Abstract
Objective: Several biologic therapies are available for the treatment of mild-to-moderate Crohn’s disease (CD). This network meta-analysis (NMA) aimed to assess the comparative efficacy of ustekinumab, adalimumab, vedolizumab and infliximab in the maintenance of clinical response and remission after 1 year of treatment.
Methods: A systematic literature search was performed to identify relevant randomized controlled trials (RCTs). Key outcomes of interest were clinical response (CD activity index [CDAI] reduction of 100 points; CDAI-100) and remission (CDAI score under 150 points; CDAI < 150). A treatment sequence Bayesian NMA was conducted to account for the re-randomization of patients based on different clinical definitions, the lack of similarity of the common comparator for each trial and the full treatment pathway from the induction phase onwards.
Results: Thirteen RCTs were identified. Ustekinumab 90 mg q8w was associated with statistically significant improvement in clinical response relative to placebo and vedolizumab 300 mg. For clinical remission, ustekinumab 90 mg q8w was associated with statistically significant improvement relative to placebo and vedolizumab 300 mg q8w. Findings from sub-population analyses had similar results but were not statistically significant.
Conclusions: The NMA suggest that ustekinumab is associated with the highest likelihood of reaching response or remission at 1 year compared with placebo, adalimumab and vedolizumab. Results should be interpreted with caution because this is a novel methodology; however, the treatment sequence analysis may be the most methodologically sound analysis to derive estimates of comparative efficacy in CD in the absence of head-to-head evidence.
Introduction
Crohn’s disease (CD) is a chronic inflammatory condition of the gastrointestinal tract that is most prevalent among adolescents and young adults between the ages of 15 and 35Citation1,Citation2. Patients with CD experience social stigma, impinging on their ability to attend work or school, and have impaired quality of lifeCitation1,Citation2. The treatment goal for patients with CD is therefore to achieve long-term remission, which provides a number of benefits for patients, their families, care providers and employersCitation3. Disease severity of patients with CD is typically measured with a Crohn’s Disease Activity Index (CDAI) scoreCitation3.
There are several biologic treatment options available for CDCitation4, including adalimumab, infliximab, vedolizumab and ustekinumab. The efficacy and safety of ustekinumab in the treatment of moderate-to-severe CD was demonstrated in two induction trials, UNITI-1Citation5 and UNITI-2Citation6 (patients received intravenous ustekinumab 6 mg/kg), and in the IM-UNITICitation6 maintenance trial (patients received subcutaneous injections of ustekinumab 90 mg every 12 or 8 weeks). UNITI-1Citation5 included patients who failed conventional therapy, whereas UNITI-2Citation6 included patients who failed anti-tumor necrosis factor alpha (anti-TNF-α) biologic therapy, and IM-UNITICitation7 included patients who failed either conventional or anti-TNF-α therapy.
No direct head-to-head comparisons are currently available for ustekinumab and other biologics in the treatment of moderate-to-severe CD. The present network meta-analysis (NMA) therefore sought to compare the efficacy of ustekinumab with other biologic treatment options during the maintenance phase. The primary objective was to compare different biologics that have never been directly compared in existing clinical trials for moderate-to-severe CD after 1 year of treatment to assess their relative effects on clinical response and remission.
Previous NMAs have not adequately accounted for heterogeneity in patient characteristics and study designCitation8–11. Specifically, these NMAs did not account for carry-over effects from induction, re-randomization and variations in placebo response rates. Therefore, this analysis was performed using a treatment sequence Bayesian NMA to account for the re-randomization of patients based on different clinical definitions, the lack of similarity of the common comparator (placebo) for each trial, and the full treatment pathway from induction phase (using clinical response: CDAI-70). Given that a treatment sequence Bayesian NMA accounts for differences in patient and study characteristics, it should yield more reliable estimates of comparative efficacy in moderate-to-severe CD.
Methods
A study protocol was drafted as a priori and followed during the process of this review. The protocol is available upon request. We also adhered to PRISMA reporting guidelines for network meta-analysis, (Appendix 13).
Literature search strategy
A systematic literature search was performed to identify English-language randomized controlled trials (RCTs) of biologic therapies used to treat moderate-to-severe CD published from 1997 to 2015. An information specialist conducted searches of electronic medical databases (MEDLINE, EMBASE and the Cochrane Library) using a predefined search strategy (Appendix 1) on 2 July 2015. Unpublished studies up to July 2015 were searched on Clinicaltrials.gov. The search strategy was independently peer reviewed by a second information specialist.
Eligibility criteria
The assumption of exchangeability of studiesCitation12,Citation13 is important for ensuring the validity of NMAs. Exchangeability suggests that the treatments are unique competing interventions that could be included in a clinical trial. In accordance with these principles, RCTs were evaluated to identify studies that had similar patient characteristics and study designs. Eligibility criteria were established using the following PICOS (Population, Intervention, Comparators, Outcomes, Study design) framework.
Population
The population comprised adult patients diagnosed with moderate-to-severe CD. Two patient sub-populations were also of interest: patients who failed conventional therapy, and patients who failed anti-TNF-α therapy.
Intervention and comparators
Relevant comparators included biologic therapies licensed for the treatment of moderate-to-severe CD: ustekinumab, infliximab, adalimumab and vedolizumab. Conventional therapy and placebo treatment were also included, given their anticipated role as a key source of indirect evidence in the network. Eligible doses of interest for each intervention are detailed in Appendix 2.
