inhibitors might infrequently be associated with hypersensitivity, skin-related reactions, and joint painCitation24,Citation25. The increased number of daily injections and increasing regimen complexity associated with intensification of basal insulin therapy with the addition of prandial insulin (basal-plus and basal-bolus regimens) or a GLP-1 receptor agonist, may further erode adherence (). For many patients, the need to inject has a significant impact on multiple aspects of their daily lifeCitation26. Being “too busy” to inject, concern about injection discomfort, having multiple daily injections, interference with daily activities, and embarrassment of injecting in public have been given as reasons for non-adherence and insulin-injection omissionCitation22,Citation27,Citation28. Not unexpectedly, most insulin-treated patients want to reduce the number of injections they administer each dayCitation28. Furthermore, having fewer injections has been shown to have a positive impact on health-related quality-of-lifeCitation29. Reducing regimen complexity/injection frequency by the use of titratable fixed-ratio co-formulations has the potential to improve adherence and patient satisfaction with treatmentCitation21,Citation22,Citation30.
Table 1. Factors affecting adherence among T2D patientsCitation19–21.
Rationale for therapy combining a basal insulin analog and a GLP-1 receptor agonist
The scientific rationale for combined therapy with a basal insulin analog and a GLP-1 receptor agonist lies in the complementary mechanisms of action of these two classes of glucose-lowering agents ()Citation31,Citation32. Basal insulin analogs are intended to mimic the normal pattern of physiological basal insulin secretion by providing consistent, long-acting insulin levels. The main role of basal insulin is, therefore, to reduce FPG levels by preventing lipolysis and hepatic glucose production in the fasting state, especially during the nightCitation33.
Table 2. The complementary mechanisms of action of basal insulin and a GLP-1 receptor agonist.
The effects of GLP-1 receptor agonists are glucose-dependent, acting to reduce blood glucose levels through the enhancement of glucose-stimulated insulin secretion by β-cells and suppression of glucagon secretion by α-cells (). GLP-1 receptor agonists can be classified as either short- or long-acting based on their duration of action. Although both classes share some effects, there are also differences (). In addition to their effects on the central nervous system and glucose dependent increase in insulin and decrease in glucagon secretion, short-acting GLP-1 receptor agonists (such as exenatide and lixisenatide) also delay gastric emptying as their primary mechanism of actionCitation34. This limits the rate and extent of meal-derived glucose presentation to β-cells, contributing to a predominant effect on postprandial glucose excursions, most markedly for the meal following administrationCitation34. Long-acting GLP-1 receptor agonists (such as semaglutide, albiglutide, dulaglutide, exenatide extended release, and liraglutide) predominantly lower blood glucose levels through stimulation of insulin secretion and reduction of glucagon levels, with a decreasing effect on gastric emptying over time likely due to tachyphylaxisCitation34–36. This results in a lesser effect on PPG excursions compared with short-acting GLP-1 receptor agonists but a longer overall effect on glucose levels reducing FPGCitation34. GLP-1 receptor agonists have also been shown to improve β-cell functionCitation37,Citation38. The action of GLP-1 receptor agonists on the central nervous system results in increased satiety, which contributes to a reduced food intakeCitation39 and helps mitigate the weight gain often associated with insulin therapy.
Figure 1. Mechanisms of GLP-1 and GLP-1 receptor agonist action. GLP-1, glucagon-like peptide 1. Reproduced with permission from Meier 201234.
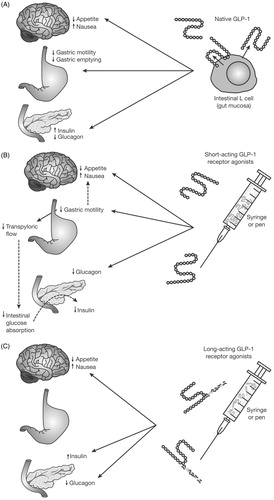
The combination of a basal insulin analog to primarily target FPG and a GLP-1 receptor agonist to benefit PPG control has, therefore, the potential to improve overall glycemic control and to provide relatively stable blood glucose levels. The combination also addresses seven of the eight pathophysiologic abnormalities contributing to hyperglycemia in T2D patients and described as the “ominous octet”Citation40. In clinical studies, adding a GLP-1 receptor agonist to basal insulin led to comparable glycemic control as adding prandial insulin, with lower risk for hypoglycemia, and weight lossCitation13,Citation41,Citation42. Although a greater proportion of patients on GLP-1 receptor agonists experienced gastrointestinal adverse events than those on prandial insulin, treatment with GLP-1 receptor agonists was associated with greater treatment satisfaction and better quality-of-lifeCitation41. Overall, systematic reviews and meta-analyses of observational/clinical practice studies and clinical trials have found that combining a basal insulin analog and a GLP-1 receptor agonist improves glycemic control without weight gain or an increased risk of hypoglycemiaCitation31,Citation43.
