Abstract
Objective: To compare treatment patterns, persistence and adherence between fixed-dose combinations (FDCs) and two-pill combinations (TPCs) of oral antidiabetic drug (OAD) classes in Japanese patients with type 2 diabetes mellitus (T2DM) using administrative claims databases (Japan Medical Data Center [JMDC] and Medical Data Vision [MDV]).
Methods: This was a retrospective, longitudinal cohort analysis conducted between 2011 and 2015, in patients with T2DM receiving OADs as FDC or TPC. Outcomes included prescribing patterns, treatment persistence and adherence.
Results: Data from 3474 and 3066 patients receiving FDCs, and 4325 and 5192 patients receiving TPCs from the JMDC and MDV databases, respectively, was extracted. The most common OAD combination received by over half of all patients was dipeptidyl peptidase-4 inhibitor (DPP-4i) + thiazolidinediones (TZDs) (64.1% [JMDC] and 70.5% [MDV]). Overall, 12-month persistence rates were higher in patients receiving FDCs compared with TPCs (70.4 vs. 66.2% [JMDC], 75.6 vs. 55.7% [MDV]). In the JMDC population receiving FDCs or TPCs, persistence rates were highest with DPP-4i schedules (67.5–83.5%). Median time to discontinuation was significantly longer with biguanide + TZD, and DPP-4i + TZD FDC schedules (p < .05) than TPC; adherence rates were ≥80% across all antidiabetic drug classes in both database populations.
Conclusions: Persistence with and adherence to OADs in Japanese patients with T2DM were greater with FDCs than with TPCs, which may suggest increased patient satisfaction due to reduced treatment burden. Further studies are warranted to investigate the impact of adherence and persistence of FDCs of OADs on glycemic control.
Introduction
The rising prevalence of type 2 diabetes mellitus (T2DM) represents a global health challenge of ever-increasing importanceCitation1. In 2017, approximately 425 million people worldwide were diagnosed with diabetes, and this is expected to reach 629 million by 2045Citation2. Asian populations constitute approximately 60% of global diabetes patientsCitation3 and, in Japan, diabetes prevalence is expected to increase from 7.9% in 2010 to 9.8% by 2030Citation4. This rise is largely attributed to increases in longevity, sedentary lifestyle and obesityCitation5,Citation6.
As the prevalence of diabetes increases, the burden of diabetes on the economy will rise substantially. This is worsened by the need to treat various diabetes-related complications such as retinopathy, and comorbidities such as hypertension and hyperlipidemiaCitation7. Adherence to oral antidiabetic drugs (OADs) and additional medications for comorbidities is important to help prevent or manage the long-term complications associated with diabetes and minimize healthcare costsCitation8. Even small improvements in adherence can have a substantial economic impact: it has been reported that a 10% increase in adherence has led to a decrease in total annual healthcare costs by 8.6%Citation9. In contrast, non-adherence is associated with poor glycemic control, increased economic burdenCitation10 and increased mortalityCitation11. Similarly, persistence with substandard treatment is commonly associated with diabetesCitation12, and reduces the effectiveness of disease managementCitation11,Citation13 and contributes to additional healthcare costsCitation14. Barriers to treatment adherence in patients with T2DM, who are largely responsible for their daily glycemic controlCitation8, may include patient factors, regimen complexity or dosing frequency, and factors such as inadequate follow-up or supportCitation8,Citation15. With respect to regimen complexity, a fixed-dose combination (FDC) can help simplify treatment regimens, enhance adherence and contribute towards improved glycemic controlCitation16.
The American Diabetes Association (ADA) recommends initial metformin monotherapy at diagnosis of T2DM followed by dual combination therapy at hemoglobin A1c (HbA1c) ≥9% to achieve glycemic controlCitation15. If target HbA1c has not been achieved after 3 months, a combination of metformin with another OAD class is recommendedCitation15, followed by metformin plus two OADs from different drug classes if target HbA1c has still not been achieved after a further 3 monthsCitation15. There are currently seven classes of OADs available in Japan for the treatment of diabetes, namely, biguanides (BGs), thiazolidinediones (TZDs), dipeptidyl peptidase-4 inhibitors (DPP-4is), sulfonylureas (SUs), rapid-acting insulin secretagogues, α-glucosidase inhibitors (α-GIs) and sodium–glucose co-transporter-2 inhibitors (SGLT-2is), which can be administered with or without insulin, in different combinationsCitation15. While DPP-4is are orally bioavailable and well tolerated with long-term efficacyCitation17, the early DPP-4is had pharmacokinetic (PK) profiles that required daily or twice daily dosing, which may have led to a decrease in patient adherence.