Outcomes
The outcomes measured were: studies that reported CDAI-70 (clinical response), and CDAI-100 (clinical response) or CDAI < 150 (clinical remission).
Study design
Eligible studies were randomized controlled trials.
Study selection
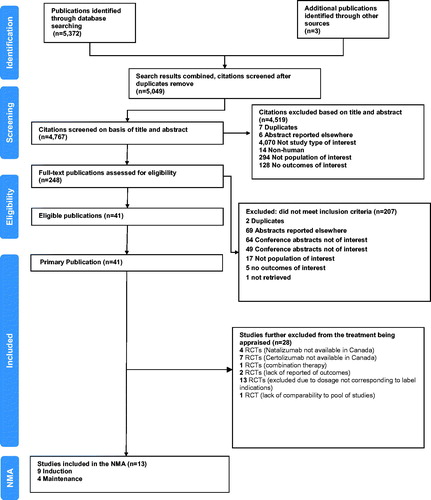
Two reviewers independently reviewed the study records, citation titles and abstracts identified in the literature search to assess study eligibility. Citations considered to describe potentially eligible articles were independently reviewed in full-text form to determine final inclusion status in the review. Disagreements between reviewers were resolved during a consensus meeting. A flow diagram documenting the process of study selection is shown in . Information on study selection and lists of included and excluded studies are presented in Appendix 3.
Data extraction and risk of bias assessment
Data was extracted independently by two reviewers using a standardized data extraction form designed in Microsoft Excel (Microsoft Corporation, Seattle, USA). The form was used to gather data related to general publication characteristics (e.g. authors, study location, year and journal of publication), intervention details (e.g. treatment, dose and frequency of administration), patient traits (e.g. disease duration, CDAI score, C-reactive protein levels, concomitant therapies and subgroup information) and study endpoints. Discrepancies in collected data were resolved by discussion.
Eligible studies were assessed for risk of bias by a single reviewer. Quality assessment was conducted using the checklist provided in the National Institute for Health and Care Excellence (NICE) single technology appraisal templateCitation14. This checklist was used to assess each study in terms of selection bias (systematic differences between the comparison groups), performance bias (systematic differences between groups in the care provided, apart from the intervention under investigation), attrition bias (systematic differences between the comparison groups with respect to loss of participants) and detection bias (bias in how outcomes are ascertained, diagnosed or verified). Results are presented in Appendices 4 and 5.
Analysis considerations – comparison of randomized controlled trial designs in moderate-to-severe Crohn’s disease and variations in placebo response rates in maintenance phase
Appendix 6 summarizes the study design of included maintenance trials. Considerable variability in study designs was found amongst the maintenance trials for patient selection and blinding.
In the included maintenance trials, entry into the maintenance phase required achievement of a clinical response (CDAI-70 or CDAI-100) at a trial-specific time point during the induction phase. Re-randomization into the maintenance phase occurred at different time points (2 to 8 weeks) and was based on achieving different outcomes (CDAI-70 or CDAI-100).
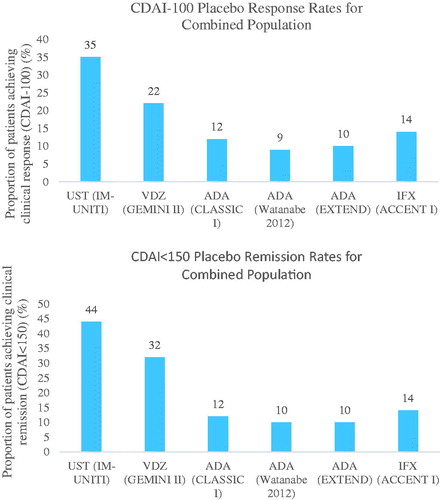
Re-randomization of patients in most trials was based on open label induction phases. Open label trials are known to have higher relative treatment effects than blinded trialsCitation15,Citation16. Therefore, trials that are open label in the induction phase have a larger number of patients who achieve clinical response and who are eligible for the maintenance phase. Therefore, a carry-over effect occurs, which causes differences in placebo response and remission rates, and relative effect estimates, as shown in and Appendix 7. shows that maintenance placebo rates are lower in trials that were open label during the induction phase. Appendix 7 shows that as placebo response (Panel A) and remission (Panel C) rates increase, the treatment response and remission rates increase. For maintenance phase clinical response and remission, higher placebo rates produce lower odds ratios, and lower placebo rates produce higher odds ratios (Panel B and D).
Figure 2. Placebo response/remission rates for clinical response and clinical remission for maintenance phase trialsCitation7,Citation25–27,Citation31,Citation32. Abbreviations. ADA, Adalimumab; CDAI, Crohn’s Disease Activity Index; IFX, Infliximab; NA, Not available; VDZ, Vedolizumab; UST, Ustekinumab.
