Abstract
Objective: Baloxavir marboxil (baloxavir) is the first cap-dependent endonuclease inhibitor being studied for the treatment of influenza in single oral dosing regimen. This network meta-analysis (NMA) evaluated the efficacy and safety of baloxavir compared to other antivirals for influenza in otherwise healthy patients.
Methods: A systematic literature review was performed on 14 November 2016 in Medline, Embase, CENTRAL, and ICHUSHI to identify randomized controlled trials assessing antivirals for influenza. A NMA including 22 trials was performed to compare the efficacy and safety of baloxavir with other antivirals.
Results: The time to alleviation of all symptoms was significantly shorter for baloxavir compared to zanamivir (difference in median time 19.96 h; 95% CrI [3.23, 39.07]). The time to cessation of viral shedding was significantly shorter for baloxavir than zanamivir and oseltamivir (47.00 h; 95% CrI [28.18, 73.86] and 56.03 h [33.74, 87.86], respectively). The mean decline in virus titer from baseline to 24 h was significantly greater for baloxavir than for the other drugs. Other differences in efficacy outcomes were not significant. No significant differences were found between baloxavir and the other antivirals for safety, except total drug-related adverse events where baloxavir demonstrated a decrease compared to oseltamivir and laninamivir.
Conclusions: The NMA suggests that baloxavir demonstrated better or similar efficacy results compared to other antivirals with a comparable safety profile. Baloxavir led to a significant decrease in viral titer versus zanamivir, oseltamivir and peramivir and decreased viral shedding versus zanamivir and oseltamivir.
Introduction
Influenza is a seasonal, acute viral infection of the respiratory tract that can cause mild to severe illness and, in some cases, can lead to death. It is characterized by fever, headache, cough, sore throat, myalgia, nasal discharge or congestion, fatigue, weakness, and appetite lossCitation1.
The symptoms of influenza are usually similar to those of other respiratory viruses, so that epidemiologists often use the term “influenza-like illness” (ILI) to refer to viral respiratory infectionsCitation2. According to the World Health Organization (WHO), ILI is defined as an acute respiratory infection with fever of ≥38 °C and cough or sore throatCitation3.
Influenza can pose a clinical and humanistic burden leading to mortality or increased morbidity, and affects quality of life. Influenza-related hospitalizations in the US ranged from 140,000 (season 2011/12) to 710,000 (season 2014/15)Citation4. A study conducted by Molinari et al. in 2007 predicted that the total economic burden of influenza would reach $87 billion in the US annuallyCitation5. In 2015, the number of medically attended influenza patients reached near epidemic level in Japan and was estimated to be 15 million. The National Epidemiological Surveillance of Infectious Diseases (NESID) reported a total of 12,705 hospitalized patients with influenza in Japan during the 2014/15 seasonCitation6.
Antiviral medications are recommended for the treatment of influenza, principally for patients at higher risk for influenza complicationsCitation7. Commonly used antivirals belong to the class of neuraminidase inhibitors, such as oseltamivir, zanamivir, laninamivir and peramivirCitation8. However, the development of resistance to this class of drugs has been identified, creating the need for more effective therapiesCitation4.
A cap-dependent endonuclease inhibitor, baloxavir marboxil, was developed to reduce the viral load of influenza, alleviate symptoms and provide administrative benefits through single oral dosing. In the CAPSTONE-1 trial, baloxavir was compared to placebo and oseltamivirCitation9. Baloxavir demonstrated significant improvement in time to alleviation of symptoms and reduced virus levels in patients compared with placeboCitation9. The percentage of patients determined positive for influenza virus titer was significantly lower in the baloxavir group compared to the oseltamivir group at 1, 2 and 4 days from the start of treatmentCitation9. Additionally, the median time to cessation of viral shedding was 24 h in patients treated with baloxavir, compared with 72 h in those treated with oseltamivir (p < .0001) and 96 h for placebo (p < .0001)Citation10. Baloxavir was generally well tolerated with lower overall incidence of adverse effects (20.7%) versus placebo (24.6%) or oseltamivir (24.8%)Citation9, and demonstrated a decrease in the incidence of treatment-related adverse effects compared with oseltamivir.
Although the efficacy of baloxavir compared to placebo and oseltamivir has been demonstrated in the treatment of otherwise healthy influenza patients, there are no head to head studies that compare the efficacy of baloxavir with other antivirals.