Titratable fixed-ratio co-formulation of a basal insulin analog and a GLP-1 receptor agonist: benefits for titration, tolerability, adherence, and persistence
Given the potential effects of complex regimens on adherence to therapy, the ability to co-administer a basal insulin analog and a GLP-1 receptor agonist as a titratable fixed-ratio co-formulation supplied in a single injection device has several advantages. In addition to the potential efficacy benefits resulting from their complementary mechanisms of action, such a co-formulations may provide additional benefits over other combination strategies. Compared with basal-bolus insulin therapy, a titratable fixed-ratio co-formulation allows a less complex and more convenient dosing schedule, with fewer insulin injections and self-monitored blood glucose (SMBG) measurements, as only pre-breakfast FPG levels are required for titration, compared with multiple daily measurements for patients on intensive insulin regimens. Titration is also easier, as one dose adjustment is required rather than two or more separate adjustments. There is also evidence that fixed ratio co-formulations may be insulin sparing compared with insulin alone. In clinical studies where insulin dose was not capped, final insulin doses were lower in the combination treatment group, while providing superior glycemic control ()Citation11,Citation13,Citation15,Citation47. Similarly, final GLP-1 receptor agonist dose in these studies was also lower with the co-formulation compared with the agent alone, due to the more gradual, incremental titration possible with the fixed-ratio co-formulationsCitation11,Citation13. Use of a titratable fixed ratio co-formulation could provide an option when changing from existing regimens with basal insulin or with a GLP-1 receptor agonist. Although it is currently off-label in the US, a titratable fixed-ratio co-formulation could also provide an “easy-start” option for initiating basal insulin therapy when patients on OADs are unable to reach glycemic target. In keeping with studies using separate injections, titratable fixed-ratio co-formulations have been shown to improve glycemic control with no increased risk of hypoglycemia, and to mitigate insulin-associated weight gainCitation11–13,Citation15–18. The slow titration of a fixed-ratio co-formulation has been shown to increase the tolerability of GLP-1 receptor agonists compared with their use individually, reducing the occurrence and severity of gastrointestinal adverse events (e.g. nausea, vomiting, and diarrhea) which often lead to decreased adherence or persistence with treatmentCitation11–13,Citation15–18. Fewer adverse events and reduced risk for insulin-associated weight gain or even leading to modest weight loss may improve patient adherence, which can lead to early and sustained achievement of glycemic control.
Table 3. Summary of key clinical trials of IDegLira and iGlarLixi, including main efficacy, safety, and tolerability findings.
Clinical evaluation of titratable fixed-ratio co-formulations of a basal insulin analog and a GLP-1 receptor agonist
iGlarLixi
iGlarLixiCitation48, a co-formulation of insulin glargine 100 U/mL (iGlar) and lixisenatide (Lixi), is a single daily dose titratable fixed-ratio co-formulation injection. It has been approved by the US Food and Drug Administration (FDA) as an adjunct to diet and exercise to improve glycemic control in adults with T2D inadequately controlled on basal insulin (less than 60 U daily) or lixisenatide. iGlarLixi has also received marketing authorization from the European Medicines Agency (EMA) for the treatment of adults with T2D to improve glycemic control when this has not been provided by metformin alone or metformin combined with another oral glucose-lowering medicinal product or with basal insulinCitation49.
The efficacy of iGlarLixi compared with iGlar was investigated in a 24-week phase 2b proof-of-concept study in insulin-naive patients with T2D on OADsCitation16. Once-daily iGlarLixi demonstrated greater A1C reduction than iGlar, with a similar rate of symptomatic hypoglycemia being observed in both groupsCitation16 (). Two phase 3 clinical trials investigated the efficacy of iGlarLixi compared with one or both of the individual components. The LixiLan-O trial compared iGlarLixi with iGlar and lixisenatide in patients with T2D inadequately controlled on metformin alone or on metformin combined with a second OADCitation17, whereas the LixiLan-L trial compared iGlarLixi with iGlar in patients inadequately controlled on basal insulin with or without OADsCitation18. In both trials, the once-daily iGlar dose was capped at 60 U. These trials showed that treatment with once-daily single injections of iGlarLixi resulted in a statistically superior reduction in A1C compared with either lixisenatide or iGlar, with a safety profile reflecting those of the individual components (). Furthermore, patients on iGlarLixi were more likely to achieve A1C < 7.0% compared with iGlar in both LixiLan-O (73.7% vs 59.4%; p < 0.0001) and LixiLan-L (54.9% vs 29.6%; p < 0.0001) or compared with lixisenatide in LixiLan-O (73.7% vs 33.0%; p < 0.0001)Citation17,Citation18.