Treatment sequence analysis to address issues with heterogeneity in randomized controlled trial design
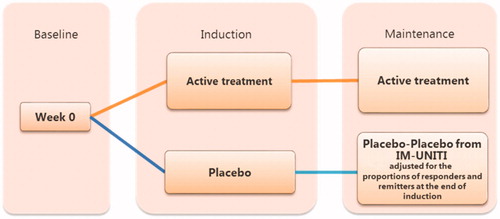
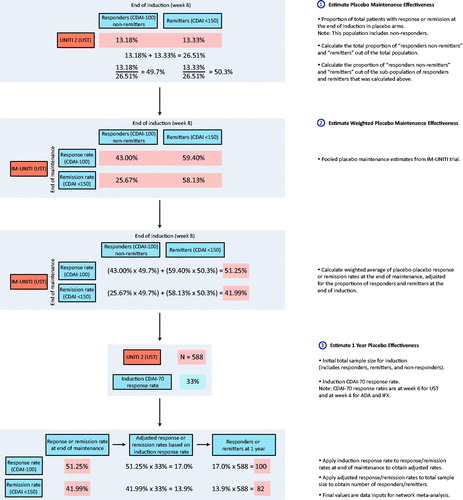
A treatment sequence analysis was conducted to address the issue of heterogeneity in placebo rates in maintenance trial data. The treatment sequence analysis utilized data from both the induction and maintenance phases to calculate relative effect estimates that account for the carry-over effects from the induction phase. To obtain relative effects, it was necessary to calculate the “probability of obtaining and maintaining response” by the end of the maintenance phase. This probability was calculated by multiplying the “probability of achieving response” during induction by the “probability of maintaining response” during the maintenance phase (). Clinical response (CDAI-70) during the induction phase was used as induction input. Clinical response (CDAI-100) and remission (CDAI < 150) at the end of maintenance were used as maintenance inputs for the treatment sequence calculation.
To address the non-comparability of placebo arms, maintenance data for placebo arms was imputed. This approach was used to reduce any bias that would occur if an NMA was conducted using unadjusted maintenance data. Placebo-to-placebo response rates at the end of maintenance were calculated using IM-UNITI individual patient level data, which was adjusted for the proportion of responders at the end of induction in each trial.
Stratification of “remitters” (defined as patients who achieved a CDAI <150) and “responders non-remitters” (defined as patients who have achieved a CDAI reduction from baseline >70 and an absolute CDAI score >150) for IM-UNITI at the end of induction was performed. A weighted average of placebo response rates for the other biologic treatments (adalimumab, infliximab and vedolizumab) was estimated using the proportion of remitters and “responders non-remitters” at the end of induction. The weighted placebo response and remission rates were used as inputs for the placebo arms during the maintenance phase ().
Summary of measures of treatment effect
A Bayesian NMA was conducted using the inputs estimated with the treatment sequence analysis. To obtain odds ratios (ORs), the number of patients estimated with the treatment sequence analysis was rounded up.
For all pairwise comparisons estimated using NMA, ORs with 95% credible intervals (CrIs) were used as a measure of the association between each treatment and its efficacy. As clinical response and remission both represent positive events for patients with CD, ORs greater than 1 corresponded to a beneficial treatment effect of the first treatment compared with the second treatment; estimates with CrIs that did not include 1 were considered to reflect statistically significant differences. Additional measures of effect that are commonly presented for Bayesian NMAs were generated, including the probability of ustekinumab being better than a comparator for a given pairwise comparison (p[better]) and Surface Under the Cumulative RAnking curve (SUCRA). The SUCRA measure is expressed as a percentage, and summarizes the relative probability of an intervention being among the best optionsCitation17. For interpretation, both p(better) and SUCRA values range between 0 and 1; values closer to 1 indicate preferred treatments.
Network meta-analysis of unadjusted and adjusted study data using treatment sequence analysis
Analyses were conducted on the overall patient population (i.e. patients who failed conventional and/or anti-TNF-α therapy), as well as on the two sub-populations of interest. Unadjusted analyses utilized data inputs that were reported directly from the maintenance phase of included RCTs, while adjusted analyses utilized data inputs derived from the treatment sequence methodology described above (which used data from the induction and maintenance phases of included clinical trials).
Primary analyses for both clinical endpoints studied were based upon a Bayesian hierarchical model for NMA recently described by Owen et al.Citation18. This model simultaneously analyzes the treatments at the class level and at the dose level, therefore identifying the most efficacious treatment at the optimal dose. This overcomes certain limitations inherent with more commonly used approaches that separately analyze treatments at the dose level and class levelCitation18. For the Bayesian hierarchy NMA models, it was not assumed that that higher doses were no worse than lower doses. Secondary analyses using well established approaches for binary endpoints described by Dias et al.Citation13,Citation19–21 were also performed to inform comparisons between the hierarchical approach and more commonly used approaches; for the latter, analyses at both the drug-dose level and the drug-class level (pooling doses together) were performed. Fixed effects (FE) models were implemented for all outcomes, given that the majority of comparisons were informed by single studies, an approach which has been previously usedCitation22. Random effects (RE) models were also conducted.
For all analyses, adequacy of model fit was assessed by comparing the posterior residual deviance with the corresponding number of unconstrained data points (i.e. the number of intervention arms across studies), which should be approximately equal if the fit is adequateCitation23. The deviance information criterion (DIC) was also assessed, where a lower DIC implies a better model fit if the difference in DIC between the two models is 3 or more. Network meta-analyses were performed using WinBUGS software (version 1.4.3, MRC Biostatistics Unit, United Kingdom) and R (version 3.3.1, R Development Core Team, University of Auckland, New Zealand), and were based on burn-in samples of ≥20,000 iterations and subsequent sampling iterations of ≥20,000 iterations. Trace plots and Gelman–Rubin plots were reviewed to assess model convergence.