The objective of this study was to assess the relative efficacy and safety of baloxavir in otherwise healthy influenza patients, compared to placebo, oseltamivir, zanamivir, laninamivir and peramivir using a network meta-analysis (NMA) based on all published randomized controlled trials (RCTs).
Methods
Systematic literature review
We conducted a systematic literature review (SLR) of comparative efficacy and safety of antivirals for the treatment of influenza in accordance with the methods developed by the Centre for Reviews and Dissemination (CRD)Citation11 and the Cochrane Handbook for Systematic Reviews of InterventionsCitation12. The SLR focused on RCTs enrolling patients with flu symptoms (ILI or confirmed influenza).
The search was performed on 14 November 2016 in MEDLINE (1946 to present); EMBASE (access via the OVID interface [1974 to present]); Cochrane Central Register of Controlled Trials (CENTRAL) and Igaku Chuo Zasshi (ICHUSHI).
Abstracts from conferences and congresses were also searched in several relevant websites. Further sources, such as ClinicalTrials.gov and regulatory websites (EMA, FDA and PMDA) were consulted in order to capture relevant documents; details of conferences, congresses, additional sources and the selection process as well as the search strategies implemented can be found in the Supplementary Appendix. No restrictions in terms of timeframe or geographical scope were applied. Searches for conference/congress abstracts were restricted to 2015 or more recent. The studies included in the SLR were selected by two reviewers and discrepancies were resolved by a third reviewer. Extracted data included publication characteristics, study details, patient characteristics, results and study limitations. A single reviewer extracted data from selected publications and quality of the extraction was checked by a second reviewer.
Network meta-analysis
A NMA allows the comparison of treatments that have not been compared in head-to-head RCTs. The estimations of relative treatments effects are based on all available evidence, both direct and indirect comparisonsCitation13. A NMA was performed to compare the relative efficacy and safety of baloxavir to neuraminidase inhibitors and placebo, focusing on the recommended doses in JapanCitation7: oseltamivir 75 mg twice a day for five days; zanamivir 10 mg twice a day for five days; laninamivir 40 mg single administration; and peramivir 300 mg single administration.
Studies enrolling patients aged 12 to 80 years with flu symptoms were included in the NMA (full network); studies focusing on patients with risk factors were excludedCitation14. Studies conducted in the general population (i.e. including a low proportion of patients outside this age range or with some risks factors) were included in the NMA. Detailed criteria for inclusion and exclusion of studies are provided in . The analysis was conducted in the intention-to-treat-infected (ITTI) population for efficacy outcomes (i.e. patients with confirmation of influenza infection based on laboratory tests) and the total trial population for safety outcomes (i.e. patients with and without confirmation of influenza infection). In a sensitivity analysis, we restricted the analysis to studies including only patients aged 12 to 65 (restricted network).
Table 1. Inclusion and exclusion criteria for the NMA.
The outcomes to be assessed in the NMA were selected based on feasibility, clinical relevance and need for a cost-effectiveness model. The following outcomes were considered: time to alleviation of all symptoms, time to resolution of fever, time to cessation of viral shedding, change in virus titer from baseline to 24 h, total adverse events (AEs), drug-related AEs and drug-related vomiting. Time to cessation of viral shedding utilized virus titer rather than RNA copy count assessed by polymerase chain reaction in defining time. Results for change in virus titer from baseline to 48 h, time to return to pre-influenza health status, incidence of pneumonia and drug-related diarrhea are provided in the Supplementary Appendix. The NMA models were estimated in a Bayesian framework using a Markov Chain Monte Carlo (MCMC) method as implemented in the WinBUGS software package. Vague prior distributions were used for the model parameters.
For time-to-event outcomes, the survival function was assumed to follow an exponential distribution; the input for the analysis was the logarithm of the hazard rate and its associated standard error (SE). For the change in virus titer from baseline, the mean change for each treatment and its associated standard errors were used as inputs. For total AEs, drug-related AEs and drug-related vomiting, the number of patients experiencing these outcomes and the total numbers of patients per arm were used.
The difference in mean change between two treatments and the difference in median time to event were considered statistically significant when the associated 95% credibility interval (CrI) did not include zero. An odds ratio (OR) was considered statistically significant if the associated 95% CrI did not include 1. Some articles reported the data in terms of time to alleviation in days while other reported data in hours, but all data was converted to hours for our analysis. Both the fixed-effects (FE) and random-effects (RE) models were fitted. The model was selected based on deviance information criterion (DIC) and the RE model was chosen if the DIC was reduced by more than 5 points compared to the FE modelCitation15,Citation16.
