Abstract
Objectives: Understanding inhaler preferences may contribute to improving adherence in COPD patients and improving long-term outcomes. This study aims to identify and quantify preferences for convenience-related inhaler attributes in French moderate-to-severe COPD patients, with discrete choice experiment (DCE) methodology.
Methods: Attributes were defined from a literature search, clinician and patient interviews: shape, dose insertion, dose preparation, dose release, dose confirmation, dose counter and reusability. An online DCE was conducted in respondents with self-reported COPD stage 2–4 recruited through a panel. The study questionnaire included twelve choice scenarios per respondent and questions on patient characteristics, treatment and disease severity. Statistical analyses used a mixed logit regression model with random effects. Utility scores were estimated for four types of inhalers: Inhaler A – soft mist inhaler; Inhaler B – reusable soft mist inhaler; Inhaler C – multi-dose dry powder inhaler; and Inhaler D – single dose dry powder inhaler.
Results: The study was completed by 153 patients (50 females); respondents were 50.4 years old on average; 13 different inhaler devices were reported. The most preferred inhaler is L-shaped, has dose preparation with capsule insertion and a dose counter, and is reusable. Inhaler profiles A and B had the highest utilities (mean of 1.2533 and 0.9578 respectively) compared to inhaler C (0.6315) and D (0.2200).
Conclusions: This study showed statistically significant results that the strongest drivers of preference in French users of inhalation devices for COPD are shape, dose counter and reusability. Convenience-related characteristics are important to patients and should be taken into account by clinicians prescribing these devices.
Introduction
Chronic obstructive pulmonary disease (COPD) is a respiratory disorder that affects more than 13 million adults in EuropeCitation1–6 and the burden of COPD is associated with considerable indirect costs due to impaired work performance, absenteeism, sick leave and loss of earnings both for patients and caregivers. Pharmacological therapy administered through an inhaler device is the preferred treatment option for COPD. A great number of inhalation devices exist, and can be subdivided into categories: metered-dose inhalers, dry powder inhalers, nebulizers and soft mist inhalers. These inhaler types present different advantages and disadvantages for use. However, a number of barriers still exist regarding optimal use of these inhalers, despite the varied profiles of the devices available. Incorrect technique can significantly impact efficacy, which in turn has considerable quality of life and public health consequencesCitation7. Lack of training and education on inhaler use has been identified as a key barrier to correct use. Moreover, taking into account patients’ preference for inhalers significantly improves adherence to asthma pharmacotherapy and clinical outcomesCitation8. Patients’ preference is increasingly recognized as an important element to be taken in to account when selecting inhalers for COPD patientsCitation9. One of the characteristics that patients take into account when considering their preference is the ease of use and convenience of inhalers.
Given that non-adherence has been identified as a significant issue in COPDCitation10,Citation11, it is of interest to better understand the drivers of patient preference in order to identify the inhaler characteristics that are of greatest importance to patients. This study aimed to identify and quantify preferences for convenience-related inhaler attributes in French moderate-to-severe COPD patients, with discrete choice experiment (DCE) methodology. DCEs are a documented and well recognized quantitative method to elicit preferences and derive values for specific characteristics of a product or situation. In DCEs, a set of characteristics (attributes) of a product or situation is defined, with possible alternatives (levels), and individuals are then asked to indicate their preference between hypothetical scenarios, comparing different levels for each attribute. Analyses allow the relative importance of different attributes to be evaluated. DCEs are frequently used in medicine and healthcareCitation12,Citation13. Despite the lack of a formal framework, the use of patient preference data using stated preference methods such as DCEs for health technology assessment negotiations has been put forward and encouragedCitation14,Citation15. Indeed, DCEs have been conducted to investigate inhaler preference in COPDCitation16,Citation17. However, no previous studies concentrate on these characteristics or report them in this population. This study is a first step in exploring patient preferences in this population, before collecting data in a larger sample of patients.