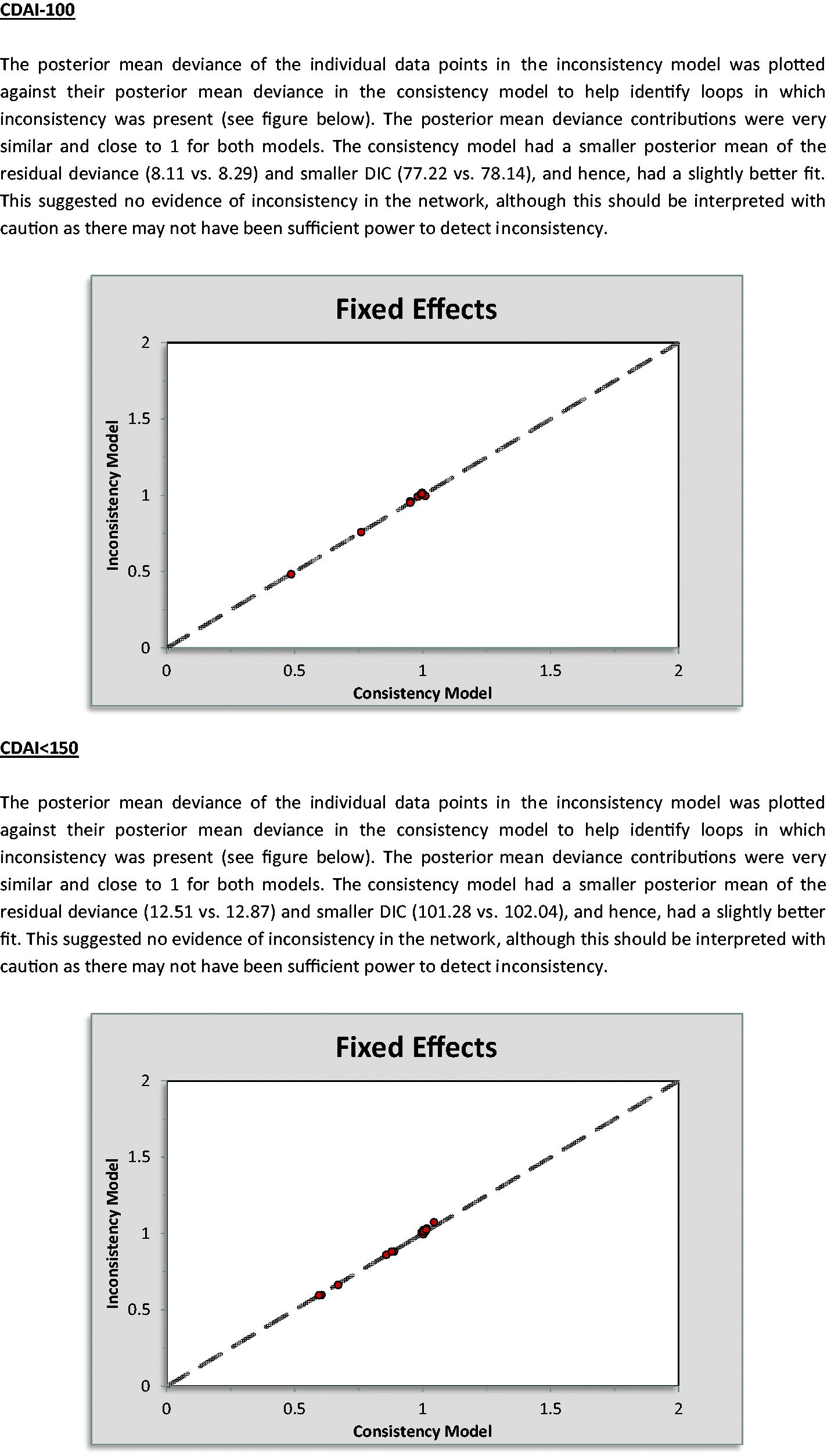
Another key assumption behind NMA is that the analyzed network is consistent; that is, there is no conflict between direct and indirect evidenceCitation21. To assess inconsistency, deviance and DIC statistics were compared in fitted consistency and inconsistency modelsCitation21. The posterior mean deviance of the individual data points in the inconsistency model was also plotted against the posterior mean deviance in the consistency model to identify any loops where inconsistency was presentCitation21.
Results
Studies included in unadjusted and adjusted treatment sequence analysis
A total of 5375 study records were identified in the search strategy and then screened. Of these records, 5127 were excluded because they did not meet the inclusion criteria and 248 were subjected to full-text screening. This resulted in 13 studiesCitation5–7,Citation24–33 that met the eligibility criteria. A list of the studies that were included in the overall population and sub-population NMAs is provided in Appendix 3. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for the selection of these studies is presented in .
Patient characteristics
Although an induction phase NMA was not conducted, available data from induction phase trials was utilized for treatment sequence calculations. For induction trials, mean age ranged from 31 to 38 years, the percentage of male patients ranged from 43% to 58%, disease duration ranged from 9 to 12 years and CDAI score ranged from 298 to 324. Among the maintenance trials, mean age ranged from 35 to 38 years, the percentage of males ranged from 38% to 47%, disease duration ranged from 7.5 to 10 years and CDAI score ranged from 299 to 320. Tables summarizing patient characteristics by study are presented in Appendix 8.