Results
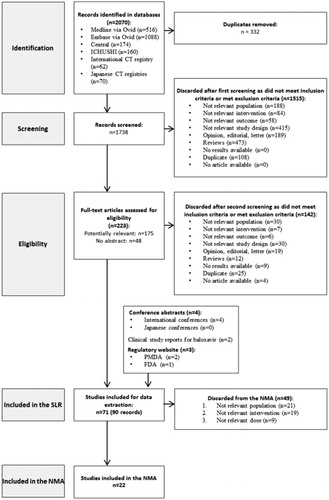
A total of 2070 references were identified through the literature search (Medline: N = 516; Embase: N = 1088; CENTRAL: N = 174; ICHUSHI: N = 160; international CT registry: N = 62; Japanese CT registries: N = 70). Finally, 22 studies were included in the NMA, after the addition of phase II and phase III trials of baloxavir. Half of the studies (n = 11) included few patients outside the 12–80 range; these studies included adults or patients older than 12 years old, without specifying a maximum age. Details on the selection process are available in and an overview of all the included studies in the NMA is reported in .
Table 2. List of studies included in the NMA.
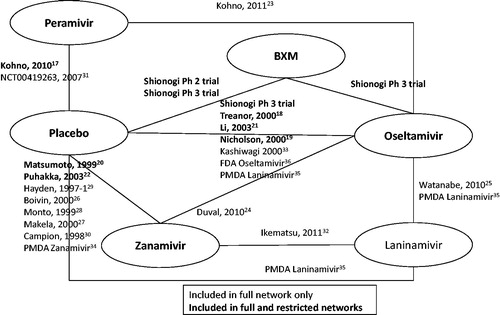
The evidence network is reported in and a summary of evidence for each outcome of interest is presented in . Based on the DIC, a random-effect model was selected for the time to alleviation of all symptoms, time to resolution of fever and change in virus titer from baseline to 24 h; while a fixed-effect model was chosen for time to cessation of viral shedding, total AEs, drug-related AEs and drug-related vomiting. The results of the meta-analyses for efficacy and safety outcomes are presented in and , respectively.
Table 3. Summary of evidence for efficacy and safety outcome measures.
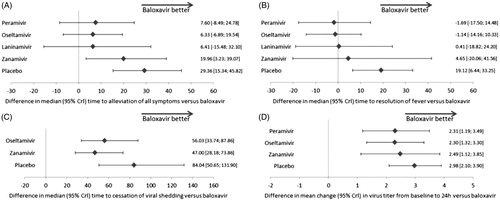
The time to alleviation of all symptoms was reported in 14 studies (six treatments; 5403 patients). Baloxavir was associated with a significantly shorter time to alleviation of all symptoms in comparison with zanamivir (difference in median time to alleviation of all symptoms [95% CrI]: 19.96 h [3.23, 39.07]) and placebo (29.36 h [15.34, 45.82]). The effect of baloxavir did not differ significantly from laninamivir (6.41 h [−15.48, 32.10]), oseltamivir (6.33 h [−6.89, 19.54]) or peramivir (7.60 h [−8.49, 24.78]).
The analysis of time to resolution of fever included nine studies (six treatments; 3772 patients). Baloxavir was associated with a significantly shorter time to resolution of fever compared to placebo (difference in median time to resolution of fever: 19.12 h [6.44, 33.25]); however, there were no significant differences between baloxavir and zanamivir (4.65 h [−20.06, 41.56]), laninamivir (0.41 h [−18.82, 24.20]), oseltamivir (−1.14 h [−14.16, 10.33]) or peramivir (−1.69 h [−17.50, 14.48]).
The time to cessation of viral shedding was reported in three studies (four treatments; 1078 patients). The use of baloxavir resulted in a significant reduction in time to cessation of viral shedding compared to zanamivir (difference in median time to cessation of viral shedding: 47.00 h [28.18, 73.86]), oseltamivir (56.03 h [33.74, 87.86]) and placebo (84.04 h [50.65, 131.90]).
The analysis of change in virus titer from baseline to 24 h included nine studies (five treatments; 3195 patients). The mean change from baseline to 24 h was significantly greater with baloxavir than zanamivir (difference in mean change in virus titer from baseline: 2.49 log10TCID50/mL [1.12, 3.85]), oseltamivir (2.30 log10TCID50/mL [1.32, 3.30]), peramivir (2.31 log10TCID50/mL [1.19, 3.49]) and placebo (2.98 log10TCID50/mL [2.10, 3.90]).
