Abstract
Objectives: Atopic dermatitis (AD) is a chronic, relapsing skin condition, with signs and symptoms that impact patients’ lives and are best measured from the patient perspective. Therefore, there is a need for AD-specific questionnaires that are consistent with Food and Drug Administration guidance and best measurement practices, assessing sign and symptom severity and associated impacts, to support treatment efficacy in regulated trials. The objectives were to develop patient-reported outcome (PRO) questionnaires assessing sign and symptom severity, as well as impacts of moderate-to-severe adult AD.
Methods: A targeted literature review and meetings with clinical experts (dermatologists) were conducted to identify AD-related sign, symptom, and impact concepts. Results were harmonized and used to construct two draft PRO questionnaires: the Atopic Dermatitis Symptom Scale (ADerm-SS; 11 items) and the Atopic Dermatitis Impact Scale (ADerm-IS; 10 items). The content validity and questionnaire content were evaluated via qualitative concept elicitation/cognitive debriefing interviews with adult patients with moderate-to-severe AD.
Results: From the literature (n = 13 articles), 13 sign and symptom and 43 impact concepts were identified, while 21 sign and symptom and 48 impacts were elicited from experts (n = 3). During the patient interviews (n = 15), 19 sign and symptom and 41 impact concepts were reported, the majority of which were evaluated by the ADerm-SS and ADerm-IS, thus substantiating the content of both questionnaires. Additionally, patients interpreted both questionnaires as intended by the developers.
Conclusions: The ADerm-SS and ADerm-IS can be regarded as content-valid PRO questionnaires for moderate-to-severe AD.
Introduction
Atopic dermatitis (AD) is a chronic, relapsing skin conditionCitation1 characterized by pruritus, redness, and pain, typically on the face, the back of the knees, and inside the elbows, but which may also be widespreadCitation2. These signs and symptoms have a considerable impact on quality-of-life (QoL) for those living with the conditionCitation1. Although AD is less common in adults compared to children (up to 3% and 15–20%, respectively, globally)Citation1,Citation3,Citation4, adults with AD usually experience more intense signs and symptoms (e.g. pruritus) that can negatively impact daily function (e.g. sleep disruptions due to itchiness and scratching, decreased work productivity, social impactCitation4) and overall QoLCitation1. A recently published report by the National Eczema Association (NEA) summarized the burden of the disease, including how pain and itch impact emotional and social functioning, QoL, work, academics, and economicsCitation5.
The authors sought to determine whether any of the existing patient-reported outcome (PRO) questionnaires were developed in a manner consistent with the US Food and Drug Administration (FDA) PRO GuidanceCitation6 and best measurement practicesCitation7,Citation8, and whether any would be appropriate to use as a daily and weekly diary for the assessment of the daily signs and symptoms, and weekly impacts of moderate-to-severe AD in adults to support efficacy and label claims in regulated clinical trialsCitation9. Because some of the most salient symptoms (e.g. pruritus, pain) and impacts (e.g. sleep disruption) of AD cannot easily be observed and evaluated by a clinician, the need for assessments from the patients themselves via PRO questionnaires is critical.
While AD-specific PRO questionnaires exist (e.g. Atopic Dermatitis Burden Scale Questionnaire for Adults [ABS-A], Dermatitis Family Impact [DFI] Questionnaire, Patient-Oriented Eczema Measure [POEM], Psychosomatic Scale for Atopic Dermatitis [PSS-AD]), and Quality of Life Index for Atopic Dermatitis [QoLIAD]), and have been evaluated by other groups (specifically, the consensus group to harmonize core outcome measures for atopic eczema/dermatitis; HOME)Citation10, only the POEM focuses on symptoms of AD. The POEM assesses the frequency of the experience of symptoms and was developed prior to the release of the FDA PRO Guidance. With respect to the impact questionnaires, the DFI is completed by an observer (i.e. a parent or caregiver reports on a child’s experience on behalf of the child), the PSS-AD focuses on stress-induced flares of symptoms and treatment effects, the QoLIADCitation11 assesses the impact of treatment on QoL, and, while the ABS-A was developed to assess burden in a broad sense (economic, work, daily life), it was not developed to be administered dailyCitation12. To our knowledge, no disease-specific PRO questionnaires have been developed following the release of the FDA PRO guidance that assess the daily/weekly signs, symptoms, and impacts of moderate-to-severe AD.