Methods
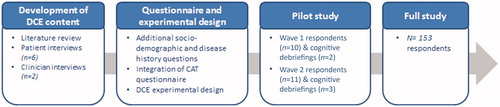
An overview of all steps of this study can be found in .
Development of the discrete choice experiment content
A literature review was conducted in order to identify possible attributes to be included in the DCE. A search strategy was developed, with the aim of identifying quantitative or qualitative studies focusing on inhaler satisfaction, inhaler preferences and inhaler characteristics that have previously been studied or reported among COPD patients (full strategy available in Supplementary online appendices). MEDLINE and Embase were searched via Ovid, and the ISPOR scientific publications database was also searched. Articles on adult (>18 years old) subjects published in English between 2007 and 14 April 2017 were included if they focused on characteristics of inhalers for COPD, regardless of geographical scope. The database search identified 421 records and the full-text articles were screened for relevant content. Twenty-three articles were included in a qualitative summary. The studies provided information on inhaler characteristics, or factors driving patient preferences for or satisfaction with different inhalers. These characteristics are listed in a table in the Supplementary online appendices, and are used to support clinician and patient interviews. The major domains covered were ease of use, training, convenience, inhalation process, hygiene, experience of use, comfort, feedback, dosing, design, type/formulation, environmental issues, efficacy, clinician preferences and other.
Following the literature search, interviews were carried out by telephone with two clinicians treating COPD and prescribing different inhalers. A semi-directive interview guide was developed and followed for interviews that lasted 60 min and were led by an experienced interviewer. Clinicians were asked about their practice, patient profiles, inhalers prescribed and experience with different inhalers. They were asked to cite inhaler characteristics spontaneously and then asked to evaluate and rank characteristics from the list of those identified during the literature reviews. Clinicians reported that the inhaler characteristics most important to COPD patients are: ease of use, ease of preparation, dose counter, dose confirmation, single dose, portability and cost. Clinicians were also asked for their input on how best to screen patients for participation in an online DCE.
As an additional step to information retrieved from the literature review, six respondents with a self-declared diagnosis of COPD were recruited for 60 min semi-structured telephone interviews conducted by an experienced researcher following a semi-directive interview guide. Respondents were recruited through a patient panel. They were included if: they were a current or former smoker having smoked ≥15 years; they had breathlessness at rest, when walking or after one or two flights of stairs; they had consulted a clinician for COPD within the last 12 months; they had a self-reported FEV1 of <80% (if they knew their FEV1); and they were currently using an inhaler for COPD. They were excluded if they declared a diagnosis of acute bronchitis. During interviews, respondents reported their symptoms and daily life with COPD and gave spontaneous feedback on their experience with different inhalers. Reasons for inhaler switches and training on using inhalers were also discussed. Respondents were asked for the most important inhaler characteristics spontaneously and then probed using the list identified in the literature review and the ranking by clinicians. The most important characteristics were identified as follows: format, dose confirmation, dose counter, reusability, dose preparation, hygiene and dose release.
Seven attributes () were selected for inclusion in the DCE. These attributes were those that were identified as the greatest drivers of preference, based on the patients’ input. The levels corresponded to the possible options for the different characteristics, for inhalers on the market in France.
Table 1. Attributes and levels.
Data collection
Data collection was done using a national online panel specific to patients with respiratory disease (n = 5000), in two phases: a pilot study followed by the full study. Inclusion criteria were as follows: breathlessness at rest, when walking or after one or two flights of stairs; having consulted a clinician for COPD within the last 12 months; a self-reported FEV1 of <80% (if they knew their FEV1); and currently using an inhaler for COPD. The sample size for the pilot study was 21 patients in two phases of ten and eleven patients respectively. The full study included 150 patients. Quotas were defined for gender (50 women and 100 men) and for the number of respondents who had never smoked, limited to twenty.