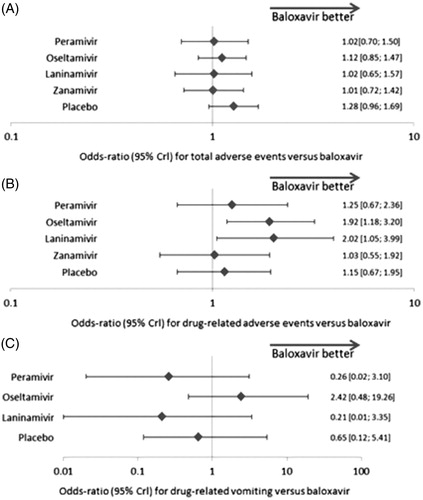
The analysis of total AEs included ten studies (six treatments; 5628 patients). AE risk did not differ significantly between zanamivir and baloxavir (odds ratio of 1.01 [0.72, 1.42]), laninamivir and baloxavir (1.02 [0.65, 1.57]), oseltamivir and baloxavir (1.12 [0.85, 1.47]), peramivir and baloxavir (1.02 [0.70, 1.50]), or placebo and baloxavir (1.28 [0.96, 1.69]).
We analyzed drug-related AEs based on eight studies (six treatments; 4528 patients). Oseltamivir and laninamivir were associated with higher odd ratios for drug-related AEs versus baloxavir (odds ratio of 1.92 [1.18, 3.20] and 2.02 [1.05, 3.99], respectively). Drug-related AE risk did not differ between zanamivir and baloxavir (1.03 [0.55, 1.92]), peramivir and baloxavir (1.25 [0.67, 2.36]), or placebo and baloxavir (1.15 [0.67, 1.95]).
For drug-related vomiting, five studies were included (five treatments; 3297 patients). No significant differences were observed between baloxavir and oseltamivir (odds ratio of 2.42 [0.48, 19.26]), peramivir (0.26 [0.02, 3.10]), laninamivir (0.21 [0.01, 3.35]) or placebo (0.65 [0.12, 5.41]) in terms of drug-related vomiting.
The sensitivity analysis focusing on studies including only patients aged 12 to 65 gave results similar to those obtained from the full network, which suggests that including a low proportion of patients outside the age range of 12 to 65 years had no major impact on the findings. Details are provided in the Supplementary Appendix.
Time to return to pre-influenza health status results revealed no significant difference between baloxavir and the other antivirals. The effect of baloxavir in change in virus titer from baseline to 48 h was significantly better than zanamivir, peramivir and placebo, and did not differ significantly from oseltamivir. For the incidence of pneumonia, there was no significant difference between baloxavir and zanamivir, oseltamivir, peramivir or placebo. The risk of drug-related diarrhea for baloxavir did not differ significantly from oseltamivir, peramivir, laninamivir or placebo (see Supplementary Appendix).
Discussion
This study investigated the efficacy and safety of baloxavir compared to other antivirals in the treatment of influenza infection using a NMA including 22 studies. Baloxavir was found to be as efficacious or more compared to oseltamivir, peramivir, laninamivir and zanamivir, especially in terms of time to alleviation of all symptoms and viral shedding, with an acceptable safety profile.
In comparison with zanamivir 20 mg, baloxavir was significantly better in terms of time to alleviation of all symptoms, time to cessation of viral shedding, change in virus titer from baseline to 24 h and change in virus titer from baseline to 48 h. Baloxavir was significantly better than oseltamivir 150 mg for time to cessation of viral shedding and change in virus titer from baseline to 24 h. In comparison with peramivir 300 mg, change in virus titer from baseline to 24 h and to 48 h was significantly better with baloxavir. No other significant differences were observed. For safety outcomes, we noted a lower odds ratio of drug-related AEs with baloxavir compared to oseltamivir and laninamivir. No other significant differences were observed for baloxavir compared to other antiviral in terms of drug-related AEs, total AEs, drug-related vomiting and drug-related diarrhea.
In a US trial of nearly 800 patients zanamivir failed to demonstrate significantly better efficacy than placeboCitation34. Also, in the trial by Duval et al., oseltamivir showed significantly higher clinical and virologic efficacy compared to zanamivirCitation24. The authors explained that it could be the consequence of a suboptimal treatment regimen in the zanamivir arm, since the IC50 values for the A(H3N2) viruses of the 2008–2009 season were two- to three-fold higher for zanamivir than oseltamivir. Given that our analysis included these studies, results for baloxavir compared to zanamivir should be interpreted with caution.