Thus, the goal of the current research was to develop and establish the content validity of two new AD-specific PRO questionnaires to assess the signs, symptoms, and impacts of moderate-to-severe AD in adults. The AD Symptom Scale (ADerm-SS) and AD Impact Scale (ADerm-IS) were developed to be an electronic diary. The ADerm-SS includes three items to be completed daily and seven items to be completed weekly, all assessing signs and symptoms over the previous 24 h. The ADerm-IS includes three items to be completed daily, assessing impact over the previous 24 h, and eight items completed weekly to assess impacts over the past 7 days. To inform the development of these PRO questionnaires, the following qualitative research activities were conducted: a concept-focused literature review, consultative advice meetings with clinicians, and interviews with moderate-to-severe AD patients.
Materials and methods
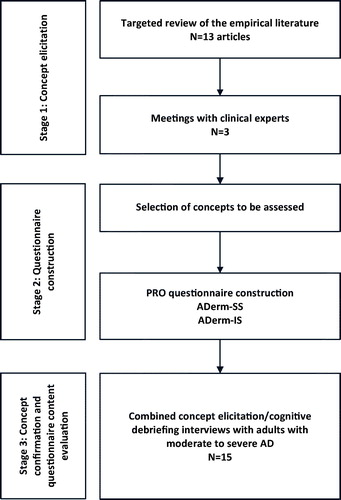
This was a qualitative study, and the research activities described herein were conducted in three stages. In concept elicitation (CE), AD-related sign, symptom, and impact concepts were identified through a review of the literature and consultation with dermatologists. During questionnaire construction, results from the literature review and expert meetings were used to construct two draft PRO questionnaires to assess signs, symptoms, and impacts. During concept confirmation and content evaluation, qualitative hybrid interviews (i.e. interviews that comprised both CE and cognitive debriefing [CD] open-ended questions)Citation13,Citation14 with adult patients of age ≥18 years with moderate-to-severe AD were conducted to evaluate the signs, symptoms, and impacts relevant and important to patients and to assess the content validity of the draft questionnaires ().
Concept elicitation
The goal of the CE stage of research was to document and gain a deeper understanding of AD-related signs, symptoms, and impacts, as reported by the literature and dermatologists.
Concept-focused literature review
A targeted search of the literature was conducted in MEDLINE, Embase, and PsycINFO in June 2016. The search terms included “atopic dermatitis OR eczema” AND “adult” AND “symptom OR sign OR impact”, and the search was limited to English-language studies in humans published in the previous 10 years. Supplemental grey literature searches were conducted in Google and Google Scholar. Abstracts returned from these searches were reviewed using a pre-defined set of selection criteria, and, through this process, publications were selected for full-text review. From these publications, primary signs and symptoms of adult AD, and associated impacts, were extracted and collated.
Qualitative meetings with clinical atopic dermatitis experts
Three dermatologists with expertise in AD, working in academic medical centers in the US, were invited to participate in 60-min telephone meetings. Trained facilitators utilized a semi-structured, open-ended meeting guide designed to elicit each clinician’s perspective on the signs, symptoms, and impacts of moderate-to-severe adult AD (i.e. concepts) experienced by patients they treat. More specifically, experts were asked to discuss in detail the common signs and symptoms experienced by adults with moderate-to-severe AD and the ways in which their patients’ lives are affected by these signs and symptoms (i.e. disease-related impacts). The experts were also asked to describe what they considered to be important treatment outcomes; which signs, symptoms, and impacts their patients reported being most bothersome; and which signs, symptoms, and impacts they considered to be the top five most important to their patients.
Audio-recordings from the meetings were transcribed verbatim, anonymized, and transcripts were subsequently uploaded into ATLAS.ti and codedCitation15. Qualitative data analysis was conducted using a directed content analysis approachCitation16, which consisted of a detailed reading of each transcript to identify and code expert discussion of sign, symptom, and impact concepts. The first transcript was coded independently by all coding team members, who then met to review the first coded transcript and discuss any discrepancies between coders until harmonization of all codes within the transcript was reached.