As new treatment regimens and approaches become available in Japan, notably once weekly dosing, it is important to compare efficacy, persistence and adherence of current therapies used in the treatment of T2DM in a practical setting using real-word data for health economic evaluations to aid reimbursement decision-making. Here, we present the findings from an observational study using data from two large health insurance claims databases in Japan. We investigated real-world trends in treatment patterns, persistence and adherence in FDCs and two-pill combinations (TPCs) of OAD classes in Japanese patients with T2DM.
Methods
Study design and data sources
This was a retrospective, longitudinal, observational cohort study, using claims from two databases in Japan between 1 January 2010 and 31 December 2016: the health insurance administrative Japan Medical Data Center (JMDC) database that included >4 million patients since January 2005 (Japan Medical Data Center Co., Ltd; Tokyo, Japan) and the hospital-based Medical Data Vision (MDV) with >21 million accumulated patients (as of December 2017) since April 2003 (Medical Data Vision Co., Ltd; Tokyo, Japan). The JMDC database contains monthly claims submitted to health insurance societies from medical institutions, while the MDV database is a nationwide hospital-based claims database, covering patients treated as inpatients or outpatients that participate in the Diagnostic Procedure Combination/Per-Diem payment system. Both databases hold anonymized information about diagnoses, patient characteristics, drug prescriptions, medical procedures, features of medical facilities and reimbursement costs. All patient data is encrypted before entry.
Study population
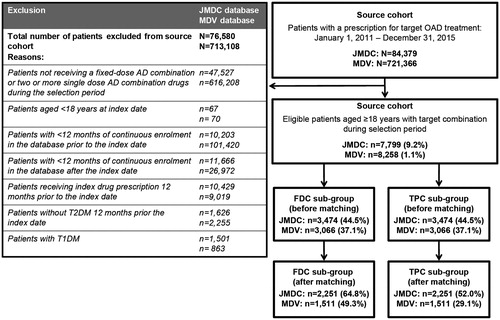
The study population consisted of all patients starting a combination of FDC and/or TPC of OADs during the selection period. Patients were included if they had a diagnosis of T2DM (International Classification of Diseases, 10th revision [ICD-10] code E11 or E14), were aged ≥18 years and received ≥1 prescription of a FDC or TPC for an OAD of interest during the selection period of 1 January 2011 to 31 December 2015. OADs of interest included DPP-4is, SUs, α-GIs, BGs, TZDs and SGLT-2is. Patients were required to have ≥12 months of continuous enrolment in the JMDC or MDV database before (pre-index period) and after (post-index period) the index date (defined as the first prescription date for a target combination initiated during the selection period), without prescription of the index combination (defined as the antidiabetic drug classes of interest prescribed at index date) within the pre-index period. The pre- and post-index periods were designed to allow for the adequate follow-up of treatment-related outcomes of interest and their relationship with baseline factors, and to ensure that the first prescription of a given OAD corresponded to initiation of that drug class. In situations where follow-up/enrolment information was missing from the MDV database, patients had to have ≥1 medical claim in each quarter of the pre-index period to allow longitudinal analysis. Patients with a diagnosis of type 1 diabetes mellitus (T1DM), within the pre- and post-index period, were excluded.
A total of five subgroups were defined based on T2DM therapies of interest that are available in Japan and can be used with or without insulin and in combination: TZD + BG, TZD + SU, α-GI + glinide, TZD + DPP-4i and DPP-4i + BG ().
Table 1. Definitions of FDCs and TPCs.