Viral shedding has been used as a proxy measure of infectivity of human influenza in many studiesCitation37–39. In a study by Tsang et al., it was found that oseltamivir treatment was associated with a reduction in infectivity for seasonal influenza A(H1N1)Citation40. Also, it was estimated that almost all secondary infections (97%) were acquired from the householdCitation40, and these findings are also consistent with other studiesCitation41–43. Therefore, by reducing the time to cessation of viral shedding and quickly reducing the viral load, baloxavir could be more effective at preventing secondary infections and the spread of disease.
Three previously published meta-analyses in the treatment of influenza were identified. Wang et al.Citation44 reported pooled results for zanamivir and oseltamivir versus placebo; however, this review focused on children, so the results were not comparable to our analysis. In the study by Burch et al.Citation45, zanamivir and oseltamivir were compared to placebo. The results were more in favor of zanamivir and oseltamivir in the ITTI analysis compared to the ITT analysis. In the study by Turner et al.Citation46, the results for efficacy outcomes were in favor of active treatments versus placebo (sometimes statistically significant) and no significant differences were found in terms of safety outcomes between active treatments and placebo. These results are in line with our findings.
The strength of this NMA was that it was based on an SLR searching for several databases including Japanese ones with extensive hand searches. The number of included trials was quite high compared to the number of treatments considered in the analysisCitation6, ensuring data availability for most relevant outcomes, and that results for each treatment were not dependent on a single trial. Also there was no major impact on the results in the sensitivity analysis with more strict inclusion criteria, suggesting that the analysis was robust regarding the selection of studies.
Seasonal influenza infection results in significant levels of morbidity and hospitalizationsCitation47. Although the influenza symptoms are generally self-limiting, the quality of life of both patients and their families may be affectedCitation48. In our study, the comparison of the relative efficacy of baloxavir in the reduction of individual influenza symptoms – cough, sore throat, headache, nasal symptoms, feverishness, muscle pain, fatigue and incidence of respiratory complications (sinusitis, bronchitis and otitis media), intra-household infection rate, hospitalizations and mortality – was unfeasible due to lack of relevant studies. More trials are needed to determine the relative effect of antivirals on these outcomes.
One limitation of this analysis was that our analysis focused on otherwise healthy patients with confirmed influenza infection. Studies focusing on high-risk patients were excluded from the NMA as their results are not comparable to those obtained in the baloxavir trials which enrolled otherwise healthy individuals. The analysis was conducted in the ITTI population for efficacy outcomes and on the total trial population for safety outcomes. Since routine testing for influenza is not common in many countries, estimates from an ITT population (without considering the infection status) would have allowed increasing the number of relevant results and could be seen as more generalizable to clinical practiceCitation49. However, in general, estimates of efficacy in ILI patients may not be comparable between studies, as results would depend on the proportion of true influenza cases among all ILI patients which may vary with the definition of ILI, as well as from one season to another.
Heterogeneity in the definition of outcomes between trials was another limitation. The primary outcome in most of the included studies was time to the alleviation of all symptoms; however, heterogeneity in the definition was observed specifically in the number of symptoms analyzed. Commonly, seven symptoms (cough, sore throat, headache, nasal symptoms, feverishness/chills, myalgia and fatigue) were includedCitation17–19,Citation23–25,Citation31. In the trial conducted by Matsumoto et al.Citation20, only three symptoms were considered (fever, headache and myalgia) but, as these are the most important ones, it was considered relevant to include these results in our analysis. Also, the study by Kashiwagi et al.Citation33 did not specify the symptoms considered, mentioning only general “influenza-duration”, but was considered relevant for our analysis.
The thresholds of fever resolution was another source of heterogeneity in outcome definitions, ranging from ≤36.9 °CCitation36 to ≤37.5 °CCitation32, which may stem from the different methods of body temperature measurement. However, determining the cause of these differences may be difficult, since a substantial number of studies did not report the method of measurement. There is no reason to believe that any of these differences would affect the treatment effect; therefore, the analysis was conducted despite the heterogeneity in outcome definitions.
The reliability of clinical diagnosis of pneumonia in the absence of criteria and without X-ray is questionableCitation49. Diagnostic criteria for pneumonia were specified in baloxavir studies in which only the radiologically confirmed cases were analyzed; in other trials no details were provided on pneumonia definition. This difference may be a potential source of between-trial heterogeneity.