Evidence networks
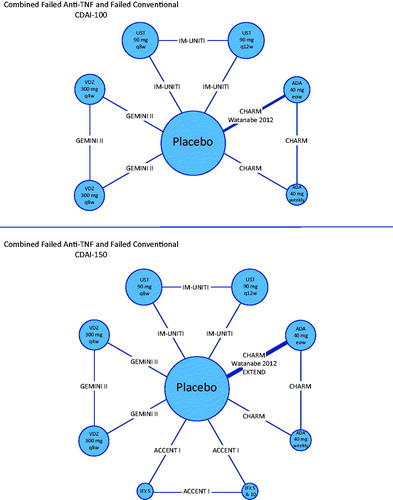
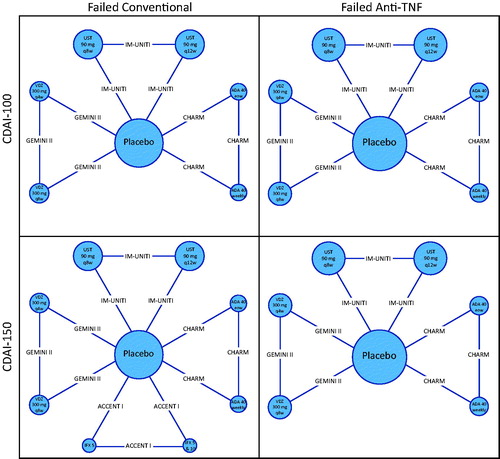
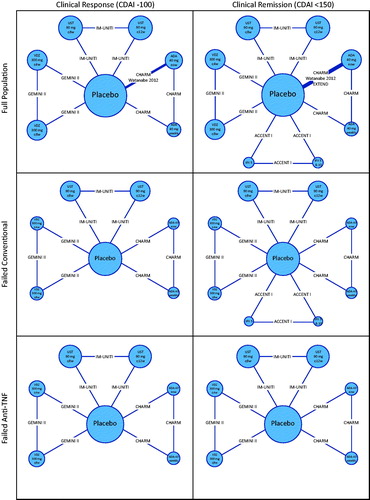
presents network diagrams that summarize the available evidence for clinical response (CDAI-100) and clinical remission (CDAI < 150) in the overall study population. For clinical response, 9 comparisons were informed by head-to-head trials, 8 of which were informed by single studies. For clinical remission, 12 comparisons were supported by data from head-to-head trials, 11 of which were informed by single studies. Additional network diagrams corresponding to the failed conventional and failed anti-TNF-α sub-populations are also presented ().
Figure 5. Evidence networks. Treatment nodes are sized to proportionately reflect the number of patients randomized to each of the interventions in the treatment networks. Widths of lines joining treatment nodes are sized to proportionately reflect the number of studies informing each comparisonCitation7,Citation24,Citation25,Citation26,Citation27,Citation31,Citation32. Abbreviations. ADA, Adalimumab; CDAI, Crohn’s Disease Activity Index; IFX, Infliximab; TNF-α, Tumor necrosis factor alpha; UST, Ustekinumab; VDZ, Vedolizumab.
Calculation of adjusted rates
The treatment sequence calculations for the overall population, and the failed conventional and failed anti-TNF-α sub-populations are shown in and Appendix 9, respectively.
Table 1. Treatment sequence calculations for the overall population.
Results from treatment sequence network meta-analysis
presents a summary of findings from the Bayesian hierarchical NMA model at the drug-dose level for ustekinumab 90 mg every 8 weeks (q8w) relative to other competing regimens. For clinical response (CDAI-100), ustekinumab 90 mg q8w was associated with numerical improvements compared to all other treatments for each population. For the overall population, statistical significance was observed for ustekinumab 90 mg q8w relative to placebo (OR 2.15; 95% CrI 1.66–2.80) and to vedolizumab 300 mg q4w (OR 1.58; 95% CrI 1.03–2.43) and q8w (OR 1.61; 95% CrI 1.04–2.47). The probability of treatment being better was in favor (≥50%) of ustekinumab 90 mg q8w relative to all other treatments.
Table 2. Results from treatment sequence network meta-analysis for the overall population, failed conventional and failed anti-TNF-α sub-populations.
For clinical remission (CDAI < 150), ustekinumab 90 mg q8w was associated with numerical improvements compared with all other treatments for each population, except for infliximab. For the overall population, statistical significance was observed for ustekinumab 90 mg q8w compared with placebo (OR 2.27; 95% CrI 1.71–3.03) and vedolizumab 300 mg q8w (OR 1.60; 95% CrI 1.01–2.53). The probability of treatment being better was in favor (≥50%) of ustekinumab 90 mg q8w relative to all other treatments, except for infliximab.
Results from unadjusted network meta-analysis
Clinical interpretations were notably different for NMA results that used data not transformed with treatment sequence methodology, with summary estimates favoring adalimumab for both endpoints (Appendix 10).
Results from additional network meta-analyses
Several sensitivity analyses were performed to assess the robustness of findings derived from the primary analyses. Results from these analyses are summarized in , which presents comparisons of the other included active interventions relative to ustekinumab from these analyses. Based on comparison of DIC, model fit of the Bayesian hierarchical model was found to be better than the dose-stratified model for both endpoints (measures of fit with the dose-pooled analyses were not directly comparable because some studies had to be removed if their study arms were redundant when collapsed).
Table 3. Results from sensitivity analyses.