Study questionnaire and experimental design
The study questionnaire comprised two parts. First, eighteen socio-demographic and disease history questions, including a confirmation of the COPD diagnosis, the type of inhalers used, respondents’ satisfaction regarding their inhaler, a description of the current inhaler, age, education and occupation. The COPD assessment test (CAT questionnaire) was also integrated into this part of the questionnaire ().
Table 2. Current inhaler(s) used.
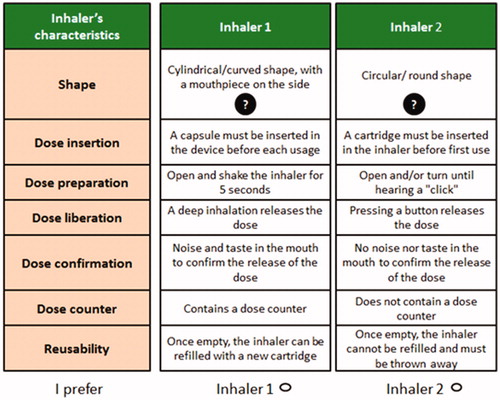
In the second part of the questionnaire, respondents were presented with binary choice scenarios in which they had to indicate their preference out of two presented inhalers. These inhalers were presented as fictitious, meaning that they were hypothetical devices described according to the defined attributes and levels, and may not exist in reality; “real” inhalers were not identified in the scenarios. In both the pilot and the full studies, respondents were randomized to one of three groups of 12 choice sets, in equal parts. This was necessary because the number of scenarios presented to each respondent would otherwise have been too high for an acceptable respondent burden.
For the pilot study, a D-efficient design with zero priors was used. The full study used a D-efficient design with priors informed by the pilot study. D-efficient designs aim to optimize the estimation power of the proposed choice situations by using the information from the pilot study in order to reduce standard errors of utility estimatesCitation18. Constraints were included in the model, in order to ensure that illogical combinations of attributes were avoided. These constraints were as follows: “Insert the capsule/pill to perforate it” could not appear in a presented inhaler together with “Pre-filled inhaler, no cartridge or capsule/pill to insert” or with “A cartridge must be inserted in the inhaler before first use”; “Pre-filled inhaler, no cartridge or capsule/pill to insert” could not appear in a presented inhaler together with “Once all the capsule/pills have been used, the inhaler can be reused with a new box of capsules/pills”.
Pilot study
First, ten patients were recruited to the study and completed both parts of the self-administered on-line questionnaire. Cognitive debriefing interviews were performed by telephone with two patients who completed the surveys in order to confirm the wording, format and comprehensibility of the survey. A second phase of pilot tests was conducted with eleven respondents, followed by three additional cognitive debriefing interviews by telephone. However, interviews after the first phase of pilot test showed that the choice task and idea of a fictitious inhaler was complicated for some respondents to understand. It was also challenging for respondents to accurately describe their own inhaler in the first part of the questionnaire. The format and wording of scenarios and instructions were altered accordingly and respondents’ understanding thereof was confirmed in pilot phase two interviews. The question regarding description of their own inhaler was simplified. The cognitive debriefing issues confirmed that, overall, the survey questionnaire was clear and comprehensible, the attributes were relevant and the completion time was acceptable. Analysis of pilot study results showed that respondents generally made rational choices.
Main study
As for the pilot study, the main study questionnaire was self-administered online and included twelve different choice scenarios per respondent. Again, in each choice scenario, respondents were asked to select the preferred inhaler from two fictitious options. The CAT questionnaire was retained in the final study questionnaire. An example of a choice situation from the final questionnaire is shown in , and the full questionnaire is available in the Supplementary online appendices.
Statistical analyses
Descriptive statistics were calculated to present respondent characteristics. These include respondent age, employment status and education, concomitant disease, smoking history, time since diagnosis, COPD stage and inhalers currently used. The data from the choice sets was analyzed using a mixed logit regression model. The probability of being chosen among two alternatives in each choice situation was modeled. The model included all seven attributes and all included variables were considered as categorical. Random effects were added on each parameter to account for the fact that answers to different choice questions by one patient were not independent. Models were tested with no random effects, with random effects assuming zero correlations between the random effects and with random effects including an estimation of correlations between the random effects. The best model was chosen based on the Akaike information criterion (AIC) criterionCitation19, which allows the assessment of the quality of statistical models and therefore the selection of the most appropriate model.