In both LixiLan-O and LixiLan-L, patients treated with iGlarLixi experienced slight weight loss, whereas patients treated with iGlar experienced weight gain (p < 0.0001 for both studies); in LixiLan-O, patients treated with lixisenatide alone had the greatest weight loss. In both of these studies, the incidences and event rates of documented symptomatic hypoglycemia were similar in iGlarLixi- and iGlar-treated patients ()Citation17,Citation18. Furthermore, in the LixiLan-O trial, the prevalence of nausea and vomiting, and associated treatment discontinuation, were lower with iGlarLixi than with lixisenatide ()Citation17.
Post-hoc analyses of the LixiLan trials have shown similar results to the primary analysesCitation50,Citation51. Data from three individual studies (ACT6011, DRI6012 [NCT00299871], and LixiLan-O) evaluating the impact of lixisenatide over the dose range delivered by iGlarLixi (5–20 μg) concluded that, even at lower doses, lixisenatide provides clinically meaningful improvements in PPG and A1C. A lixisenatide dose of 5 μg gave a 44% reduction in PPG under the curve compared with a 20 μg dose, and the maximum reduction in PPG area under the curve was achieved at 12.5 μg, indicating that a 20 μg dose is not necessary for the greatest reduction in PPGCitation51. Efficacy and safety of iGlarLixi also appear to be maintained in special populations. In older patients (≥65 years) more iGlarLixi-treated patients achieved A1C < 7.0% than those treated with iGlar alone in both LixiLan-O (78.0% vs 54.4%; p < 0.001) and LixiLan-L (51.8% vs 21.0%; p < 0.001). In addition, there were reductions in the number of hypoglycemic events in older patients treated with iGlarLixi compared with those treated with iGlar alone in both LixiLan-O (1.42 vs 1.89 events/patient-year; p = 0.035) and LixiLan-L (2.84 vs 4.91 events/patient-year; p < 0.001). The proportion of older adults achieving the composite endpoint of A1C < 7.0% with no weight gain and no documented hypoglycemia (SMPG ≤70 mg/dL) was also greater with iGlarLixi than with iGlar alone in both LixiLan-O (30.1% vs 14.0%; p = 0.002) and LixiLan-L (26.4% vs 8.4%; p < 0.001)Citation52. Results from another post-hoc analysis of the LixiLan trials indicate that the lixisenatide component of iGlarLixi mitigates iGlar-associated weight gain while being associated with better gastrointestinal toleranceCitation53. This result is attributed to the more gradual titration of lixisenatide when it is a component of iGlarLixi, rather than a separate injectionCitation53.
A further post-hoc analysis of the LixiLan trials investigated the time taken to achieve glycemic control with iGlarLixi vs iGlar alone. In LixiLan-O, the A1C < 7.0% target was reached by 50% of patients in approximately half the time when treated with iGlarLixi compared with those patients treated with iGlar (median time to target = 85.0 days vs 166.0 days; p < 0.0001). In LixiLan-L, it took a median time of 153.0 days for 50% of iGlarLixi-treated patients to reach the A1C target, whereas the target was never achieved by 50% of patients treated with iGlar (p < 0.0001). The time to FPG control was similar between the two treatment arms, indicating that the PPG-lowering effect of lixisenatide contributes to patients achieving overall glycemic controlCitation54. An additional post-hoc analysis of LixiLan-L determined that patients with A1C levels at screening that were either ≤8%, 8% < A1C ≤ 9%, or >9%, receiving iGlarLixi, had greater reductions in A1C at study end compared with those receiving iGlar, indicating that iGlarLixi is more effective than iGlar at controlling A1C, regardless of A1C value at screeningCitation55.
IDegLira
IDegLiraCitation56 is a once-daily single-injection of a titratable fixed-dose co-formulation of the long-acting basal insulin analog degludec (IDeg) and the once-daily GLP-1 receptor agonist liraglutide (Lira) that has been approved by the FDA as an adjunct to diet and exercise to improve glycemic control in adults with T2D inadequately controlled on basal insulin (<50 U daily) or liraglutide (≤1.8 mg daily). IDegLira has also been granted marketing authorization by the EMA for treatment of adults with T2D to improve glycemic control in combination with oral glucose-lowering medicinal products when these alone, or combined with a GLP-1 receptor agonist or basal insulin, do not provide adequate glycemic controlCitation57.