Study objectives and outcomes
The objectives of the study were to describe patterns of OAD use and to compare treatment persistence and adherence rates between FDCs and TPCs of OAD classes in Japanese patients with T2DM (treatment-naïve and treatment-experienced) listed in the JMDC and MDV database population. Adherence to treatment was measured as the proportion of days covered (PDC) 12 months after initiation of the OAD category in treatment-naïve and treatment-experienced patients with T2DM. The PDC was calculated as the number of days the treatment was available to the patient divided by the number of days in the treatment period. For this study, patients were considered adherent if they had a PDC ≥0.80. Persistence was defined as the time from the index date to the first discontinuation of the index OAD. Adherence analyses were performed for patients with at least two prescriptions of the index OAD class(es) during the 12 month post-index follow-up period.
Statistical analysis
Data management was handled by Creativ-Ceutical and analyses were performed using SAS version 9.3 (SAS Institute; Cary, NC, USA) and conducted on all patients who met the inclusion criteria. Treatment patterns were reported descriptively. Time to treatment-related events in both treatment-naïve and treatment-experienced patients, including time to switch and time to treatment discontinuation, was analyzed per line-specific strategies using Kaplan–Meier methodology.
Due to the potential imbalance in baseline covariates between patients receiving FDCs and TPCs, propensity score matching was performed to eliminate the impact of confounding parametersCitation18,Citation19. Each patient in the FDC group was matched with one patient in the TPC group with the closest propensity score, using a greedy algorithm without replacement (i.e. once a match had been made, the match was not reconsidered). The maximum tolerated difference between matched patients in a “non-perfect” matching (i.e. calliper matching) was 0.2 of a standard deviation of the logit of the propensity score. The propensity score was assessed using a multivariable logistic regression model. The potential confounders included: age at index date, gender, polypharmacy in the pre-index period, Charlson Comorbidity Index (CCI), treatment status, comorbidities of interest (hyperlipidemia, hypertension, dementia, diabetic nephropathy), follow-up duration and the number of OAD classes at index-date.
An adjusted Cox regression model was used to compare time from index date to drug discontinuation between comparative subgroups. Treatment persistence was described per Kaplan–Meier and subgroup differences were evaluated using log-rank test; summary statistics were also provided. For all analyses, a two-sided p <.05 was considered statistically significant. For patient characteristics to be included in regression models, a threshold level of α = .10 was used.
Statement of ethics
Based on Ethical Guidelines for Epidemiological ResearchCitation20 issued by the Japanese Ministry of Health, Labor and Welfare, ethics approval and informed consent were not applicable for this study. The study complied with the International Society for Pharmacoepidemiology Guidelines for Good Pharmacoepidemiology PracticesCitation21. All authors had full access to all the data, and take responsibility for its integrity and the data analysis.
Patient involvement
No patients were involved in setting the research question or outcome measures, and no patients were involved in developing plans for study implementation. Furthermore, no patients were asked for advice about interpretation or writing up of results. There are no plans to distribute the research findings to study participants or the specific patient community. Individual patient consent was not required for this study, as the trial was based on anonymized administrative claims data.
Results
Patient disposition
Between January 2011 and December 2015, 84,379 patients in the JMDC database and 721,366 patients in the MDV database with at least one prescription for an OAD class of interest were identified. Of these, 7799 patients (9.2%) in the JMDC database and 8258 patients (1.1%) in the MDV database met the inclusion criteria and were included in the analyses (). After propensity score matching to discard patients without complementary matches, there were a total of 4502 patients in the JMDC database and 3022 in the MDV database ().
Patient characteristics
Patient demographics and clinical characteristics before and after matching are presented in and respectively. In the matched patient population, mean duration of follow-up was similar in each database and across both sub-cohorts (FDC and TPC). In the JMDC database across both sub-cohorts, patients were younger (aged 53.2–53.4 vs. 67.1–67.5 years), there was a greater proportion of males (77.5–78.5% vs. 67.1–67.5%), the mean number of concurrent medications was lower (2.0 vs. 5.1), and incidence rates for comorbid hyperlipidemia (46.5–47.4 vs. 68.2–74.1%), hypertension (54.8–55.2 vs. 69.1–77.1%) and dementia (0–0.1 vs. 2.2–3%) were lower than in the MDV database. Diabetic nephropathy was higher in the JMDC than in the MDV database (19.1–20.2 vs. 16.5–17.5%).