The quality of RCT reporting is not optimalCitation50. Some heterogeneity in outcome definitions remains undetectable due to the lack of adequate and transparent reporting of outcome definitions.
Baloxavir demonstrated a significant decrease in total drug-related AEs compared to oseltamivir and laninamivir; however, a difference in the total AEs was not seen. In influenza, some of the reported AEs are attributable to the natural course of the infection, while drug-related AEs are considered as these having a potentially causal relationship to the intervention. Therefore, the observed difference may be explained by the novel mechanism of action which is different from other antiviral treatments and which may impact the occurrence of drug-related AEs.
The definition of drug-related AEs has been modified over years which could create a source of heterogeneity between oseltamivir and zanamivir studies (conducted between 1997 and 2003) and laninamivir, peramivir and baloxavir studies which are more recent (2007 and onwards). Thus, the results for drug-related AEs need to be interpreted with caution.
The comparability among studies may be questioned as the response to treatment could vary between seasons or countries, depending on circulating strains of the virusCitation45,Citation49. The random-effects model accounts for heterogeneity between studies, and therefore takes into account variations due to differences between seasons. The random-effects model was not selected for some analyses, suggesting that for these endpoints either the heterogeneity between studies or seasons was minor or there was not enough data to disentangle differences between treatments from differences between seasons.
Conclusion
This study estimated the relative efficacy and safety of baloxavir compared to other antivirals for the treatment of influenza using a systematic literature review and network meta-analysis. The results suggest that baloxavir was associated with a time to alleviation of all symptoms that was significantly shorter compared to zanamivir and placebo and similar to other neuraminidase inhibitors. Baloxavir was more efficacious in controlling the viral load (time to cessation of viral shedding and change in virus titer from baseline to 24 h) than other antivirals. The safety profile of baloxavir was comparable to other antivirals. These findings support the use of baloxavir in otherwise healthy influenza patients and might help to inform clinicians’ decisions when considering therapeutic options. The efficacy of baloxavir in controlling complicated influenza in high-risk individuals remains to be seen.
Transparency
Declaration of funding
This study (design and conduct, data collection, management, analysis and interpretation of the data, review and approval of the manuscript) was funded by Shionogi & Co. Ltd.
Author contributions
Conception and design: H.I., K.T., V.T., S.A. Acquisition of data: V.T., S.A., K.B., F.M. Analysis and interpretation of the data: H.I., K.T., V.T., S.A., K.B., F.M., N.H. Drafting of the manuscript: V.T., S.A., F.M., H.I., K.T. Critical revision of the manuscript for important intellectual content: V.T., S.A., F.M., H.I., K.T., K.B., N.H. Obtaining funding: H.I., K.T.
Declaration of financial/other relationships
V.T., F.M., K.B. and S.A. have disclosed that they have received grant funding from Shionogi & Co. Ltd during the conduct of the study and outside of the submitted work. K.T. has disclosed that she is an employee of Shionogi Limited. H.I. has disclosed that he is an employee of Shionogi & Co. Ltd. N.H. has disclosed that he has received personal fees and other from Shionogi & Co. Ltd, outside the submitted work.
Supplemental Material
Download MS Word (107.9 KB)Acknowledgements
Creativ-Ceutical Ltd performed the NMA, funded by Shionogi & Co. Ltd. The authors wish to thank Takahiro Hasegawa (Shionogi) for biostatistics analysis support and Yutaka Saisho (Shionogi) for medical input. We also thank Yoshie Onishi (Creativ-Ceutical) for collaboration and valuable technical input, and Nawel Jaafer and Monique Dabbous (Creativ-Ceutical) for medical writing support.
References
- Nabeshima S, Kashiwagi K, Ajisaka K, et al. A randomized, controlled trial comparing traditional herbal medicine and neuraminidase inhibitors in the treatment of seasonal influenza. J Infect Chemoth. 2012;18:534–543.
- Kelly H, Birch C. The causes and diagnosis of influenza-like illness. Aust Fam Physician. 2004;33:305–309.
- National Institute of Infectious Diseases. IASR 36(11), Influenza 2014/15 season/Japan [Internet; cited 2016 Nov 7]. Available from: https://www.niid.go.jp/niid/en/iasr-e/865-iasr/6096-tpc429.html.
- Centers for Disease Control and Prevention. Estimated Influenza Illnesses, Medical Visits, Hospitalizations, and Deaths Averted by Vaccination in the United States [Internet]. 2016 [updated 2016 Dec 13; cited 2017 Jan 18]. Available from: https://www.cdc.gov/flu/about/disease/2015-16.htm
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086–5096.