Questionnaire construction
Drawing upon data from the literature review and meetings with experts, the questionnaire development team met to select the sign, symptom, and impact concepts to be included in and assessed by the PRO questionnaires being developed. Concept selection was largely driven by (1) the frequency each sign, symptom, or impact was reported during the meetings with dermatologists; (2) whether the concept was reported by dermatologists as bothersome to patients with AD; and (3) whether the concept would be important to track in the context of a clinical trial or real world setting. During this process, two PRO questionnaires were constructed: the ADerm-SS () and the ADerm-IS (). Once the concepts were selected, the formatting of the questionnaires was defined, including the recall period, instructions, phrasing of the items, response options, and mode of administration.
Table 1. Structure of the atopic dermatitis symptom scale (ADerm-SS).
Table 2. Structure of the atopic dermatitis impact scale (ADerm-IS).
Concept confirmation and questionnaire content evaluation
Confirmation of the concepts included in the draft PRO questionnaires and content evaluation of the ADerm-SS and ADerm-IS were assessed through hybrid CE and CD interviews with adult patients with moderate-to-severe AD (n = 15). Sample size estimates were based on predictions about the number of patients needed to achieve saturation, which is the point at which no new relevant information is gained from additional interviewsCitation7,Citation17,Citation18.
Ethics committee approval and recruitment
Ethics review board approval from the Quorum Review Independent Review Board (https://quorumreview.com/) was granted on 27 November 2016 and 9 December 2016 (QR#32117/1), and all study participants provided written consent. Participants were recruited from three clinical sites in the US (New Orleans, LA; St. Louis, MO; and Chicago, IL) and were included if they met the Eczema Area and Severity Index (EASI) score criteria (≥16) and body surface area (BSA) criteria (≥10%) for moderate-to-severe AD, and had a documented history (within 1 year prior to screening) of inadequate response to treatment with topical corticosteroids (TCS) or topical calcineurin inhibitors (TCI). Individuals were excluded if they had been previously treated with a Janus kinase (JAK) inhibitor or dupilumab; had been recently treated with TCS, TCI, omalizumab, H1-antihistamine treatments, or any investigational systemic treatment; or if they had other active skin diseases or infections requiring systemic treatment. These criteria were chosen to ensure that participants were similar to patients who will be enrolled in future clinical trials for moderate-to-severe AD.
Interview conduct and data analysis
During CE, participants were asked a series of open-ended questions about the signs, symptoms, and impacts they experienced due to AD to confirm the content of the questionnaires. During CD, participants were asked to complete the preliminary versions of the ADerm-SS and ADerm-IS questionnaires via screenshots of a handheld electronic devise using a “think-aloud” methodCitation19,Citation20, and to provide feedback on the instructions, items, response options, and recall period of each.
Each interview was audio-recorded, transcribed verbatim, de-identified, imported into ATLAS.tiCitation15, and then qualitatively coded and analyzed in a manner similar to what was described earlier for the meetings with experts. Based on the content evaluation process, suggestions for questionnaire modifications were made.
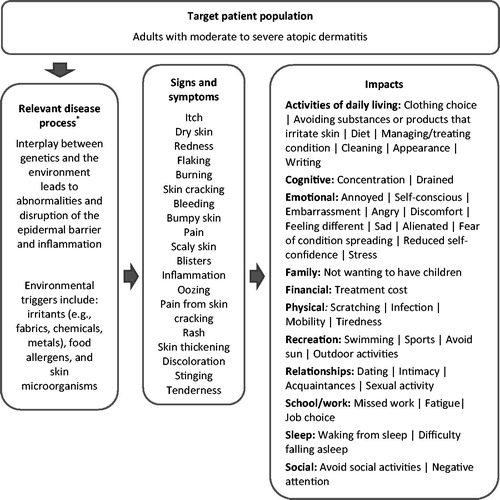
The CE data were utilized to develop a patient-centered conceptual model ()Citation21, and the sign-, symptom-, and impact-related concepts described by participants were analyzed and tabulated with regard to (1) the frequency of participant report, (2) the words used by participants to describe the concept, (3) the bothersomeness of the concept to participants, and (4) the importance participants attached to improvement in the concept as an outcome of effective treatment. Additionally, the CE data were analyzed in regard to saturation, providing evidence that the study sample was large enough that additional interviews were unlikely to produce new relevant and important conceptsCitation7,Citation17,Citation18.