Analyses were carried out on the full population, as well as on the following subgroups: respondents aged <50 vs. >50; low vs. medium vs. high CAT scores. Data in the results tables is presented as parameter estimates, corresponding to respondents’ preference for attribute levels. Preferences were considered as statistically significant when the p value for an attribute level was <.05. The regression coefficients represent marginal utilities for different attribute levels. If the marginal utility for an attribute level is higher than zero, this indicates that this level is preferred over the reference level of the same attribute – the higher the figure, the greater the preference. If marginal utilities are below zero, the reference option is preferred. The comparative utility of different inhaler profiles was calculated by summing the parameter estimates corresponding to the characteristics of each inhaler.
Results
The total study population included 103 males and 50 females with COPD.
Patient characteristics
Patients were on average 50.4 years old (range 40–70 years). Seventy-one percent (n = 109) of respondents were employed at least part-time and 27% (n = 42) had no more education than a high school diploma or the equivalent. Asthma was the most frequent concomitant respiratory disease, with 31% (n = 47) self-declaring this diagnosis. Seventy-one percent of the respondent population were current smokers (n = 109) and only 3% (n = 4) had never smoked. The large majority (92%, n = 141) of respondents had received a COPD diagnosis no more than 10 years ago. Three percent (n = 5) had received a diagnosis more than 20 years ago. Eight percent (n = 13) did not remember their FEV1, 46% (n = 70) declared a FEV1 of 50–80% (stage 2), 44% (n = 63) declared a FEV1 between 30% and 49% (stage 3) and 2% (n = 3) declared under 30% (stage 4). Thirteen different inhalers were reported as being currently used, with most respondents (n = 101, 66%) reporting more than one inhaler currently prescribed.
Sixty-five percent of respondents received at least one training session from a healthcare professional on the use of their inhaler. Twenty-three percent watched a video in order to learn, and twelve percent had no training in any form. The most frequent level of satisfaction with their current inhaler was 8/10 (n = 74), followed by 9/10 (n = 38) and 7/10 (n = 27). The average CAT score was 28.11 (range: 11–38).
Patient preferences in the full study population
presents results for the full study population.
Table 3. Results for full study population.
Inhaler shape, dose counter and reusability after 1 month of treatment were the greatest drivers of patient preference. L-shaped inhalers were the preferred shape. This preference was statistically significant. Pre-filled inhalers appeared to be preferred vs. inhalers with a capsule, and results did not reach statistical significance. Regarding dose preparation, inhalers requiring capsule insertion and “open and turn” were almost equally preferred. “Open and shake” inhalers were the least preferred, and this was statistically significant vs. capsule insertion. Preferences for dose liberation and dose confirmation revealed small trends towards preference for button-activated inhalers and a sensation in the mouth to confirm dose. However, these were not statistically significant. Inhalers with a dose counter and reusable inhalers were preferred and results were statistically significant.
reports the comparative utility of Inhaler A, a reusable soft mist inhaler, Inhaler B – a soft mist inhaler, Inhaler C – a multi-dose dry powder inhaler and Inhaler D – a single dose dry powder inhaler. The reusable soft mist inhaler and the soft mist inhaler profiles had the highest utilities (mean of 1.2533 and 0.9578 respectively) compared to the multi-dose dry powder inhaler (0.6315) and single dose dry powder inhaler (0.2200).
Table 4. Comparative utility of four inhaler profiles.