- National institute of infectious diseases. Influenza 2014/15 season, Japan [Internet]. 2015 [cited 2016 Nov 7]; Available from: http://www.nih.go.jp/niid/en/component/content/article/865-iasr/6096-tpc429.html
- Takaku F. Manual of therapeutic agents 2018. Igaku Syoin. 2018:1576–1581.
- Moscona A. Neuraminidase inhibitors for influenza. N Engl J Med. 2005;353:1363–1373.
- Osaka JaFP, NJ – Shionogi & Co., Ltd. Shionogi to present S-033188 phase 3 CAPSTONE-1 study results for treatment of influenza at IDWEEK 2017 [Internet]. 2017 [cited 2018 Mar 7]; Available from: https://www.prnewswire.com/news-releases/shionogi-to-present-s-033188-phase-3-capstone-1-study-results-for-treatment-of-influenza-at-idweek-2017-300531077.html
- Portsmouth S, Kawaguchi K, Arai M, et al. Cap-dependent endonuclease inhibitor S-033188 for the treatment of influenza: results from a phase 3, randomized, double-blind, placebo- and active-controlled study in otherwise healthy adolescents and adults with seasonal influenza. Open Forum Infectious Dis. 2017;4:S734.
- Centre for Reviews and Dissemination, U.o.Y. Systematic Reviews – CRD’s guidance for undertaking reviews in health care. York: University of York; 2008.
- Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [Internet]. 2011 [cited 2016 Nov 7]; Available from: http://handbook-5-1.cochrane.org/
- Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med. 2002;21:2313–2324.
- Centers for Disease Control and Prevention. People at High Risk of Developing Flu-Related Complications [Internet] [cited 2017 March 6]. Available from: https://www.cdc.gov/flu/about/disease/high_risk.htm
- Spiegelhalter D, Best N, Carlin B, et al. Bayesian measures of model complexity and fit. Quality Control Appl Statistics. 2003;48:431–432.
- Dias S, Sutton AJ, Ades AE, et al. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33:607–617.
- Kohno S, Kida H, Mizuguchi M, et al. Efficacy and safety of intravenous peramivir for treatment of seasonal influenza virus infection. Antimicrob Agents Chemother. 2010;54:4568–4574.
- Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA. 2000;283:1016–1024.
- Nicholson KG, Aoki FY, Osterhaus AD, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet. 2000;355:1845–1850.
- Matsumoto K, Ogawa N, Nerome K, et al. Safety and efficacy of the neuraminidase inhibitor zanamivir in treating influenza virus infection in adults: results from Japan. GG167 Group. Antivir Ther. 1999;4:61–68.
- Li L, Cai B, Wang M, et al. A double-blind, randomized, placebo-controlled multicenter study of oseltamivir phosphate for treatment of influenza infection in China. Chin Med J (Engl). 2003;116:44–48.
- Puhakka T, Lehti H, Vainionpaa R, et al. Zanamivir: a significant reduction in viral load during treatment in military conscripts with influenza. Scand J Infect Dis. 2003;35:52–58.
- Kohno S, Yen MY, Cheong HJ, et al. Phase III randomized, double-blind study comparing single-dose intravenous peramivir with oral oseltamivir in patients with seasonal influenza virus infection. Antimicrob Agents Chemother. 2011;55:5267–5276.
- Duval X, van der Werf S, Blanchon T, et al. Efficacy of oseltamivir–zanamivir combination compared to each monotherapy for seasonal influenza: a randomized placebo-controlled trial. PLoS Med. 2010;7:e1000362.
- Watanabe A, Chang SC, Kim MJ, et al. Long-acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: a double-blind, randomized, noninferiority clinical trial. Clin Infect Dis. 2010;51:1167–1175.
- Boivin G, Goyette N, Hardy I, et al. Rapid antiviral effect of inhaled zanamivir in the treatment of naturally occurring influenza in otherwise healthy adults. J Infect Dis. 2000;181:1471–1474.
- Makela MJ, Pauksens K, Rostila T, et al. Clinical efficacy and safety of the orally inhaled neuraminidase inhibitor zanamivir in the treatment of influenza: a randomized, double-blind, placebo-controlled European study. J Infect. 2000;40:42–48.
- Monto AS, Fleming DM, Henry D, et al. Efficacy and safety of the neuraminidase inhibitor zanamivirin the treatment of influenza A and B virus infections. J Infect Dis. 1999;180:254–261.