Figure 2. Patient-centered atopic dermatitis conceptual model. *Napolitano M, Megna M, Patruno C, et al. Adult atopic dermatitis: a review. G Ital Dermatol Venereol 2016;151:403–411Citation27.
The CD data were analyzed and tabulated with regard to (1) any difficulties experienced by participants in reading and understanding the questionnaires; (2) whether participants felt the questionnaires comprehensively assessed relevant and important sign-, symptom-, and impact-related concepts; and (3) whether participants were able to interpret the questionnaires’ instructions, items, and response options in a manner that enabled them to provide meaningful responses to the items.
Results
Concept elicitation
Concept-focused literature review
Thirteen full-text articles were included in the analysis. From these, 13 sign and symptom concepts were identified: bleeding, blisters, burning, dry skin, fissures, inflammation, itching, pain, rash, redness, scaling, skin thickening, and swelling. Additionally, 43 impact concepts were identified and organized into eight domains: activities of daily living (ADLs), cognitive, emotional, financial, physical, sleep, social, and work/school. The most frequently reported impacts were sleep disturbances, followed by work/school activities, social withdrawal, anxiety, depressed feelings, embarrassment, and the inability to participate in ADLs.
Qualitative meetings with clinical experts
The expert advice meetings were conducted with three dermatologists who had 3–25 years of experience treating adult patients with AD and were working in US academic medical centers. A total of 21 signs and symptoms associated with moderate-to-severe AD were identified from the experts: bleeding, blisters, burning, dermatographism, discoloration/scars, excoriations, fissures, flaking, hair loss, inflammation, itching, nail changes, oozing/crusting, prurigo, rash, redness, skin thickening, pain, scaling, skin dryness, and swelling. All experts agreed that itching and discoloration/scars were the most bothersome to patients, and all experts ranked itching among what they considered to be the top five signs or symptoms associated with moderate-to-severe AD.
Experts also reported 48 disease-related impacts across 12 domains: ADLs, cognitive, emotional, leisure, physical, relationships, school, sexual, sleep, social, treatment, and work. The most frequently reported impact concepts were concentration, damage by alternative/non-evidence-based treatment, depressed mood, embarrassment, feeling helpless, frustration, scratching, self-esteem, sleep disruption, steroid phobia, stigmatization, work absenteeism (missed work), and work performance. All experts (n = 3, 100.0%) ranked sleep disruption among the top five impacts most important to their patients.
Experts were asked to discuss the treatment outcomes that they and their patients considered important, which included reduction in dermatitis, reduction in itch, patient happiness, and improvement in sleep.
Questionnaire construction
Drawing on data from the literature review and meetings with experts, the sign, symptom, and impact concepts were selected for measurement by the ADerm-SS and ADerm-IS. Both questionnaires are designed for self-administration as a patient diary completed on a handheld electronic device. and Citation2 summarize the structure of the ADerm-SS and ADerm-IS, respectively.
Concept confirmation and questionnaire content evaluation
The hybrid CE/CD interviews included 15 participants, interviewed in three waves (n = 5 each) across three clinic sites in the US. Participants’ ages ranged from 21.2–75.4 (mean = 47.3, standard deviation [SD] = 20.3) years. Approximately half were male (n = 8, 53.3%), and the majority were White/Caucasian (n = 11, 73.3%).
All (n = 15, 100.0%) met the EASI score criteria (≥16) and BSA criteria (≥10%) for moderate-to-severe AD, and had a documented history (within 1 year prior to screening) of inadequate response to treatment with TCS or TCI ().
Table 3. Participant demographic and health characteristics.