The DUAL clinical development program for IDegLira encompasses a series of phase 3, 26-week, randomized clinical trials in patients with T2D. Data indicate that treatment with IDegLira results in superior A1C reductions compared with basal insulin or liraglutide alone. This was with a lower risk of hypoglycemia than with IDeg (in trials where the comparator insulin was not capped) and with additional benefits in terms of weight change in both insulin-naive and insulin-experienced patientsCitation11,Citation12,Citation15,Citation44 (). In DUAL III, patients who switched to IDegLira showed superior glycemic control to those who continued on GLP-1 receptor agonist therapy (liraglutide once daily or exenatide twice daily) (). A significantly higher proportion of IDegLira-treated patients achieved the secondary end-point of A1C < 7.0% compared with IDeg alone in both DUAL I (81% vs 65%; p < 0.0001) and DUAL II (60.3% vs 23.2%; p < 0.0001)Citation11,Citation12. Similar results were shown in the DUAL V study of patients with T2D not adequately controlled on iGlar, where A1C < 7% was an exploratory pre-specified end-point; 76% of patients treated with IDegLira achieved an A1C < 7.0% vs 47% who escalated their dose of insulin glargine (p < 0.001)Citation15. Data from the 26-week extension study to DUAL I demonstrated that the benefits of IDegLira were sustained, with a greater reduction in A1C, significantly greater weight loss, and lower rate of hypoglycemia at 52 weeks with IDegLira compared with IDeg. Rates of hypoglycemia were higher, and weight loss lower, for IDegLira compared with liraglutide alone ()Citation13. A prospective sub-study from DUAL I using continuous glucose monitoring showed that, compared with each of its components, IDegLira improved mean PPG excursions in patients with T2D; this was possibly due to the greater endogenous insulin secretion and improved β-cell function observed in those patients, rather than to enhanced glucagon suppressionCitation58. Overall, patients receiving IDegLira in the DUAL studies required a significantly lower dose of insulin by the end of the study than those using IDeg or iGlar (especially in trials where the comparator basal insulin was not capped)Citation13,Citation15. In addition, a post-hoc analysis of data from the DUAL II and DUAL IV studies reported that the proportion of patients experiencing gastrointestinal adverse events during treatment with IDegLira, IDeg alone, or placebo was similar, and lower than that reported in patients treated with liraglutide alone in DUAL I. This is likely due to the slow, steady titration of IDegLiraCitation59.
DUAL VIICitation47 compared IDegLira with basal-bolus therapy (insulin glargine plus insulin aspart). Data suggested that, at least in basal-insulin-experienced patients, transition to IDegLira may be superior to addition of a fast-acting insulin. The decrease in mean A1C was similar between the two regimens (−1.49% vs −1.48%, respectively; p < 0.0001 for non-inferiority). FPG at week 26 was also comparable for patients treated with IDegLira and insulin glargine plus insulin aspart (109.7 mg/dL vs 115.1 mg/dL, respectively). Patients treated with IDegLira had weight loss rather than weight gain (−0.92 kg vs +2.64 kg; p < 0.0001) and had markedly fewer episodes per patient year of overall hypoglycemia (1.07 vs 8.17; p < 0.0001) and nocturnal hypoglycemia (0.13 vs 1.66; p < 0.0001). Additionally, a greater number of patients achieved the triple composite endpoint of A1C < 7%, with no hypoglycemia episodes and no weight gain with IDegLira (34.9% vs 4.7%; p < 0.0001)Citation47. To date, no similar trial has been conducted using iGlarLixi; however, GetGoal-Duo-2 compared the addition of lixisenatide (20 μg once daily) or insulin glulisine (once daily or thrice daily) in patients treated with insulin glargine, allowing for an indirect comparisonCitation14. Changes in glycemic parameters were similar between treatment groups. Lixisenatide-treated patients experienced weight loss (−0.6 kg), whereas those treated with insulin glulisine experienced weight gain in both once daily (+1.0 kg) and thrice daily (+1.4 kg) treatment groups. Furthermore, a higher percentage of patients did not experience weight gain when treated with lixisenatide (64.7%) compared with insulin glulisine once (36.6%) or thrice daily (30.5%). Symptomatic hypoglycemia occurred in fewer patients treated with lixisenatide compared with insulin glulisine once daily (35.9% vs 46.5%; p = 0.01) and thrice daily (35.9% vs 52.4%; p = 0.0001). No severe symptomatic hypoglycemia occurred in the lixisenatide or thrice-daily insulin glulisine groups, and only two (0.7%) occurred in the insulin glulisine once-daily groupCitation14.