Table 2. Patient demographics and clinical characteristics (before matching).
Table 3. Patient demographics and clinical characteristics (after matching).
Treatments
In the JMDC database, approximately half of the patients were taking 1–3 medications in the FDC and TPC group, whereas an approximate equal number of patients in the MDV database were receiving 1–3, 4–5 or 6–8 medications. The majority of patients in both the JMDC and MDV databases were treatment-experienced across both groups. The most common therapy received by over half of all patients was a combination of DPP-4i + TZD in both the JMDC and MDV databases (64.1 and 70.5%). Only 0.6% of patients were receiving DPP-4i + BG in the MDV group compared with 12.1% in the JMDC database. The least common therapy across both groups was SU + TZD, received by only 1.3% of patients in the JMDC database and 1.8% of patients in the MDV database ().
Persistence
Overall, treatment continuation rate in the FDC group was high across both databases ( and ). In the JMDC database and in both groups (FDC and TPC), 12 month persistence rates, described as the probability of remaining on treatment with the index OAD class at 12 months, were highest with the DPP-4i schedules (range: 67.5–83.5%) and lowest with the α-GI + glinide schedule (range: 53.8–60.4%) (). In the MDV database persistence rates were highest with BG schedules (range: 60.0%–88.9%) and lowest with SU schedules (range: 48.1–59.3%) (). In the JMDC database, persistence rates were numerically higher in the FDC group than the TPC group (range: 66.5–83.5 vs. 58.5–72.5%), except for α-GI + glinide, which was higher in the TPC group (60.4 vs. 53.8%), and for SU + TZD which was comparable in both groups (66.7%). In the MDV database persistence rates were also numerically higher in the FDC group compared with TPC except for SU + TZD, which was higher in the TPC group (). Persistence rate of approximately 50% or less were recorded for SU + TZD as a FDC, and for α-GI + glinide as a TPC.
Table 4. Time to treatment discontinuation and 12 month persistence rate (JMDC).
Table 5. Time to treatment discontinuation and 12 month persistence rate (MDV).
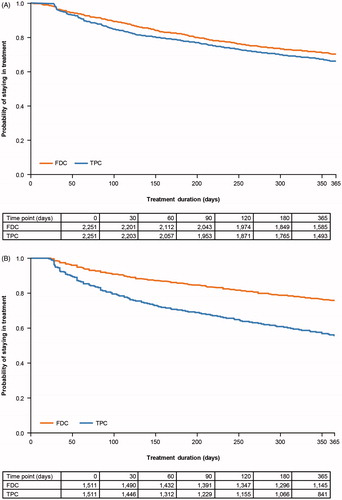
Overall median time to discontinuation in the FDC group was significantly longer across both databases compared with TPC (p = .002 [JMDC], p < .0001 [MDV], ). In both databases, median time to discontinuation was significantly longer in the FDC group compared with TPC for the BG + TZD and DPP-4i + TZD schedules (p < .05). The median time to discontinuation for the DPP-4i + BG schedule in the JMDC database and α-GI + glinide schedule in the MDV database was significantly longer in the FDC group compared with the TPC group (p < .05) ( and ).
Figure 2. KM curve of time to discontinuation according to FDC or TPC OAD class in: (A) the JMDC patient population; (B) the MDV patient population. Abbreviations. FDC, fixed-dose combination; JMDC, Japan Medical Data Center; KM, Kaplan–Meier; MDV, Medical Data Vision; OAD, oral antidiabetic drug; TPC, two-pill combination.
Adherence
Overall, adherence to OAD classes was high (mean PDC 0.90–0.97) across both databases and in both patient groups ( and ). Adherence rates were ≥80% across all combinations in both database populations, although slightly higher in the MDV database (88.9–98.6%, ) than in the JMDC database (82.1–93.6%, ). In the JMDC database, the lowest adherence rates were with SU + TZD in the FDC group and α-GI + glinide in the TPC group, and DPP-4i + BG in both the FDC and TPC groups in the MDV database ( and ). For the remaining antidiabetic drug classes in the JMDC database, adherence rates were ≥86%, and ≥90% in the MDV database in the FDC and TPC groups ( and ).