A total of 19 signs and symptoms of moderate-to-severe adult AD were reported by participants during the CE portion of the interviews (). All symptoms assessed by the ADerm-SS were reported by participants (as well as by the literature and experts): itching, dry skin, flaking, skin cracking, bleeding, pain, oozing, pain from skin cracking, rash, and skin thickening. Additionally, participants reported experiencing redness, burning, bumpy skin, scaly skin, blisters, inflammation, discoloration, stinging, and tenderness. Participants also indicated symptom bothersomeness and which symptoms should improve with treatment.
Table 4. Participant-reported signs and symptoms of moderate-to-severe AD.
Participants reported a total of 41 disease-related impacts across 11 domains: ADLs, cognitive, emotional, family, financial, physical, recreation, relationships, school/work, sleep, and social (). All impact concepts assessed by the ADerm-IS were reported by participants.
Table 5. Participant-reported impacts of moderate-to-severe AD.
Participant-reported signs, symptoms, and impacts were organized into a patient-centered conceptual model ().
Saturation analysis found that all 19 (100.0%) sign and symptom concepts, and 95.1% (n = 39) of all impact concepts reported by participants emerged during the first 75% of interviews. The two concepts that emerged during the final 25% of interviews either overlapped with concepts that were previously elicited or were unique to a single participant. Taken together, these results provide evidence for the adequacy of the sample size.
During CD, participants were asked to complete the ADerm-SS and ADerm-IS and provide feedback on the recall period, instructions, items, and response options. Following each wave, results were reviewed to determine whether revision of either questionnaire was warranted. No revisions were made between interview waves, but, following completion of the interviews, two modifications were suggested: revise instructions asking participants to complete the diary “before you go to bed at night” to “each night”, and the instructions asking participants to complete the diary “once a week before you go to bed at night” to “once a week at night”. A decision about the recommended revision will be made following evaluation of data on the draft questionnaires after future quantitative analyses are conducted.
In the debriefing of the ADerm-SS and ADerm-IS daily and weekly items, generally participants interpreted the instructions, items, and response options as intended by the developers.
The CD results demonstrate that participants were able to complete and comprehend both the ADerm-SS and ADerm-IS as intended. All of the sign and symptom concepts evaluated by the ADerm-SS were found to be relevant to the AD experience, as described during CE. In addition, the majority of participants reported experiencing the impact concepts evaluated by the ADerm-IS. These results substantiate the content validity of the ADerm-SS and ADerm-IS PRO questionnaires.
Discussion
The results of this research support the content validity and use of the ADerm-SS and ADerm-IS as endpoints in clinical trials of patients with moderate-to-severe AD. This qualitative development work (including the conduct of conceptual research across multiple data sources: literature, experts, and patients) addresses the need for PRO questionnaires specific for moderate-to-severe AD and developed in accordance with regulatory guidelinesCitation6 and scientific best practicesCitation8. While AD-specific tools are already in existence, many were developed prior to the FDA PRO Guidance. While some include some of the key concepts that emerged from the current qualitative work, none assess all of the key signs, symptoms, and impacts of moderate-to-severe AD. In addition, none of the existing tools were designed to assess the daily and weekly symptoms and impacts of the condition.
The ADerm-SS and ADerm-IS questionnaires include daily items that are assessed using a 24-h recall period and weekly items that are assessed every 7 days. Minimizing the recall period was essential to reduce the potential for bias in the assessments, which aligns with regulatory and scientific recommendationsCitation6,Citation22. Previous literature has documented that concepts such as pain are susceptible to recall bias, as people tend to remember and attend to the most salient times, specifically the times when the concept is experienced the most (e.g. highest pain), and the most recently experienced time (e.g. the pain experienced when the person is answering the questionnaire)Citation23–25. Therefore, to evaluate the symptoms of itch and skin pain, and the impact on sleep, the ADerm-SS and ADerm-IS use a 24-h recall period for these daily items, administered via an electronic device (which includes time/date stamps to track the compliance with the diary assessment schedule). In addition, to reduce patient burden for the daily diary, additional symptoms of AD that have less daily variability (e.g. bleeding, dry skin, flaking, oozing, rash, skin cracking and pain, and skin thickening) were designed to be administered by the ADerm-SS on the electronic diaries every 7 days, but still use a 24-h recall period. Finally, the ADerm-IS includes physical and emotional impacts that were also assessed each week, but using a 7-day recall period. In this way, the tools were designed to track the key signs, symptoms, and impacts of moderate-to-severe AD, minimizing recall bias and patient burden. Too, the fact that the ADerm-SS and ADerm-IS questionnaires both have items assessed daily and weekly may be a potential source of confusion for respondents, especially those with a low education level. To mitigate this potential, the questionnaires are intended to be completed on an electronic device which would prompt respondents to complete the items necessary for that particular day.