Although the LixiLan trials and the DUAL studies differed in design, their findings indicate that such co-formulations can indeed improve glycemic control and tolerability compared with either basal insulin or GLP-1 receptor agonists alone, with an increase in the number of patients reaching glycemic targets and low rates of adverse events (hypoglycemia, weight gain, and gastrointestinal adverse events). Furthermore, data from patient-reported outcomes questionnaires completed by participants in the DUAL V study showed that, compared to iGlar, IDegLira-treated patients had significantly greater improvements in treatment-related satisfaction, particularly treatment burden and diabetes management, and in patient-reported physical healthCitation15. A possible limitation of the LixiLan and DUAL trials is that neither co-formulation was compared directly with co-administration of the GLP-1 receptor agonist and basal insulin as separate injections. Head-to-head trials would be needed to determine any additional benefit of the co-formulations over co-administration of the individual agents. However, indirect comparisons of the results of the LixiLan and GetGoal-Duo trials suggest favorable results with the co-formulation in comparable populationsCitation17,Citation18. Overall, results of the trials to date suggest that titratable fixed-ratio co-formulations of a basal insulin analog and a GLP-1 receptor agonist are effective therapies for both insulin-naive and insulin-experienced patients, although labeling in the US is limited to insulin-experienced patients only or those inadequately controlled on lixisenatide or liraglutide, respectivelyCitation11–13,Citation15–18,Citation44,Citation45.
Optimizing the use of titratable fixed-ratio co-formulation in T2D therapy
The optimal stage in the progression of T2D at which to add a titratable fixed-ratio co-formulation of basal insulin and a GLP-1 receptor agonist will depend on individual patient characteristics and preferences. A potential limitation of the currently available fixed ratio co-formulations is the top dose of insulin available in each formulation (50 U IDegLira and 60 U for iGlarLixi), influenced by the maximum approved GLP-1 receptor agonist dose and the co-formulation ratio of the basal insulin and the GLP-1 receptor agonist. There are also no studies directly comparing sequential administration of basal insulin and a GLP-1 receptor agonist with simultaneous administration of a co-formulation. Despite this lack of head-to-head studies, comparison of results from separate studies suggests that co-administration, rather than sequential addition of a basal insulin analog and a GLP-1 receptor agonist, might be an optimal strategy for many patientsCitation11,Citation12,Citation60, as these results indicate that early simultaneous treatment with a titratable fixed-ratio co-formulation of basal insulin plus a GLP-1 receptor agonist may be more effective than sequential administration. Real-world evidence also suggests limited persistency with the free dose combination of a GLP-1 receptor agonist and basal insulin, with a median time to treatment discontinuation of 133 daysCitation61. Furthermore, a titratable fixed-ratio co-formulation of these therapies may provide more effective treatment, with potentially improved gastrointestinal tolerability, than administration of the two components separately. Given the multiple pathophysiological abnormalities observed in patients with T2D, and the progressive nature of β-cell dysfunction, there might well be a potential benefit in initiating combination therapy with agents that correct different pathogenic defects in T2D earlier in the disease courseCitation62.
Summary
The underlying pathophysiology of T2D involves the progressive loss of pancreatic β-cell function and incretin activity. As the disease progresses, most patients will eventually require the use of combinations of anti-hyperglycemic agents with complementary mechanisms of action.
The combination of a basal insulin analog together with a GLP-1 receptor agonist is currently recommended in ADA and American Association of Clinical Endocrinologists (AACE)/American College of Endocrinology (ACE) guidelines, algorithms, and position statements as an initial strategy for patients with T2D not achieving A1C goal on basal insulin plus OADs, and is also indicated for patients with T2D inadequately controlled on GLP-1 receptor agonist and OADsCitation3,Citation9,Citation10,Citation48,Citation56. The use of titratable fixed-ratio co-formulations of a basal insulin analog and a GLP-1 receptor agonist obviates the need for two separate injections and reduces regimen complexity associated with the administration of each agent individually, and so may have a positive effect on treatment initiation and adherence. In addition to this benefiting the patient, the simplified dosing regimen could also benefit healthcare professionals, therefore reducing a potential barrier to treatment initiation or intensification. Data from clinical trials show that titratable fixed-ratio co-formulations improve glycemic control, allowing more patients with T2D (both insulin-naive and insulin-experienced) to reach their glycemic targets when compared with each medication used separately. The use of these titratable fixed-ratio co-formulations has often been associated with less weight gain, or even weight loss, compared with basal insulin alone, and a lower rate of gastrointestinal adverse events compared with a GLP-1 receptor agonist alone. Reducing regimen complexity has the potential to curtail clinical inertia and, along with fewer adverse events, improve adherence and persistence to therapy, which may improve clinical outcomes.