Table 6. Treatment adherence assessed by PDC in patients using FDC and TPC (JMDC).
Table 7. Treatment adherence assessed by PDC in patients using FDC and TPC (MDV).
Discussion
This retrospective, longitudinal, observational cohort study assessed treatment patterns, persistence and adherence to OAD classes in patients receiving FDC or TPC using medical claims information from two large administrative claims databases in Japan. The analysis was undertaken to evaluate adherence to prescribed OAD class(es) and continuation of treatment over its prescribed course in Japanese patients.
Analysis of OAD classes used as index therapy in the FDC and TPC groups revealed that approximately three quarters of patients were receiving DPP-4i + TZD irrespective of the database. Analysis of the data revealed that adherence to OADs was generally high (≥80% for all drug classes) irrespective of FDC or TPC. For persistence rates at 12 months, generally more patients receiving FDC continued treatment after 12 months compared with TPC, irrespective of the database. Overall across all OAD classes, the median time to discontinuation was significantly shorter for TPC compared with FDC in both databases (JMDC, p = .002; MDV, p < .0001). Time to discontinuation appeared to be significantly different in patients receiving a DPP-4i + TZD or DPP-4i + BG schedule and a BG + TZD schedule in the JMDC database (p < .05), and patients receiving a BG + TZD, DPP-4i + TZD or α-GI + glinide schedule in the MDV database (p < .05). In contrast, median time to discontinuation was not significantly different between patients receiving TZD + SU or α-GI + glinide, irrespective of receiving FDC or TPC, which may be related to the lack of statistical power and the number of patients in each group.
Adherence to antidiabetic treatment is a driver of clinical and economic outcomesCitation14. The results of this study indicate that many Japanese patients with T2DM with comorbidities receiving multiple medications may not be receiving optimal treatment with TPC, as evidenced by the lower rates of treatment adherence and persistence. Consequently, this may hinder patients from achieving their glycemic target. It is important to note that many patients with T2DM are unable to achieve satisfactory glycemic control by monotherapy alone, and often require several antidiabetic agentsCitation16. Combining antidiabetic agents with complementary mechanisms of action is fundamental to the management of diabetesCitation16. While FDCs have only recently been launched in Japan, they offer a method of simplifying complex regimens, reducing treatment burden through less frequent dosing and allowing multiple medications with complementary mechanisms of actions to be given in a single formulationCitation22. In addition, novel once weekly administration schedules also allow regimen simplification, which may help improve treatment adherenceCitation23,Citation24. Some FDC OADs are available in Japan, namely, pioglitazone/glimepiride, pioglitazone/metformin, pioglitazone/alogliptin and voglibose/mitiglinideCitation16, and more recently vildagliptin/metformin, alogliptin/metformin and teneligliptin/canagliflozin. Reducing the complexity of the treatment regimen may contribute to an improvement in adherence, which may lead to improved glycemic control. Notably, the results of a previously published study showed that patients adherent to treatment were more likely to achieve glycemic control than non-adherent patientsCitation13. In the same study it was reported that each 10% increase in oral anti-diabetic medication adherence was associated with a 0.1% decrease in HbA1c (p = .0004)Citation13. More recently, the relationship between medication adherence and improved glycemic control has also been publishedCitation25. Reducing treatment complexity will help meet the current ADA Standards of Medical Care in Diabetes recommendations, which advocate a patient-centered approach to managing diabetes care and the encouragement of treatment adherenceCitation26.
This analysis provides valuable information from a large number of patients with T2DM with continuous enrolment included in two large insurance claims databases. However, the study is limited by its observational nature. The study was not randomized; impact of selection bias was reduced using adjusted models. There was limited follow-up (12 months), and a lack of statistical power to detect differences between subgroups of interest. There was also an assumption that all patients who filled their prescriptions actually took their medication. Further information missing from both databases included reasons for discontinuing treatment, whether medication was taken correctly and at the correct time of day, incidence of pill dumping or stockpiling, and details of non-reimbursed treatments. Specific limitations associated with the JMDC database included small numbers of elderly patients aged ≥65 years and lack of information for this group (as beneficiaries were working adults and their family members) and non-validated diagnoses. For the MDV database, there is no linkage of data between medical care facilities. Therefore, the data will be incomplete if a patient receives care in different institutions. There were large differences in the number of medications that were received by patients in the JMDC and MDV databases. As previously mentioned the JMDC database comprised health insurance claims data from medium-to-large-scale company employees and their family members only and thus the majority of patients were aged <65 years with only approximately 12% of patients aged ≥65 years. The MDV database included patients regardless of their working status and therefore comprised more patients aged ≥65 years (more than 60%) with multiple comorbidities who were more exposed to polypharmacy than patients in the JMDC database.