For clinical response (CDAI-100), summary estimates of effect were similar in magnitude relative to those derived from the dose-stratified analysis; however, precision was improved using the hierarchical model, a consequence of borrowing strength from this approach. Findings from the pooled-dose NMA showed very similar summary estimates compared to those estimated at the drug level from the hierarchical model, with minimal differences in precision. Results from a RE analysis for the hierarchical model had slightly larger model fit statistics values than the FE model (Appendix 11). No inconsistencies in the primary NMAs were detected (Appendix 12). Lastly, analyses of clinical response in the sub-populations of patients failing conventional therapy and patients failing anti-TNF-α therapy showed similar trends in terms of the benefits of ustekinumab; however, results were associated with more uncertainty due to a reduction in available sample size, and differences between therapies were not statistically significant. The probability of a treatment being better than a comparator for pairwise comparisons and SUCRA were in favor of ustekinumab.
For clinical remission (CDAI < 150), similar patterns were observed in terms of comparability in summary estimates from the different models as well as differences in precision of the effect estimates. With the exception of infliximab, the probability of a treatment being better than a comparator for pairwise comparisons and SUCRA were in favor of ustekinumab.
Discussion
The objective of the current study was to evaluate the efficacy of ustekinumab relative to infliximab, adalimumab and vedolizumab. This study represents the most up-to-date systematic review and NMA to synthesize data comparing biologic treatments for moderate-to-severe CD. In total, 13 RCTs were identified that compared treatments for the maintenance phase of clinical response and clinical remission in patients with moderate-to-severe CD. Due to variations in study designs (i.e. lack of similarity amongst control groups in terms of response and remission rates), a treatment sequence calculation was deemed appropriate, and this data was used to inform NMAs. Bayesian hierarchical models for network meta-analysis were employed to account for the presence of different doses of the therapies of interest.
For the overall patient population, who failed conventional and/or anti-TNF-α therapy, ustekinumab was associated with statistically significant improvement in clinical response (CDAI-100) relative to placebo and vedolizumab. It was also associated with the highest probability of being better than a comparator and had the highest SUCRA. For clinical remission (CDAI < 150), ustekinumab was associated with statistically significant improvement relative to placebo and vedolizumab. Ustekinumab was also associated with the highest probability of being better than a comparator and had the highest SUCRA, with the exception of infliximab.
The treatment sequence NMA was also run by stratifying the overall population into two sub-populations: failed conventional and failed anti-TNF-α. Ustekinumab showed statistically significant improvement in both CDAI-100 and CDAI < 150 relative to placebo and was also associated with the highest probability of being better than a comparator and had the highest SUCRA, except for infliximab for CDAI < 150 in the failed conventional sub-population.
Others have faced similar challenges in attempts to synthesize the available evidence on biologics in moderate-to-severe CD. For instance, Hazlewood et al.Citation9 conducted an NMA in the overall population. A commentary on this NMA, published by Bonovas et al.Citation8, pointed out that results should be interpreted with caution due to various non-negligible issues such as pooling dosages, different assessment times for each trial and differences in patients’ baseline characteristics. Another NMA conducted by Stidham et al.Citation34 raised similar concerns. Singh et al.Citation35 also conducted an NMA for patients in the failed conventional sub-population, raising issues such as variation in placebo response rates, study design differences and assessment times across studies. To address these issues, a treatment sequence analysis was performed. The treatment sequence analysis used a Bayesian hierarchical model to account for heterogeneity among the trials, but also the presence of different doses of the therapies of interest.
Despite the strengths of the treatment sequence analysis, there are also limitations with the current analysis that should be noted. Most trials evaluating comparators used in the treatment sequence analysis were open label during the induction phase. This would in turn bias results against RCTs that were double-blinded during the induction phase (Appendix 6). The re-randomization into the maintenance phase was also non-homogenous across the included trials, therefore contributing to variations in placebo response/remission rates between maintenance trials. Another potential limitation to this study was the systematic literature review (SLR) was conducted on 2 July 2015. New studies and treatments may have been approved after this date which may have not been included in the treatment sequence analysis. Certolizumab, for instance, was excluded since it was not available in Canada at the time of the SLR search. However, a methodological case study such as the present study is not required to include all treatments to illustrate the methodological application. Additionally, we did not consider a range of imputed values for IM-UNITICitation7 for our treatment sequence analysis, but it is reassuring that the sensitivity analyses to assess robustness were comparable to the primary analysis. A final limitation is that many NMAs have been published in this therapeutic area; however, given that the focus of this study was to illustrate the novel method of treatment sequence NMAs, these findings should be of interest and help to guide interpretation of future NMAs.
Conclusions
The results of the current NMA suggest that ustekinumab is associated with significantly improved clinical response and clinical remission compared with placebo and vedolizumab, and similar efficacy compared to adalimumab and infliximab. Results should be interpreted with caution because this is a novel methodology; however, treatment sequence analysis may be the most appropriate method given the lack of head-to-head evidence and the heterogeneity across maintenance trials.
Transparency
Declaration of funding
This study was funded by Janssen Inc. Cornerstone received financial support from Janssen Inc. for the conduct of this study.
Author contributions: A.V. was involved with analysis of the data, drafting the manuscript and approving the final version to be published. F.R.W., P.D., M.H., B.H. and C.C. assisted with interpretation of data, critically reviewed for important intellectual content for the work and approved the final version to be published.
Declaration of financial/other relationships
P.D. and M.H. have disclosed that they are employees of Janssen Inc. B.H. has disclosed that he is an employee of the Ottawa Hospital Research Institute and provides methodological advice for Cornerstone Research Group Inc. C.C. has disclosed that she/he is an employee and shareholder of Cornerstone Research Group Inc. A.V. and F.R.W. have disclosed that they are employees of Cornerstone Research Group Inc. Cornerstone consults for various pharmaceutical, medical device and biotech companies.