Transparency
Declaration of funding
Writing/editorial support funded by Sanofi US, Inc.
Declaration of financial/other relationships
L.B. has disclosed he has received grants/research support from AstraZeneca, Janssen Pharmaceuticals, Inc., Lexicon Pharmaceuticals, Inc., Merck & Co., Novo Nordisk, and Sanofi; is a Speaker for AstraZeneca, Janssen Pharmaceuticals, Inc., Merck & Co., Novo Nordisk, and Sanofi; and a Consultant for AstraZeneca, GlaxoSmithKline, Intarcia Therapeutics, Inc., Janssen Pharmaceuticals, Inc., Merck & Co., Inc., Novo Nordisk, and Sanofi. J.E.A. has disclosed that he is an Advisory Board member for Abbott Diabetes, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, and Sanofi; and a Speakers Bureau member for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, and Sanofi; he is a stock shareholder for NuSirt Biopharma. P.C. has disclosed that he has received a research and travel grant from Sanofi; is a Speaker for Janssen and Novo Nordisk; and a Consultant for Pfizer. J.A.D. has no relevant financial or other relationships to disclose. CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors would like to thank Dr R. Aronson for his contributions and critical review of the manuscript. The authors received writing/editorial support in the preparation of this manuscript provided by Catarina Fernandes, PhD, and Keisha Peters, MSc, of Excerpta Medica, funded by Sanofi US, Inc.
References
- Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care 2003;26:881–5
- Riddle M, Umpierrez G, DiGenio A, et al. Contributions of basal and postprandial hyperglycemia over a wide range of A1C levels before and after treatment intensification in type 2 diabetes. Diabetes Care 2011;34:2508–14
- American Diabetes Association. Standards of Medical care in diabetes – 2018. Diabetes Care 2018;41(Suppl 1):S1–S159.
- Blonde L, Brunton SA, Chava P, et al. Achievement of goal A1C (<7%) by U.S. patients with T2DM on basal insulin in both randomized, controlled trials (RCTs) and in clinical practice. Diabetes 2014;63:A235
- Brunton SA, Blonde L, Chava P, et al. Characteristics of patients with T2DM on basal insulin (INS) who do not achieve glycemic goals. Diabetologia 2014;57:S54
- Khunti K, Nikolajsen A, Thorsted BL, et al. Clinical inertia with regard to intensifying therapy in people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab 2016;18:401–9
- Shaefer C, Traylor L, Gao L, et al. Exploratory study of a dose–response curve for basal insulin. Diabetes 2015;64:A253
- Reid T, Gao L, Gill J, et al. How much is too much? Outcomes in patients using high-dose insulin glargine. Int J Clin Pract 2016;70:56–65
- Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract 2015;21(Suppl 1):1–87
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm – 2016 Executive Summary. Endocr Pract 2018;24:91–120
- Gough SC, Bode B, Woo V, et al. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol 2014;2:885–93
- Buse JB, Vilsbøll T, Thurman J, et al. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care 2014;37:2926–33
- Gough SC, Bode BW, Woo VC, et al. One-year efficacy and safety of a fixed combination of insulin degludec and liraglutide in patients with type 2 diabetes: results of a 26-week extension to a 26-week main trial. Diabetes Obes Metab 2015;17:965–73
- Rosenstock J, Guerci B, Hanefeld M, et al. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: the GetGoal Duo-2 trial. Diabetes Care 2016;39:1318–28
- Lingvay I, Pérez Manghi F, García-Hernández P, et al. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized clinical trial. JAMA 2016;315:898–907
- Rosenstock J, Diamant M, Aroda VR, et al. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of lixisenatide and insulin glargine, versus insulin glargine in type 2 diabetes inadequately controlled on metformin monotherapy: the LixiLan proof-of-concept randomized trial. Diabetes Care 2016;39:1579–86
- Rosenstock J, Aronson R, Grunberger G, et al. Benefits of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled on oral agents: the LixiLan-O randomized trial. Diabetes Care 2016;39:2026–35