Subpopulation results
L-shaped inhalers were the preferred shape in all subpopulations. This preference was statistically significant in all subgroups but one (respondents with 29 ≤ CAT < 31 [n = 33] [high impact]). Respondents with a moderate-to-high CAT score (26 < CAT < 29) had very strong and statistically significant preference for inhalers with a cartridge. Inhalers requiring capsule insertion and “open and turn” were almost equally preferred. “Open and shake” inhalers were the least preferred, and this was statistically significant vs. capsules in some subpopulations, as it was in the full study population. Inhalers with a dose counter were preferred in all subpopulations. Results were statistically significant in some subpopulations. Reusable inhalers were preferred in all subpopulations but results were not statistically significant.
Discussion
Our study allowed us to identify key drivers of patient preference for inhalers: shape, dose counter and reusability were the three attributes with the strongest influence on preference. This supports previous evidence showing that the portability and environmental aspects of inhalers are important to patientsCitation20,Citation21. The preference for L-shaped devices may have been influenced by the fact that a large proportion of the study population reported using “other types” of inhalers (35% of respondents reported using “other types” of inhalers without an inhalation chamber, 16% with an inhalation chamber) which notably include Ventolin and Flixotide. Subpopulation results overall showed the same trends as for the full population.
The growing importance of the patient’s voice in healthcare decision-making is supported by the Haute Autorité de Santé that recommends taking patients’ preferences into account in shared decision-makingCitation22. DCE methodology has been used infrequently in healthcare in France in the past; it is now increasingly used and will support these organizations.
Ease of use has previously been reported as of key importance to patientsCitation23. This may be particularly true for older patients who find it harder to manipulate devices. In our study we explored the specific aspects that constitute ease of use (dose preparation, dose insertion, dose release, dose confirmation, dose counter) and although preference trends were identified for these characteristics, the majority of these attributes specific to ease of use were not among the main drivers of preference. The soft mist inhaler and reusable soft mist inhaler (not currently on the market) described according to the attributes included in our study seemed to have significantly higher utility values than those of the single dose dry powder inhaler and multi-dose dry powder inhaler in this study with a specific COPD population. These findings support the conclusions of Dekhuijzen et al.Citation24 who carried out a review of inhalers from the patient perspective, underlining the importance of patient preference and the advantages of the soft mist inhaler. A previous cross-sectional study comparing a soft mist inhaler to a dry powder inhaler did not show statistically significant results regarding satisfaction between the two devicesCitation25. It should be noted that the two devices were evaluated by different user groups in this study, whereas in our study one single population was used to evaluate preference. The “Inhalator study” conducted by Oliveira et al.Citation26 also investigated inhaler preference and satisfaction and reported a preference for Breezhaler® over Respimat®. The study was limited to a trial of each device for only 7 days and among a population with mild-to-moderate COPD and is not therefore comparable to our study population or to inhaler use for COPD in real life. Moreover, the study did not meet its primary endpoint – correct inhaler use – which means care must be taken when interpreting the preference results of this study.
Limitations
Previous evidence shows that patients make mistakes when using their inhalers. This suggests that patients may also have had difficulties understanding inhaler descriptions. This could have contributed to large variability in patients’ choices, and it is possible that preferences were not found to be significantly associated with some attributes because of that. Cognitive debriefing interviews suggested a satisfactory understanding of inhaler attributes, but additional interviews would be useful to confirm this. It should also be noted that patient preferences in reality may differ from preferences elicited in this survey if patients are not aware of all the features of their inhaler, such as confirmation of dose intake.
The online format of this study limited the population sample to include only respondents with internet access and who are sufficiently computer literate to complete a web-based survey, and respondents’ experience of and satisfaction with different inhalers in the past may have influenced study results. The study population was not representative of the COPD population in clinical practice due to the bias towards a younger population that is inherent in online studies. No respondents without any qualifications were included in the study. One can also note an over-sampling of certain inhaler users, notably Spiromax. However, the subgroup analyses suggested that results were relatively stable across subgroups, and the influence of these biases on results is therefore probably small.