Results from a previous meta-analysis in patients with conditions other than diabetes revealed that the administration of FDCs improved medication adherence compared with separate pillsCitation27. It has been reported that FDCs decreased the risk of medication non-compliance and should thus be considered in patients with chronic conditions for improving medication complianceCitation28. In addition, it is known that diabetes is associated with comorbiditiesCitation26, and thus polypharmacy is considered integral for the management of diabetesCitation29. In particular, older adults with diabetes are more likely to receive polypharmacy than those without diabetesCitation26. FDCs may be an appropriate therapeutic regimen for patients with multiple comorbidities receiving polypharmacy to alleviate treatment burden, which can be extended to patients with medical conditions other than diabetes.
Conclusions
Overall, consistent findings from these two large administration claims databases confirm the central role of FDCs in the management of Japanese patients with T2DM. The findings indicate high adherence and persistence rates with FDCs, implying patient satisfaction with treatment, which may ultimately result in improved glycemic control and hence fewer diabetes-related complications. For this reason, FDCs may be especially appropriate for patients with comorbidities treated with multiple antidiabetic drug classes. Further studies are warranted to understand the impact of adherence to and persistence with FDCs on patient outcomes with respect to glycemic control.
Transparency
Declaration of funding
Funding for this research was provided by Takeda Pharmaceutical Company Limited, Tokyo, Japan.
Author contributions: R.N., H.K., A.O., Y.O., F.G. and Y.S. are responsible for the work described in this paper. R.N., H.K., A.O., Y.O., F.G. and Y.S. were involved in the conception, design or planning of the study. Y.O. and F.G. were involved in the analysis of data. R.N., H.K., K.K., A.O. and Y.S. were involved in the interpretation of results. R.N., H.K., K.K., A.O. and Y.O. contributed substantially to drafting of the manuscript.
Declaration of financial/other relationships
H.K., K.K., A.O. and Y.S. have disclosed that they are employees of Takeda Pharmaceutical Co. Ltd. F.G. and Y.O. have disclosed that they are employees of Creativ-Ceutical KK. R.N. has disclosed that she/he has received speaker honoraria from Astellas Pharma Inc., Nippon Boehringer Ingelheim Co. Ltd, Eli Lilly Japan KK, Kissei Pharmaceutical Co. Ltd, Medtronic Japan Co. Ltd, MSD, Novartis Pharma KK, Novo Nordisk Pharma Ltd, Sanofi KK and Takeda Pharmaceutical Co. Ltd.; and contract research fees for collaborative research with the Japan Diabetes Foundation.
A CMRO peer reviewer on this paper discloses being a member of the Invokana Speakers Bureau for Janssen Pharmaceuticals. The other peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
We acknowledge Sabah Farooq of FireKite, an Ashfield company, part of UDG Healthcare plc, for writing support during the development of this manuscript, which was funded by Takeda Pharmaceutical Co. Ltd (Tokyo, Japan), and complied with Good Publication Practice 3 ethical guidelines (Battisti et al. Ann Intern Med 2015;163:461-4).
Data sharing: Given the administrative nature of the data, no informed consent was required for patient data; however, all data is fully anonymized.
Disclaimer: The study made use of de-identified data from the JMDC and MDV databases. The opinions, results and conclusions reported are those of the authors. No endorsement by JMDC or MDV or any of its funders or partners is intended or should be inferred.