- Aroda VR, Rosenstock J, Wysham C, et al. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L randomized trial. Diabetes Care 2016;39:1972–80
- Vijan S, Hayward RA, Ronis DL, et al. Brief report: the burden of diabetes therapy: implications for the design of effective patient-centered treatment regimens. J Gen Intern Med 2005;20:479–82
- Ross SA. Breaking down patient and physician barriers to optimize glycemic control in type 2 diabetes. Am J Med 2013;126(9 Suppl 1):S38–S48
- García-Pérez LE, Alvarez M, Dilla T, et al. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther 2013;4:175–94
- Peyrot M, Barnett AH, Meneghini LF, et al. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med 2012;29:682–9
- Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care 2011;34(Suppl 2):S279–S84
- Karagiannis T, Boura P, Tsapas A. Safety of dipeptidyl peptidase 4 inhibitors: a perspective review. Ther Adv Drug Saf 2014;5:138–46
- FDA safety warning [Internet]. Drug Safety Communication: FDA warns that DPP-4 inhibitors for type 2 diabetes may cause severe joint pain. Silver Spring, MD: FDA; 2015. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm459579.htm [Last accessed April 9, 2017]
- Abu Hassan H, Tohid H, Mohd Amin R, et al. Factors influencing insulin acceptance among type 2 diabetes mellitus patients in a primary care clinic: a qualitative exploration. BMC Fam Pract 2013;14:164
- Davies MJ, Gagliardino JJ, Gray LJ, et al. Real-world factors affecting adherence to insulin therapy in patients with Type 1 or Type 2 diabetes mellitus: a systematic review. Diabet Med 2013;30:512–24
- Rubin RR, Peyrot M, Kruger DF, et al. Barriers to insulin injection therapy: patient and health care provider perspectives. Diabetes Educ 2009;35:1014–22
- Evans M, Jensen HH, Bøgelund M, et al. Flexible insulin dosing improves health-related quality-of-life (HRQoL): a time trade-off survey. J Med Econ 2013;16:1357–65
- Pan F, Chernew ME, Fendrick AM. Impact of fixed-dose combination drugs on adherence to prescription medications. J Gen Intern Med 2008;23:611–14
- Balena R, Hensley IE, Miller S, et al. Combination therapy with GLP-1 receptor agonists and basal insulin: a systematic review of the literature. Diabetes Obes Metab 2013;15:485–502
- Baruah MP, Kalra S. The novel use of GLP-1 analogue and insulin combination in type 2 diabetes mellitus. Recent Pat Endocr Metab Immune Drug Discov 2012;6:129–35
- Arnolds S, Kuglin B, Kapitza C, et al. How pharmacokinetic and pharmacodynamic principles pave the way for optimal basal insulin therapy in type 2 diabetes. Int J Clin Pract 2010;64:1415–24
- Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2012;8:728–42
- Nauck MA, Kemmeries G, Holst JJ, et al. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes 201;60:1561–5
- Rodbard HW. The clinical impact of GLP-1 receptor agonists in type 2 diabetes: focus on the long-acting analogs. Diabetes Technol Ther 2018;20:S233–S241
- Bunck MC, Diamant M, Cornér A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2009;32:762–8
- Bunck MC, Cornér A, Eliasson B, et al. Effects of exenatide on measures of β-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care 2011;34:2041–7
- Seufert J, Gallwitz B. The extra-pancreatic effects of GLP-1 receptor agonists: a focus on the cardiovascular, gastrointestinal and central nervous systems. Diabetes Obes Metab 2014;16:673–88
- Defronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–95
- Diamant M, Nauck MA, Shaginian R, et al. Glucagon-like peptide 1 receptor agonist or bolus insulin with optimized basal insulin in type 2 diabetes. Diabetes Care 2014;37:2763–73
- Mathieu C, Rodbard HW, Cariou B, et al. A comparison of adding liraglutide versus a single daily dose of insulin aspart to insulin degludec in subjects with type 2 diabetes (BEGIN: VICTOZA ADD-ON). Diabetes Obes Metab 2014;16:636–44
- Eng C, Kramer CK, Zinman B, et al. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet 2014;384:2228–34
- Linjawi S, Bode BW, Chaykin LB, et al. The efficacy of IDegLira (insulin degludec/liraglutide combination) in adults with type 2 diabetes inadequately controlled with a GLP-1 receptor agonist and oral therapy: DUAL III randomized clinical trial. Diabetes Ther 2017;8:101–14