The signs and symptoms assessed by the ADerm-SS are in line with recently published articles from the NEACitation5 and the HOME initiativeCitation10, including pain, which was noted by both groups as a concept that should be explored more fully in future research. From the summary of the international consensus meeting of the HOME initiative, while QoL questionnaires were reviewed by the group, no impacts/QoL questionnaire was recommended for inclusion in the core set of PRO questionnaires at that meetingCitation10. Given this lack of consensus in the field, the ADerm-IS was developed to be appropriate for patients with moderate-to-severe AD, and to assess the sleep impacts daily and physical and emotional functioning impacts weekly.
A limitation of this research is that the ADerm-SS and ADerm-IS were not developed to be used with pediatric patients or those with milder AD. Further research would be necessary to evaluate the use of these questionnaires in those broader samples. Another limitation of the present study is that the hybrid CE/CD interviews included a single participant with an education level of high school or lower. As noted above, given the different recall periods and administration schedules for each questionnaire, care should be taken to train all participants, included those with low education, on how to complete the questionnaires electronically before implementation in future studies. Finally, it is important to note that the first version of the questionnaires was developed prior to the patient interviews, due to timeline constraints of the clinical development program. However, during meetings with experts, they were queried on the signs, symptoms, and impacts that their patients report as more important or bothersome. In addition, the methodological approach of developing a draft questionnaire based on a targeted literature review and expert input, and then subsequently conducting hybrid CE/CD interviews to confirm the key concepts of assessment and evaluate the comprehensibility and comprehensiveness of the questionnaires does align with best measurement practices and has been used in other PRO development studiesCitation13,Citation14,Citation26.
Conclusions
The ADerm-SS and ADerm-IS can be regarded as content-valid PRO questionnaires for the assessment of the signs, symptoms, and impacts of adult moderate-to-severe AD. Next steps in the development of the ADerm-SS and ADerm-IS involve an evaluation of their psychometric properties and development of score interpretation guidelines for use in the target patient population, the results of which are intended to be reported separately.
Transparency
Declaration of funding
Study design, conduct, and financial support were provided by AbbVie Inc., and AbbVie Inc. participated in the interpretation of data, review, and approval of the manuscript.
Declaration of financial/ other interests
ES reports grants, personal fees, and non-financial support from Eli Lilly and Company, grants and personal fees from Anacor Pharma, Glaxo Smith Kline, Regeneron Pharmaceuticals, Sanofi Genzyme, Pfizer, Leo, and Eli Lilly and Valeant Pharmaceuticals, personal fees from AbbVie, Celgene Corporation, Dermira, Galderma, Genentech, Leo Pharma, Menlo Therapeutics, and grants from MedImmune, Novartis, Roivant Sciences, Tioga Pharmaceuticals, Vanda Pharmaceuticals. AB, HDT, and NT are employees of AbbVie Inc. and may own AbbVie stock or stock options. LLK and CF are employed by Adelphi Values LLC, which received payment from AbbVie Inc. to support the research activities presented in this manuscript. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors would like to acknowledge Roger E. Lamoureux and Masami Kelly of Adelphi Values, and Clark Jackson, formerly of Adelphi Values, all of whom contributed to this research and provided scientific writing assistance. Data availability statement: The authors confirm that the data supporting the findings of this study are available within the article.
References
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(Suppl 1):8–16
- National Eczema Association. Atopic dermatitis. NEA; 2016 [cited 2016 July 7]. San Rafael, California, USA. Available from: https://nationaleczema.org/eczema/types-of-eczema/atopic-dermatitis-2/.
- National Institute of Arthritis and Musculoskeletal and Skin Diseases. Who has atopic dermatits? NIAMSD; 2013 [cited 2016 July 7]. Bethesda, Maryland, USA. Available from: http://www.niams.nih.gov/health_info/atopic_dermatitis/#b.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23:S115–S123.