A high proportion (71%) of the study population declared to be current smokers, and data suggests that the prevalence in primary care of current smoking in COPD patients in France is higher than in many other countries, at around 47–55%Citation27–29. Respondents were included based on a self-report of their diagnosis and COPD stage. It may have been the case that some respondents are not reliably at the stage reported or may have a different diagnosis. This study included a relatively small sample size for a DCE, and although subgroups were analyzed, it is not possible to draw clear conclusions from the subgroup analysis.
A DCE is a stated preference study, not based on respondents’ observed preferences. In real life, respondents do not always go on to choose the product or service for which they indicated a preference. Indeed, making choices based on descriptions is not the same as making choices based on testing or experience.
Conclusions
The results show that a number of convenience-related inhaler characteristics are strong drivers of COPD patient preference in France and should be taken into account by clinicians prescribing these devices. The strongest drivers of preference were shape, dose counter and reusability after 1 month of treatment. The DCE survey should be replicated in a larger sample of patients with clinician-confirmed diagnosis of COPD and its stage, and inhaler use representative of French patients, in order to provide more robust estimates of utility scores by inhaler and further explore how patient characteristics influence their preferences.
Transparency
Declaration of funding
This study was funded by Boehringer Ingelheim. Creativ-Ceutical received professional fees from Boehringer Ingelheim for the conduct of this study.
Author contributions: N.G. led the study, conducted interviews with patients and clinicians, developed the study questionnaire, and contributed to writing the manuscript. C.C., G.D.P. and P.D. contributed to the development of the study questionnaire, gave clinical and methodological input throughout the study, and were involved in the manuscript development. S.A. designed the study methodology, and gave scientific and strategic direction to the project, and input into the manuscript. D.K. carried out the literature review and statistical analyses, and gave input into the manuscript. M.T. provided scientific guidance and reviewed the manuscript. L.L., K.L.L. and M.B. reviewed all materials, gave input throughout the study and reviewed the manuscript.
Declaration of financial/other relationships
N.G., S.A., D.K. and M.T. have disclosed that they are employed by Creativ-Ceutical. C.C., G.D.P. and P.D. have disclosed that they received professional fees from Boehringer Ingelheim for their work in this study. M.B., L.L. and K.L.L. have disclosed that they are employed by Boehringer Ingelheim.
CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
Thank you to Magali Desroches for her assistance with the study and the preparation of the manuscript. Magali Desroches is an employee of Creativ-Ceutical.
References
- British Lung Foundation. Chronic obstructive pulmonary disease (COPD) statistics [Internet]. 2011. Available from: https://statistics.blf.org.uk/copd
- Pauwels R. COPD: the scope of the problem in Europe. Chest. 2000;117(5 Suppl 2):332S–335S.
- Direction des études de l’évaluation et des statistiques. L’état de santé de la population en France – Suivi des objectifs annexés à la loi de santé publique – Rapport [Internet]. 2011 [cited 2018 Oct 3]. Available from: http://drees.social-sante.gouv.fr/IMG/pdf/etat_sante_2011.pdf
- Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33(5):1165–1185.
- Fuhrman C, Delmas MC, pour le groupe epidemiologie et recherche clinique de la S. [Epidemiology of chronic obstructive pulmonary disease in France]. Rev Mal Respir. 2010;27(2):160–168.
- Laurendeau C, Chouaid C, Roche N, et al. [Management and costs of chronic pulmonary obstructive disease in France in 2011]. Rev Mal Respir. 2015;32(7):682–691.
- Molimard M, Raherison C, Lignot S, et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J. 2017;49:1601794.
- Wilson SR, Strub P, Buist AS, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010;181(6):566–577.
- Global Initiative for Chronic Obstructive Lung Disease. Pocket Guide to COPD Diagnosis, Management, and Prevention – 2016 [Internet]. 2015 [cited 2018 Oct 3]. Available from: http://goldcopd.org/pocket-guide-copd-diagnosis-management-prevention-2016/
- Bender BG. Nonadherence in chronic obstructive pulmonary disease patients: what do we know and what should we do next? Curr Opin Pulm Med. 2014;20(2):132–137.