- Hayden FG, Osterhaus AD, Treanor JJ, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group. N Engl J Med. 1997;337:874–880.
- Randomised trial of efficacy and safety of inhaled zanamivir in treatment of influenza A and B virus infections. The MIST (Management of Influenza in the Southern Hemisphere Trialists) Study Group. Lancet. PubMed PMID. 1998;352:1877–1881.
- Management of Influenza in the Southern Hemisphere Trialists (MIST). BioCryst Pharmaceuticals. Evaluation of the Efficacy and Safety of Peramivir in Subjects With Uncomplicated Acute Influenza [Internet]. 2007 [cited 2016 Nov 25]. Available from: https://clinicaltrials.gov/ct2/show/results/NCT00419263?term=NCT00419263&rank=1
- Ikematsu. Clinical efficacy at the early stage of inhaled anti-influenza drug zanamivir after inhalation randomized open label test. J Japan Clin Inter Med Assoc. 2011;26(2):215–219.
- Kashiwagi S, Kudoh S, Watanabe A, et al. [Clinical efficacy and safety of the selective oral neuraminidase inhibitor oseltamivir in treating acute influenza – placebo-controlled double-blind multicenter phase III trial]. kansenshogakuzasshi. 2000;74:1044–1061.
- Pharmaceuticals and Medical Devices Agency. Relenza (Zanamivir) package insert. 2017.
- Pharmaceuticals and Medical Devices Agency. Report on the Deliberation Results (Laninamivir). 2010.
- Food and Drug Administration. Center for drug evaluation and research (Application number: 021246Orig1s045 and 021087Orig1s062) Labeling. 2012.
- Suess T, Remschmidt C, Schink SB, et al. Comparison of shedding characteristics of seasonal influenza virus (sub)types and influenza A(H1N1)pdm09; Germany, 2007–2011. PLoS One. 2012;7:e51653.
- Lau LL, Ip DK, Nishiura H, et al. Heterogeneity in viral shedding among individuals with medically attended influenza A virus infection. J Infect Dis. 2013;207:1281–1285.
- Fielding JE, Kelly HA, Mercer GN, et al. Systematic review of influenza A(H1N1)pdm09 virus shedding: duration is affected by severity, but not age. Influenza Other Resp. 2014;8:142–150.
- Tsang TK, Cowling BJ, Fang VJ, et al. Influenza A virus shedding and infectivity in households. J Infect Dis. 2015;212:1420.
- Gubareva LV, Novikov DV, Hayden FG. Assessment of hemagglutinin sequence heterogeneity during influenza virus transmission in families. J Infect Dis. 2002;186:1575.
- Poon LL, Chan KH, Chu DK, et al. Viral genetic sequence variations in pandemic H1N1/2009 and seasonal H3N2 influenza viruses within an individual, a household and a community. J Clin Virol. 2011;52:146–150.
- Papenburg J, Baz M, Hamelin ME, et al. Household transmission of the 2009 pandemic A/H1N1 influenza virus: elevated laboratory-confirmed secondary attack rates and evidence of asymptomatic infections. Clin Infect Dis. 2010;51:1033. America.
- Wang K, Shun-Shin M, Gill P, et al. Neuraminidase inhibitors for preventing and treating influenza in children (published trials only). Cochrane Database Syst Rev. 2012;18:CD002744.
- Burch J, Paulden M, Conti S, et al. Antiviral drugs for the treatment of influenza: a systematic review and economic evaluation. Health Technol Assess. 2009;13:1–265, iii–iv.
- Turner D, Wailoo A, Nicholson K, et al. Systematic review and economic decision modelling for the prevention and treatment of influenza A and B. Health Technol Assess. 2003;7:iii–iv, xi–xiii, 1–170.
- Gums JG, Pelletier Em, Blumentals WA. Oseltamivir and influenza-related complications, hospitalization and healthcare expenditure in healthy adults and children. Expert Opin Pharmacother. 2008;9:151–161.
- Chow MY, Morrow AM, Booy R, et al. Impact of children’s influenza-like illnesses on parental quality of life: a qualitative study. J Paediatr Child Health. 2013;49(8):664–670.
- Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2012;1:CD008965.
- To MJ, Jones J, Emara M, et al. Are reports of randomized controlled trials improving over time? A systematic review of 284 articles published in high-impact general and specialized medical journals. PLoS One. 2013;8:e84779.