References
- Seino Y, Kuwata H, Yabe D. Incretin-based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig 2016;7(0):102-9
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed. Brussels, Belgium: International Diabetes Federation, 2017
- Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009;301:2129-40
- Charvat H, Goto A, Goto M, et al. Impact of population aging on trends in diabetes prevalence: a meta-regression analysis of 160,000 Japanese adults. J Diabetes Investig 2015;6:533-42
- Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus – present and future perspectives. Nat Rev Endocrinol 2011;8:228-36
- Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001;414:782-7
- Neville SE, Boye KS, Montgomery WS, et al. Diabetes in Japan: a review of disease burden and approaches to treatment. Diabetes Metab Res Rev 2009;25:705-16
- Garcia-Perez LE, Alvarez M, Dilla T, et al. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther 2013;4:175-94
- Balkrishnan R, Rajagopalan R, Camacho FT, et al. Predictors of medication adherence and associated health care costs in an older population with type 2 diabetes mellitus: a longitudinal cohort study. Clin Ther 2003;25:2958-71
- DiBonaventura M, Wintfeld N, Huang J, et al. The association between nonadherence and glycated hemoglobin among type 2 diabetes patients using basal insulin analogs. Patient Prefer Adherence 2014;8:873-82
- Currie CJ, Peyrot M, Morgan CL, et al. The impact of treatment noncompliance on mortality in people with type 2 diabetes. Diabetes Care 2012;35:1279-84
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487-97
- Rozenfeld Y, Hunt JS, Plauschinat C, et al. Oral antidiabetic medication adherence and glycemic control in managed care. Am J Manag Care 2008;14:71-5
- Asche C, LaFleur J, Conner C. A review of diabetes treatment adherence and the association with clinical and economic outcomes. Clin Ther 2011;33:74-109
- American Diabetes Association. Promoting health and reducing disparities in populations. Diabetes Care 2017;40(Suppl 1):S6-S10
- Nagai Y. [Fixed-dose combination]. In Japanese. Nihon Rinsho 2015;73:465-9
- Fujibayashi K, Hayashi M, Yokokawa H, et al. Changes in antidiabetic prescription patterns and indicators of diabetic control among 200,000 patients over 13 years at a single institution in Japan. Diabetol Metab Syndr 2016;8:72
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399-424
- Rosenbaum PR, Rubin DB. The bias due to incomplete matching. Biometrics 1985;41:103-16
- Council for International Organizations of Medical Sciences (CIOMS) in collaboration with the World Health Organization (WHO). International Ethical Guidelines for Epidemiological Studies. Geneva: CIOMS, 2009
- Public Policy Committee International Society of Pharmacoepidemiology. Guidelines for Good Pharmacoepidemiology Practice (GPP). Pharmacoepidemiol Drug Saf 2016;25:2-10
- Aoki K, Nagakura M, Taguri M, et al. Effect of switching from an anti-diabetic loose dose combination to a fixed dose combination regimen at equivalent dosage for 6 months on glycemic control in Japanese patients with type 2 diabetes: a pilot study. J Clin Med Res 2017;9:719-24
- Inagaki N, Onouchi H, Maezawa H, et al. Once-weekly trelagliptin versus daily alogliptin in Japanese patients with type 2 diabetes: a randomised, double-blind, phase 3, non-inferiority study. Lancet Diabetes Endocrinol 2015;3:191-7
- Kaku K. First novel once-weekly DPP-4 inhibitor, trelagliptin, for the treatment of type 2 diabetes mellitus. Expert Opin Pharmacother 2015;16:2539-47
- de Vries McClintock HF, Morales KH, Small DS, et al. A brief adherence intervention that improved glycemic control: mediation by patterns of adherence. J Behav Med 2015;38:39-47
- American Diabetes Association. Standards of medical care in diabetes – 2018. Diabetes Care 2018;41(Suppl 1):S1-S2
- van Galen KA, Nellen JF, Nieuwkerk PT. The effect on treatment adherence of administering drugs as fixed-dose combinations versus as separate pills: systematic review and meta-analysis. AIDS Res Treat 2014;2014:967073
- Bangalore S, Kamalakkannan G, Parkar S, et al. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med 2007;120:713-19
- Indu R, Adhikari A, Maisnam I, et al. Polypharmacy and comorbidity status in the treatment of type 2 diabetic patients attending a tertiary care hospital: an observational and questionnaire-based study. Perspect Clin Res 2018;9:139-44