- Drucker AM, Wang AR, Li WQ, et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:26–30.
- US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. Silver Spring, Maryland, USA. US DoHHS; 2009.
- Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity-establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1-eliciting concepts for a new PRO instrument. Value Health. 2011;14:967–977.
- Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity-establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 2-assessing respondent understanding. Value Health. 2011;14:978–988.
- Ando T, Hashiro M, Noda K, et al. Development and validation of the psychosomatic scale for atopic dermatitis in adults. J Dermatol. 2006;33:439–450.
- Chalmers JR, Simpson E, Apfelbacher CJ, et al. Report from the fourth international consensus meeting to harmonize core outcome measures for atopic eczema/dermatitis clinical trials (HOME initiative). Br J Dermatol. 2016;175:69–79.
- Whalley D, McKenna SP, Dewar AL, et al. A new instrument for assessing quality of life in atopic dermatitis: international development of the Quality of Life Index for Atopic Dermatitis (QoLIAD). Br J Dermatol. 2004;150:274–283.
- Taieb A, Boralevi F, Seneschal J, et al. Atopic dermatitis burden scale for adults: development and validation of a new assessment tool. Acta Derm Venereol. 2015;95:700–705.
- Marquis P, Lasch KE, Delgado-Herrera L, et al. Qualitative development of a patient-reported outcome symptom measure in diarrhea-predominant irritable bowel syndrome. ClinTranslGastroenterol. 2014;5:e59.
- Trask PC, Trivedi B, Palsgrove A, et al. Symptoms of multiple myeloma: results of hybrid concept elicitation/cognitive debriefing interviews. Blood. 2014;124:5973.
- Weitzman EA, Miles MB. Computer programs for qualitative data analysis. London: Sage Publications; 1995.
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277–1288.
- Lamoureux R, Galipeau N, Yaworsky A, et al. How many subjects are enough for symptom-focused concept elicitation studies? A retrospective analysis of saturation across twenty-six studies. Abstract presented at: the 20th Annual International Meeting of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR), 16–20 May, 2015.; Philadelphia, Pennsylvania, USA. 2015.
- Turner-Bowker DM, Lamoureux RE, Stokes J, et al. Informing a priori sample size estimation in qualitative concept elicitation interview studies for clinical outcome assessment instrument development. Value Health. 2018;21:839–842.
- Willis GB. Cognitive interviewing: a tool for improving questionnaire design. Thousand Oaks, CA: Sage Publications; 2005.
- Ericsson KA, Simon HA. Protocol analysis: Verbal reports as data. Cambridge, MA: MIT Press; 1993.
- Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273:59–65.
- Stull DE, Leidy NK, Parasuraman B, et al. Optimal recall periods for patient-reported outcomes: challenges and potential solutions. Curr Med Res Opin. 2009;25:929–942.
- Gorin AA, Stone AA. Recall biases and cognitive errors in retrospective self-reports: a call for momentary assessments. In: Baum A, Revenson T, Singer J, editors. Handbook of health psychology. Mahwah, NJ: Erlbaum; 2001. p. 405–414.
- Stone AA, Broderick JE, Shiffman SS, et al. Understanding recall of weekly pain from a momentary assessment perspective: absolute agreement, between- and within-person consistency, and judged change in weekly pain. Pain. 2004;107:61–69.
- Broderick JE, Schwartz JE, Vikingstad G, et al. The accuracy of pain and fatigue items across different reporting periods. Pain. 2008;139:146–157.
- Benjamin K. Workshop 13: Application of the Report’s Final Recommendations: Clinical Outcomes Assessment (COA) Measurement in Rare Disease Clinical Trials Emerging Good Practices Task Force. ISPOR 22nd Annual International Meeting; 20–24 May 2017. Boston, Massachusetts, USA.
- Napolitano M, Megna M, Patruno C, et al. Giornale italiano di dermatologia e venereologia: organo ufficiale. [Adult atopic dermatitis: a review.] Societa italiana di dermatologia e sifilografia. 2016;151:403–411