CMRO peer reviewers on this manuscript have received an honorarium from CMRO for their review work but have no relevant financial or other relationships to disclose.
Previous presentation
The work from this study has been previously presented as an abstract at the 2017 Canadian Digestive Diseases Week. Our work builds off initial concepts presented by Pacou and colleagues at ISPOR in 2016.
Acknowledgments
The authors would like to acknowledge Meaghan Bartlett for writing support, and the peer reviewers for their helpful comments.
References
- Floyd DN, Langham S, Severac HC, et al. The economic and quality-of-life burden of Crohn’s disease in Europe and the United States, 2000 to 2013: a systematic review. Dig Dis Sci. 2015;60:299–312.
- van der Have M, van der Aalst KS, Kaptein AA, et al. Determinants of health-related quality of life in Crohn’s disease: a systematic review and meta-analysis. J Crohns Colitis. 2014;8:93–106.
- Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223.
- World Gastroenterology Organisation. Inflammatory Bowel Disease [Internet]. 2015 [cited 2018 Apr 5]. Available from: http://www.worldgastroenterology.org/UserFiles/file/guidelines/inflammatory-bowel-disease-english-2015-update.pdf
- A Study to Evaluate the Safety and Efficacy of Ustekinumab in Patients With Moderately to Severely Active Crohn’s Disease Who Have Failed or Are Intolerant to Tumor Necrosis Factor (TNF) Antagonist Therapy (UNITI-1) [Internet]. 2015 [cited 2018 Apr 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT01369329
- A Study to Evaluate the Safety and Efficacy of Ustekinumab Induction Therapy in Patients With Moderately to Severely Active Crohn’s Disease (UNITI-2) [Internet]. 2015 [cited 2018 Apr 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT01369342
- A Study to Evaluate the Safety and Efficacy of Ustekinumab Maintenance Therapy in Patients With Moderately to Severely Active Crohn’s Disease (IM-UNITI) [Internet]. 2016 [cited 2018 Apr 5]. Available from: https://clinicaltrials.gov/ct2/show/NCT01369355
- Bonovas S, Moja L, Danese S. In the presence of conceptual heterogeneity, results of network meta-analysis comparing therapies in Crohn’s disease need to be interpreted with caution. Gastroenterology. 2015;148:1483–1484.
- Hazlewood G, Rezaie A, Borman M, et al. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn’s disease: a network meta-analysis. Gastroenterology. 2015;148:344–354.
- Su C, Lichtenstein G, Krok K, et al. A meta-analysis of the placebo rates of remission and response in clinical trials of active Crohn’s disease. Gastroenterology. 2004;126:1257–1269.
- Thorlund K, Druyts E, Toor K, et al. Comparative efficacy of golimumab, infliximab, and adalimumab for moderately to severely active ulcerative colitis: a network meta-analysis accounting for differences in trial designs. Expert Rev Gastroenterol Hepatol. 2015;9:693–700.
- Caldwell DM, Ades AE, Higgins JPT. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. Br Med J. 2005;331:897.
- Dias S, Sutton A, Ades A, et al. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33:607–617.
- NICE. Single technology appraisal: user guide for company evidence submission template. 2015 [cited 2018 Apr 5]. Available from: https://www.nice.org.uk/process/pmg24/chapter/instructions-for-companies.
- Beyer-Westendorf J, Büller H. External and internal validity of open label or double-blind trials in oral anticoagulation: better, worse or just different? J Thromb Haemost. 2011;9:2153–2158.
- Schulz K, Chalmers I, Hayes R, et al. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–412.
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–171.
- Owen R, Tincello D, Abrams K. Network meta-analysis: development of a three-level hierarchical modeling approach incorporating dose-related constraints. Value Health. 2015;18:116–126.
- Dias S, Sutton A, Welton N, et al. Evidence synthesis for decision making 3: heterogeneity – subgroups, meta-regression, bias, and bias-adjustment. Med Decis Making. 2013;33:618–640.
- Dias S, Welton N, Sutton A, et al. Evidence synthesis for decision making 1: introduction. Med Decis Making. 2013;33:597–606.
- Dias S, Welton N, Sutton A, et al. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making. 2013;33:641–656.
- Cameron C, Coyle D, Richter T, et al. Systematic review and network meta-analysis comparing antithrombotic agents for the prevention of stroke and major bleeding in patients with atrial fibrillation. BMJ Open. 2014;4:e004301.
- Spiegelhalter DJ, Best NG, Carlin BP, et al. Bayesian measures of model complexity and fit. J Royal Statistical Soc B. 2002;64:583–639.
- Colombel J, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65.
- Hanauer S, Feagan B, Lichtenstein G, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549.
- Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333.
- Rutgeerts P, Van Assche G, Sandborn WJ, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology. 2012;142:1102–1111.
- Sandborn WJ, Rutgeerts P, Enns RA, et al. Adalimumab rapidly induces clinical remission and response in patients with moderate to severe Crohn’s disease who had secondary failure to infliximab therapy: results of the GAIN study. Am J Gastroenterol. 2006;101:448.
- Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed: GEMINI III study. Gastroenterology. 2014;147:618–627.
- Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor α for Crohn’s disease. N Engl J Med. 1997;337:1029–1036.
- Watanabe M, Hibi T, Lomax KG, et al. Adalimumab for the induction and maintenance of clinical remission in Japanese patients with Crohn’s disease. J Crohns Colitis. 2012;6:160–173.
- Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease: GEMINI II study. N Engl J Med. 2013;369:711–721.
- Sandborn WJ, Gasink C, Gao LL, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367:1519–1528.
- Stidham RW, Lee TCH, Higgins PDR, et al. Systematic review with network meta-analysis: the efficacy of anti-TNF agents for the treatment of Crohn’s disease. Aliment Pharmacol Ther. 2014;39:1349–1362.
- Singh S, Garg SK, Pardi DS, et al. Comparative efficacy of biologic therapy in biologic-naive patients with Crohn disease: a systematic review and network meta-analysis. Mayo Clinic Proc. 2014;89:1621–1635.
- D’Haens G, Van Deventer S, Van Hogezand R, et al. Endoscopic and histological healing with infliximab anti–tumor necrosis factor antibodies in Crohn’s disease: a European multicenter trial. Gastroenterology. 1999;5:1029–1034.
- Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. BMJ. 2007;56:1232–1239.
- Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. New England J Med. 2010;15:1383–1395.
- Rutgeerts P, D’Haens G, Targan S, et al. Efficacy and safety of retreatment with anti–tumor necrosis factor antibody (infliximab) to maintain remission in Crohn’s disease. Gastroenterology. 1999;117:761–769.
- Feagan BG, Greenberg GR, Wild G, et al. Treatment of active Crohn’s disease with MLN0002, a humanized antibody to the α4β7 integrin. Clin Gastroenterol Hepatol. 2008;6:1370–1377.
- Hanauer SB, Feagan BG, Macintosh DG, et al. Tu1138 efficacy of vedolizumab in Crohn’s disease by prior treatment failure in Gemini II, a randomized, placebo-controlled, double-blind, multicenter study. Gastroenterology. 2013;5:S-772.
- Schreiber S, Rutgeerts P, Fedorak RN, et al. A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn’s disease. Gastroenterology. 2005;129:807–818.
- Winter TA, Wright J, Ghosh S, et al. Intravenous CDP870, a PEGylated Fab′ fragment of a humanized antitumour necrosis factor antibody, in patients with moderate-to-severe Crohn’s disease: an exploratory study. Aliment Pharmacol Therapeutics. 2004;201337–1346.
- Sandborn WJ, Feagan BG, Stoinov S, et al. Certolizumab pegol for the treatment of Crohn’s disease. New England J Med. 2007;357:228–238.
- Feagan BG, Reilly MC, Gerlier L, et al. Clinical trial: the effects of certolizumab pegol therapy on work productivity in patients with moderate-to-severe Crohn’s disease in the PRECiSE 2 study. Aliment Pharmacol Therapeutics. 2010;31:1276–1285.
- Rutgeerts P, Schreiber S, Feagan B, et al. Certolizumab pegol, a monthly subcutaneously administered Fc-free anti-TNFα, improves health-related quality of life in patients with moderate to severe Crohn’s disease. Int J Colorectal Disease. 2008;23:289–296.
- Sandborn WJ, Abreu MT, D’Haens G, et al. Certolizumab pegol in patients with moderate to severe Crohn’s disease and secondary failure to infliximab. Clin Gastroenterol Hepatol. 2010;8:688–695.
- Feagan BG, Sandborn WJ, Wolf DC, et al. Randomised clinical trial: improvement in health outcomes with certolizumab pegol in patients with active Crohn’s disease with prior loss of response to infliximab. 2011;33:5.
- Sandborn WJ, Schreiber S, Feagan BG, et al. Certolizumab pegol for active Crohn’s disease: a placebo-controlled. Randomized Trial. 2011;9:8.
- Sandborn WJ, Colombel JF, Enns R, et al. Natalizumab induction and maintenance therapy for Crohn’s disease. New England J Med. 2005;353:1912–1925.
- Targan SR, Feagan BG, Fedorak RN, et al. Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE Trial. Gastroenterology. 2008;132:1672–1683.
- Ghosh S, Goldin E, Gordon FH, et al. Natalizumab for active Crohn’s disease. New England J Med. 2003;348:24–32.
Appendices
Appendix 1. Search strategy.MEDLINE and MEDLINE-IN-PROCESS (date of the search: 2 July 2015)
EMBASE (date of the search: 2 July 2015)
The Cochrane Library (date of the search: 2 July 2015)
Appendix 2. Treatment doses in moderate-to-severe Crohn’s disease.
Appendix 3. Detailed rationale for included and excluded studies.Included studies for primary dosage network meta-analysis
Included studies for failed conventional sub-population primary dosage network meta-analyses
Included studies for the failed anti-TNF sub-population primary dosage network meta-analyses
Excluded studies from primary dosage network meta-analysis
Appendix 4. Quality assessment of included studies.
Appendix 5. ARisk of bias assessment.
Appendix 6. Study design of maintenance phase trials.
Appendix 7. Placebo response/remission rates versus treatment response/remission rates and odds ratios.
Appendix 8. Detailed summary of randomized controlled trial characteristics.Induction
Maintenance
Appendix 9. Treatment sequence calculations for sub-populations.
Treatment sequence calculations for failed anti-TNF sub-population
Appendix 10. Results from network meta-analysis based on data prior to treatment sequencing adjustments.
Appendix 11. Random effects model results.Results from primary analyses for random effects model
Appendix 12. Inconsistency models
Appendix 13. PRISMA network meta-analysis checklist.