- Lareau SC, Hodder R. Teaching inhaler use in chronic obstructive pulmonary disease patients. J Am Acad Nurse Pract. 2012;24(2):113–120.
- Pfarr C, Schmid A, Schneider U. Using discrete choice experiments to understand preferences in health care. Dev Health Econ Public Policy. 2014;12:27–48.
- Ryan M. Discrete choice experiments in health care. BMJ. 2004;328(7436):360–361.
- FDA. Patient Preference Information – Voluntary Submission, Review in Premarket Approval Applications, Humanitarian Device Exemption Applications, and De Novo Requests and Inclusion in Decision Summaries and Device Labeling 2016 [Internet]. 2016 [cited 2018 Oct 26]. Available from: https://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm446680.pdf
- Hughes D, Waddingham E, Mt-Isa S, et al. Recommendations for benefit–risk assessment methodologies and visual representations. Pharmacoepidemiol Drug Saf. 2016;25(3):251–262.
- Hawken NA, Aballéa S, Torvinen S, et al. Identification of dry powder inhaler attributes, and their relative importance to asthma and chronic obstructive pulmonary disease patients, to inform a discrete choice experiment. Value Health. 17(7):A600.
- Kawata AK, Kleinman L, Harding G, et al. Evaluation of patient preference and willingness to pay for attributes of maintenance medication for chronic obstructive pulmonary disease (COPD). Patient. 2014;7(4):413–426.
- Reed Johnson F, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16(1):3–13.
- Akaike A. Information theory and an extension of the maximum likelihood principle. In: Parzen E, Tanabe K, Kitagawa G, editors. Selected papers of Hirotugu Akaike. New York (NY): Springer; 1998. p. 199–213.
- Ding B, Small M, Scheffel G, et al. Maintenance inhaler preference, attribute importance, and satisfaction in prescribing physicians and patients with asthma, COPD, or asthma–COPD overlap syndrome consulting for routine care. Int J Chron Obstruct Pulmon Dis. 2018;13:927–936.
- Molimard M, Colthorpe P. Inhaler devices for chronic obstructive pulmonary disease: insights from patients and healthcare practitioners. J Aerosol Med Pulm Drug Deliv. 2015;28(3):219–228.
- Éléments pour élaborer une aide à la prise de décision partagée entre patient et professionnels de santé [Internet]. 2018. [cited 2018 Oct 3] Available from: https://www.has-sante.fr/portail/upload/docs/application/pdf/2018-03/elaborer_une_aide_a_la_prise_de_decision_partagee_mel.pdf
- Lavorini F, Fontana GA, Usmani OS. New inhaler devices – the good, the bad and the ugly. Respiration. 2014;88(1):3–15.
- Dekhuijzen PN, Lavorini F, Usmani OS. Patients’ perspectives and preferences in the choice of inhalers: the case for Respimat or HandiHaler. Patient Prefer Adherence. 2016;10:1561–1572.
- Miravitlles M, Montero-Caballero J, Richard F, et al. A cross-sectional study to assess inhalation device handling and patient satisfaction in COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:407–415.
- Oliveira MVC, Pizzichini E, da Costa CH, et al. Evaluation of the preference, satisfaction and correct use of Breezhaler and Respimat inhalers in patients with chronic obstructive pulmonary disease – INHALATOR study. Respir Med. 2018;144:61–67.
- Perez T, Serrier P, Pribil C, et al. [COPD and quality of life: impact of the disease in primary care in France]. Rev Mal Respir. 2013;30(1):22–32.
- Piperno D, Huchon G, Pribil C, et al. The burden of COPD in France: results from the Confronting COPD survey. Respir Med. 2003;97(Suppl C):S33–S42.
- Roche N, Pribil C, Van Ganse E, et al. Real-life use of fluticasone propionate/salmeterol in patients with chronic obstructive pulmonary disease: a French observational study. BMC Pulm Med. 2014;14:56.