- Rodbard HW, Bode BW, Harris SB, et al. Safety and efficacy of insulin degludec/liraglutide (IDegLira) added to sulphonylurea alone or to sulphonylurea and metformin in insulin-naïve people with Type 2 diabetes: the DUAL IV trial. Diabet Med 2017;34:189–96
- Harris SB, Kocsis G, Prager R, et al. Safety and efficacy of IDegLira titrated once weekly versus twice weekly in patients with type 2 diabetes uncontrolled on oral antidiabetic drugs: DUAL VI randomized clinical trial. Diabetes Obes Metab 2017;19:858–65
- Billings LK, Doshi A, Gouet D, et al. Efficacy and safety of IDegLira versus basal-bolus insulin therapy in patients with type 2 diabetes uncontrolled on metformin and basal insulin: the DUAL VII randomized clinical trial. Diabetes Care 2018;41:1009–16
- Soliqua prescribing information [Internet]. Bridgewater, NJ: Sanofi-aventis U.S. Updated 2016. Available at: http://products.sanofi.us/Soliqua100-33/Soliqua100-33.pdf [Last accessed April 9, 2017]
- Suliqua summary of product characteristics [Internet]. Paris, France: Sanofi-aventis Groupe. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_ Product_Information/human/004243/WC500224673.pdf [Last accessed April 9, 2017]
- Blonde L, Bailey TS, Chao J, et al. Characteristics and glycemic outcomes of T2D patients (PTS) titrated to 60 U/day with insulin glargine/lixisenatide fixed-ratio combination (iglarlixi) vs. insulin in the lixilan-L trial [abstract]. Diabetes 2017;66(Suppl 1):A294 (abstract 1107-P)
- Frias J, Hurst W, Newton J, et al. Impact of lixisenatide (LIXI) dose range on clinical outcomes with fixed-ratio combination (FRC) iglarlixi in patients with T2D [abstract]. Diabetes 2017;66(Suppl 1):A291–292 (abstract 1100-P)
- Handelsman Y, Chovanes C, Dex T, et al. Efficacy and safety of insulin glargine/lixisenatide fixed-ratio combination in elderly patients with T2D. American Diabetes Association - 76th Annual Scientific Sessions 2016;65:A246
- Skolnik N, Dupree RS, Johnson EL. iGlarLixi, a titratable once-daily fixed-ratio combination of basal insulin and lixisenatide for intensifying type 2 diabetes management for patients inadequately controlled on basal insulin with or without oral agents. Curr Med Res Opin 2017;33:2187–94
- Frias JP, Domingo MP, Meneghini LF, et al. Shorter time to glycemic control with fixed-ratio combination of insulin glargine and lixisenatide compared with insulin glargine treatment alone [abstract]. Diabetes 2017;66(Suppl 1):A287 (abstract 1084-P)
- Niemoeller E, Souhami E, Wu Y, et al. iGlarLixi reduces glycated hemoglobin to a greater extent than basal insulin regardless of levels at screening: post hoc analysis of LixiLan-L. Diabetes Ther 2018;9:373–82.
- Xultophy prescribing information [Internet]. Bagsvaerd, Denmark: Novo Nordisk A/S. Updated 2016. Available at: http://www.novo-pi.com/xultophy10036.pdf [Last accessed April 9, 2017]
- Xultophy summary of product characteristics [Internet]. Bagsvaerd, Denmark: Novo Nordisk A/S. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPA R_-_Product_Information/human/002647/WC500177657.pdf [Last accessed April 9, 2017]
- Holst JJ, Buse JB, Rodbard HW, et al. IDegLira improves both fasting and postprandial glucose control as demonstrated using continuous glucose monitoring and a standardized meal test. J Diabetes Sci Technol 2015;10:389–97
- Aroda V, Jaekel E, Jarlov H, et al. Similar incidence of gastrointestinal side effects between IDegLira and non-glucagon-like peptide-1 receptor agonist comparators [abstract]. Diabetologia 2015;58(Suppl 1):S1–S607; abstract 833
- DeVries JH, Bain SC, Rodbard HW, et al. Sequential intensification of metformin treatment in type 2 diabetes with liraglutide followed by randomized addition of basal insulin prompted by A1C targets. Diabetes Care 2012;35:1446–54
- Lin J, Lingohr-Smith M, Fan T. Real-world medication persistence and outcomes associated with basal insulin and glucagon-like peptide 1 receptor agonist free-dose combination therapy in patients with type 2 diabetes in the US. Clinicoecon Outcomes Res 2017;9:19–29
- DeFronzo RA, Eldor R, Abdul-Ghani M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care 2013;36(Suppl 2):S127–S138
